三氟甲磺酸铜催化新型苯并恶唑烷衍生物的合成
2017-12-15李肖微穆婉露李惠静
李肖微, 穆婉露, 陈 永, 李惠静
[哈尔滨工业大学(威海) 海洋科学与技术学院,山东 威海 264209]
三氟甲磺酸铜催化新型苯并恶唑烷衍生物的合成
李肖微, 穆婉露, 陈 永, 李惠静*
[哈尔滨工业大学(威海) 海洋科学与技术学院,山东 威海 264209]
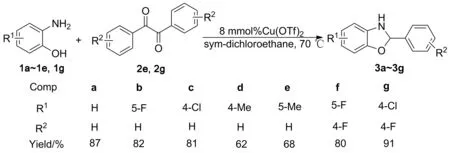
以三氟甲磺酸铜[Cu(OTf)2]为催化剂,取代邻氨基苯酚(1a~1g)和取代联苯甲酰(2a~2g)为原料,合成了7个苯并恶唑烷衍生物(3a~3g,其中3b~3g为新化合物),其结构经1H NMR,13C NMR, IR和HR-MS(ESI)表征。以3a的合成为例,研究了催化剂、溶剂、催化剂用量、物料比γ[n(1) ∶n(2)]和反应温度对3产率的影响。结果表明:在最佳反应条件[8 mmol%Cu(OTf)2, 1,2-二氯乙烷为溶剂,1a~1g1.5 mmol,γ=3 ∶1,于70 ℃反应10 h]下,3a~3g产率62%~91%。
三氟甲磺酸铜; 催化; 苯并恶唑衍生物; 合成; 条件优化
[School of Marine Science and Technology, Harbin Institute of Technology(Weihai), Weihai 264209, China]
多功能杂环化合物,尤其是含氮杂环化合物,在中间体合成和药物化学领域扮演了重要角色[1-3]。其中,同时含有氮原子和氧原子的恶唑烷类化合物可作为合成各种手性化合物的手性助剂[4]和氨基醇的链保护基[5-6],在不对称转化和天然产物合成中有较广泛的应用。目前,多种含恶唑烷环的药物已成功用于临床治疗,如抗癌前药多佐唑烷,多西维酸和多沙唑氨基甲酸酯[7-8]。恶唑烷环还是具有抗肿瘤作用的四氢异喹啉类天然药物[9](如喹喔啉和四嗪)的重要药效基团,以及作为前体药物用于改善部分β-氨基醇的药代动力学[10-12]。此外,恶唑烷衍生物在农药和染料合成中也有一定的应用价值[13-14]。对含恶唑烷骨架化合物合成方法的研究具有现实意义[15-16]。Yoon等[17-18]报道了一种通过铁或铜催化烯烃的不对称氨羟基化反应合成恶唑烷的方法。Zhang等[19]利用偶氮甲基叶立德与羰基化合物的[3+2]环加成反应合成了恶唑烷。Meng等[20]报道了一种新的有机催化策略,在手性配体存在下Pd催化的不对称脱羧反应合成恶唑烷。
Scheme1
本文以Cu(OTf)2为催化剂,取代邻氨基苯酚(1a~1e,1g)和联苯甲酰(2e,2g)为原料,合成了7个苯并恶唑烷衍生物(3a~3g, Scheme 1),其中3b~3g为新化合物,其结构经1H NMR,13C NMR, IR和HR-MS(ESI)表征。以3a的合成为例,研究了催化剂、溶剂、催化剂用量、物料比{γ[n(1) ∶n(2)]}和反应温度对3产率的影响。
1 实验部分
1.1 仪器与试剂
BrukerBiospin ADVANCE III 400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);Nicolet 380型傅里叶变换红外光谱仪(KBr压片);API 3200型质谱仪。
所用试剂均为分析纯。
1.2 3a~3g的合成(以3a为例)
在反应管中加入1a164 mg(1.5 mmol),2e105 mg(0.5 mmol), 8 mmol%Cu(OTf)2和1,2-二氯乙烷2 mL,于70 ℃反应10 h。用乙酸乙酯(3×10 mL)萃取,合并有机相,用饱和食盐水洗涤,无水硫酸钠干燥,过滤,滤液浓缩后经硅胶柱层析[洗脱剂:V(石油醚)/V(乙酸乙酯)=20/1]纯化得3a86 mg。
用类似的方法合成3b~3g。
3a: 乳白色固体,产率87%;1H NMRδ: 4.80(s, 1H), 6.75(d,J=6.7 Hz, 1H), 6.90(d,J=7.4 Hz, 3H), 7.00(d,J=7.9 Hz, 2H), 7.12(t,J=7.4 Hz, 2H), 7.28(t,J=4.8 Hz, 1H);13C NMRδ: 85.8, 115.3, 115.5, 120.8, 121.2, 127.2, 127.6, 128.8, 129.5, 138.9,144.1; IRν: 3 898, 3 743, 3 730, 3 708, 3 518, 3 456, 3 401, 3 370, 3 342, 2 959, 2 933, 2 423, 2 361, 2 341, 2 241, 2 204, 2 158, 2 064, 2 040, 2 012, 1 990, 1 607, 1 501, 1 418, 1 292, 1 261, 1 235, 1 169, 1 093, 1 018, 905, 844, 800, 668 cm-1; HR-MS(ESI)m/z: Calcd for C13H12NO{[M+H]+}198.091 3, found 198.091 3。
3b: 棕色固体,产率82%;1H NMRδ: 4.75(s, 1H), 6.66(dd,J=14.1 Hz, 9.4 Hz, 3H), 6.97(d,J=7.6 Hz, 1H), 7.14(t,J=6.9 Hz, 2H), 7.30(s, 1H);13C NMRδ: 86.7, 102.8, 103.0, 103.2, 107.4, 107.7, 115.4, 115.5, 125.3, 127.3, 127.6, 129.1, 138.2, 144.8, 144.9, 156.5, 158.9; IRν: 3 369, 2 971, 2 832, 2 463, 2 183, 1 633, 1 517, 1 488, 1 385, 1 305, 1 276, 1 247, 1 172, 1 143, 1 102, 1 009, 1 005, 969, 905, 842, 785, 782, 744 cm-1; HR-MS(ESI)m/z: Calcd for C13H11NOF{[M+H]+}216.081 9, found 216.082 3。
3c: 黄色固体,产率81%;1H NMRδ: 4.81(s, 1H), 6.70(s, 1H), 6.81(s, 2H), 6.90(d,J=7.6 Hz, 2H), 7.09(t,J=7.8 Hz, 2H), 7.25(dd,J=12.0 Hz, 4.4 Hz, 1H);13C NMRδ: 85.6, 115.1, 116.6, 120.7, 126.1, 127.4, 127.5, 129.2, 130.5, 138.1, 142.5; HR-MS(ESI)m/z: Calcd for C13H11NOCl{[M+H]+}232.052 4, found 232.051 8。
3d: 黄棕色固体,产率62%;1H NMRδ: 2.28(s, 3H), 4.72(s, 1H), 6.54(s, 1H), 6.65(d,J=8.0 Hz, 1H), 6.79(d,J=8.0 Hz, 1H), 6.99(d,J=7.6 Hz, 2H), 7.09(t,J=7.5 Hz, 2H), 7.23(d,J=7.1 Hz, 1H);13C NMRδ: 21.1, 85.9, 115.6, 116.1, 121.5, 127.4, 127.9, 129.0, 130.9, 139.5, 142.1, 146.4; HR-MS(ESI)m/z: Calcd for C14H14NO{[M+H]+}212.107 0, found 212.107 4。
3e: 黄棕色固体,产率68%;1H NMRδ: 2.28(s, 3H), 4.68(s, 1H), 6.63(d,J=7.8 Hz, 1H), 6.69(d,J=7.9 Hz, 1H), 6.73(s, 1H), 7.00(d,J=7.7 Hz, 2H), 7.10(t,J=7.7 Hz, 2H), 7.26(d,J=14.4 Hz, 1H);13C NMRδ: 20.8, 86.2, 115.1, 116.0, 121.6, 126.9, 127.1, 127.7, 128.7, 130.4, 139.2, 144.2; HR-MS(ESI)m/z: Calcd for C14H13NO{[M+H]+}234.099 7, found 234.099 3。
3f: 黄棕色固体,产率80%;1H NMRδ: 4.67(s, 1H), 6.64(d,J=9.0 Hz, 3H), 6.83(t,J=8.3 Hz, 2H), 6.92~6.95(m, 2H);13C NMRδ: 86.3, 103.1, 103.4, 107.8, 108.0, 114.2, 114.5, 115.6, 115.7, 125.1, 129.5, 129.6, 134.1, 134.1, 144.5, 144.6, 156.6, 159.0, 161.9, 164.4; IRν: 3 357, 2 923, 2 850, 2 362, 2 283, 1 607, 1 509, 1 473, 1 405, 1 305, 1 288, 1 239, 1 164, 1 141, 1 106, 1 018, 1 002, 978, 907, 835, 797, 767, 734 cm-1; HR-MS(ESI)m/z: Calcd for C13H10NOF2{[M+H]+}234.072 5, found 234.071 7。
3g: 黄棕色固体,产率91%;1H NMRδ: 4.84(s, 1H), 6.71(s, 1H), 6.81(d,J=9.5 Hz, 4H), 6.88~6.91(m, 2H);13C NMRδ: 85.2, 114.3, 114.5, 115.2, 116.7, 120.9, 126.4, 129.4, 129.5, 130.2, 134.0, 134.0, 142.0, 161.8, 164.3; IRν: 3 336, 2 926, 1 724, 1 607, 1 509, 1 496, 1 409, 1 381, 1 300, 1 277, 1 233, 1 163, 1 118, 1 103, 1 086, 1 019, 997, 949, 924, 908, 837, 797, 733, 694 cm-1; HR-MS(ESI)m/z: Calcd for C13H10NOFCl{[M+H]+}250.042 9, found 250.043 3。
2 结果与讨论
2.1 合成条件优化
(1) 催化剂和溶剂
表1为催化剂和溶剂对3a产率的影响。由表1可见,No.1~No.8为催化剂对产率的影响。结果表明:以1,2-二氯乙烷为溶剂,Cu(OTf)2作催化剂时,产率最高(No.8, 52%)。No.8~No.14为溶剂对产率的影响。结果表明,1,2-二氯乙烷作溶剂,产率最高(No.8, 52%)。

表1 催化剂和溶剂对3a产率的影响*
*11.0 mmol,20.5 mmol,于70 ℃反应10 h,其余反应条件同1.2;a5 mmol%;b2 mL。

表2 催化剂用量对3a产率的影响*Table 2 Effect of catalyst dosage on yield of 3a
*11.0 mmol,20.5 mmol, 1,2-二氯乙烷2 mL,其余反应条件同表1。
(2) 催化剂用量
表2为催化剂用量对3a产率的影响。由表2可见,不加催化剂Cu(OTf)2时,反应无法进行,随着Cu(OTf)2用量增加,3a产率呈增高趋势,催化剂用量为8 mmol%时,产率最高(No.3, 68%),继续增加催化剂用量,产率反而呈下降趋势。这是因为催化剂Cu(OTf)2的用量较少时,会造成底物反应不充分;催化剂Cu(OTf)2的用量过多,副反应也会进一步增强,主反应的竞争优势变弱,目标产物的产率降低。因此催化剂的最佳用量为8 mmol%。
(3)γ和反应温度
表3为γ和反应温度对3a产率的影响。No.1~No.4为γ对产率的影响,γ=3 ∶1时,产率最高(No.3, 85%)。 No.3和No.5~No.8为反应温度对3a产率的影响。反应温度为70 ℃时,产率最高(No.3, 85%)。

表3 γ和反应温度对3a产率的影响Table 3 Effects of γ and temperature on yield of 3a
*11.0 mmol,20.5 mmol, 1,2-二氯乙烷2 mL, Cu(OTf)28 mmol%,其余反应条件同表2。
2.2 底物适应性
为研究优化后的反应条件对反应底物的适应性,考察了3b~3g的合成情况(Scheme 1)。当邻氨基苯酚芳环上带有吸电子基团(如F和Cl),比带有供电子基团(如甲基)的产率要高。当邻氨基苯酚的芳环上带有吸电子基团,同时联苯甲酰的芳环上也带有吸电子基团时,在最优条件下反应都较容易进行,产率较高。因此,该反应条件具有良好的底物适应性。
以三氟甲磺酸铜[Cu(OTf)2]为催化剂,取代邻氨基苯酚(1a~1e,1g)和取代联苯甲酰(2e,2g)为原料,合成了7个苯并恶唑烷衍生物(3a~3g,其中3b~3g为新化合物)。在最佳反应条件[8 mmol%Cu(OTf)2, 1,2-二氯乙烷为溶剂,1a~1g1.5 mmol,γ=3 ∶1,于70 ℃反应10 h]下,3a~3g产率62%~91%。该反应具有条件温和,绿色环保,操作简单,底物适应性好等特点,为恶唑烷类衍生物的合成提供了一定借鉴。
[1] BARGHILISH A, FARZANEH S, MAMAGHANI M,etal. One-pot,three-component,catalyst-free synthesis of novel derivatives of pyrido-[2,3-d] pyrimidines under ultrasonic irradiations[J].Synthetic Communications,2016,46(14):1209-1214.
[2] PATIL N T, YAMAMOTO Y. Coinage metal-assisted synthesis of heterocycles[J].Chemical Reviews,2008,108(8):3395-3442.
[3] JAKOPIN Z, DOLENC M S. Advances in the chemistry of saccharins:From synthetic novelties towards biologically active compounds[J].Current Medicinal Chemistry,2010,17(7):651-671.
[4] NAKANO H, OKUYAMA Y, KWON E,etal. Chiral oxazolidine catalyst for asymmetric synthesis[J].Heterocycles,2014,89(1):1-26.
[5] PASTOR A, ADAM W, WIRTH T,etal. Diastereoselective reactions of the tiglic acid functionality mediated by oxazolidine chiral auxiliaries:A mechanistic comparison of DMD andm-CPBA epoxidations versus singlet oxygen and PTAD enereactions[J].European Journal of Organic Chemistry,2005,2005(14):3075-3084.
[6] LEE S H, YANG J, HAN T D,etal. Synthesis of 1,3-oxazolidines by copper-catalyzed addition ofacetone and ethyl diazoacetate to imines[J].Tetrahedron Letters,2001,42(20):3487-3490.
[7] BURKHART D J, BARTHEL B L, Post G C,etal. Design,synthesis,and preliminary evaluation of doxazolidinecarbamates as prodrugs activated by carboxylesterases[J].Journal of Medicinal Chemistry,2006,49(24):7002-7012.
[8] POST G C, BARTHEL B L, BURKHART D J,etal. Doxazolidine,a proposed active metabolite of doxorubicin that cross-links DNA[J].Journal of Medicinal Chemistry,2005,48(24):7648-7657.
[9] YOTSU-YAMASHITA M, KIM Y H, DUDLEY S C,etal. The structure of zetekitoxin AB,a saxitoxin analog from the Panamanian golden frog Atelopuszeteki:A potent sodium-channel blocker[J].Proc Natl Acad Sci U S A,2004,101(13):4346-4351.
[10] DANIELSSON J, TOOM L, SOMFAI P,etal. 1,3-Dipolar cycloaddition of azomethine ylides to aldehydes:Synthesis of antiα-amino-β-hydroxy esters[J].European Journal of Organic Chemistry,2011,2011(3):607-613.
[11] SEASHORE L B, TORSSELL S, SOMFAI P,etal. Addition of azomethine ylides to aldehydes:Mechanistic dichotomy of differentially substitutedα-imino esters[J].European Journal of Organic Chemistry,2010,2010(20):3927-3933.
[12] GOSSELIN F, ROY A, O SHEAPD,etal. Oxazolidine ring opening and isomerization to (E)-imines:Asymmetric synthesis of aryl-α-fluoroalkyl amino alcohols[J].Organic Letters,2004,6(4):641-644.
[13] PITT W R, PARRY D M, PERRY B G,etal. Heteroaromatic rings of the future[J].Journal of Medicinal Chemistry,2009,52(9):2952-2963.
[14] ANUMULA R R, KAGGA M, GHANTA M R,etal. Synthesis of new oxazolidinonyl/oxazolidinylcarbazole derivatives forβ-blocking activity[J].Heterocyclic Communications,2007,13(5):187-194.
[15] MICHAELIS D J, ISCHAY M A, YOON T P,etal. Activation ofN-sulfonyloxaziridines using copper(II) catalysts:Aminohydroxylations of styrenes and 1,3-dienes[J].Journal of the American Chemical Society,2008,130(20):6610-6615.
[16] SHAGHAFI M B, GROTE R E, JARVO E R,etal. Oxazolidine synthesis by complementary stereospecific and stereoconvergent methods[J].Organic Letters,2011,13(19):5188-5191.
[17] MICHAELIS D J, ISCHAY M A, YOON T P,etal. Activation ofN-sulfonyl oxaziridines using copper(II) catalysts:Aminohydroxylations of styrenes and 1,3-dienes[J].Journal of the American Chemical Society,2008,130(20):6610-6615.
[18] WILLIAMSON K S, YOON T P. Iron-catalyzed aminohydroxylation of olefins[J].Journal of the American Chemical Society,2010,132(13):4570-4571.
[19] WU X, LI L, ZHANG J,etal. Nickel(II)-catalyzed diastereoselective [3+2] cycloaddition ofN-tosyl-aziridines and aldehydesviaselective carbon-carbon bond cleavage[J].Chemical Communications,2011,47(27):7824-7826.
[20] ZHANG L B, YANG S R, WANGJ Q,etal, A facile preparation and electrochemical properties of nickel based compound graphene sheet composites for supercapacitors[J].Chinese Chemical Letters,2015,26(5):522-528.
Copper(II)Trifluomethanesulfonate-catalyzedSynthesisofNovelBenzoxazolidineDerivatives
LI Xiao-wei, MU Wan-lu, CHEN Yong, LI Hui-jing*
Seven benzoxazolidine derivatives(3a~3g), among them3b~3gwere novel compounds, were synthesized with the substrates of substitutedo-aminophenols(1a~1g) and substituted 1,2-diphenylethane-1,2-dione(2a~2g), using copper(II) trifluoromethanesulfonate as catalyst. The structures were characterized by1H NMR,13C NMR, IR and HR-MS(ESI). The effects of catalyst, solvent, catalyst dosage, mole ratio{γ[n(1) ∶n(2)]} and reaction temperature on yield of3were investigated using synthesis of3aas the template reaction. The results showed that under the optimum reaction conditions[8 mmol% Cu(OTf)2, 1,2-dichloroethane as solvent,1a~1g1.5 mmol,γ=3 ∶1, reaction at 70 ℃ for 10 h], the yield of3a~3gwere 62%~91%.
copper(II) trifluoromethanesulfonate; catalysis; benzoxazolidine derivative; synthesis; optimization
2017-05-16;
2017-10-18
国家自然科学基金资助项目(21272046)
李肖微(1992-),女,汉族,河南周口人,硕士,主要从事有机合成的研究。 E-mail: lixiaoweihit@126.com
李惠静,教授,博士生导师, E-mail: lihuijing@iccas.ac.cn
O626.2
A
10.15952/j.cnki.cjsc.1005-1511.2017.12.17113
