棒状CuFe4Ox的可控合成及其异戊醇脱氢反应的催化性能
2017-12-13马宏彬马令娟侯梦宁岳明波
马宏彬 马令娟 侯梦宁 岳明波
棒状CuFe4Ox的可控合成及其异戊醇脱氢反应的催化性能
马宏彬 马令娟*侯梦宁 岳明波
(曲阜师范大学化学与化工学院,曲阜 273165)
利用液相沉淀法可控合成了均匀的棒状CuFe4Ox催化剂。通过原位X射线粉末衍射(XRD)、高分辨透射电子显微镜(TEM)及程序升温还原(TPR)等手段表征其晶相结构、形貌和还原性能。通过还原棒状CuFe4Ox获得Cu0/Fe3O4纳米棒,原位X射线光电子能谱(XPS)用于确定Cu0/Fe3O4表面的相组成。通过液相沉淀法制备棒状CuFe4Ox,在120℃保持3 h后加入Na2CO3溶液至pH等于9时所得棒状形貌最为规整。以异戊醇脱氢反应作为探针反应,比较了Cu0/Fe3O4纳米棒和Cu0/Fe3O4纳米颗粒的催化反应性能,发现Cu0/Fe3O4纳米棒比Cu0/Fe3O4纳米粒子具有更好的活性和稳定性,表明棒状Fe3O4担载的Cu纳米粒子具有更好的结构稳定性。
CuFe4Ox复合物;XPS;异戊醇脱氢
0 Introduction
Morphology-dependent nanocatalysts have been intensively explored over metal and metal oxides[1-3],but less attention has been paid to binary metal oxidesand oxide-supported metals.Copper-based catalysts are extensively studied due to their good catalytic performance in many industrial reactionssuch as methanol synthesis and water gas shift reactions[4-6].And,Cu/Fe3O4catalysts are one of the most effective catalysts and their catalytic properties strongly depended on the properties of Fe3O4particles[7].The major drawbacks of Cu-based catalyst are still the difficulties of homogeneous dispersion of Cu particles on supports and poor thermal stability[8].Yang et al.proposed a space-confined synthesis method to prepare rod-shaped CuFe2O4which was reduced to Cu/Fe3O4catalyst with fine Cu0nanoparticles supported on Fe3O4rod[9].Additionally,the precursors of copper are also very important for the stability and activity of copper species.Kameoka et al.[10]proposed that spinel CuFe2O4was an effective precursor for a high performance copper catalyst which showed high thermal stability and activity.However,Cu content in stoichiometric CuFe2O4is too high to be dispersed on the surface of Fe3O4support.As a result,controllable synthesized rod-shaped CuFe4Oxwas used as the precursor of Cu/Fe3O4and the composition of reduced product are well studied.
It is also known that copper-based catalysts show high activity in the dehydrogenation of alcohols[11].Isovaleraldehyde is an important industrial intermediary in the manufacturing of synthetic resins,special chemicals and isovaleric acid which is widely used in the medical industry and Shiau et al.indicated that Cu-based catalysts have a good activity for isoamylic alcohol dehydrogenation[12].Crivello et al.[13]also studied the performance ofCr-Cu-Mg catalysts in the dehydrogenation of isoamylic alcohol.In this paper dehydrogenation of isoamylic alcohol was used to assess the catalytic performance of Cu/Fe3O4-nanorods and Cu/Fe3O4-nanoparticle was also prepared to compare the performance with Cu/Fe3O4-nanorods.
1 Experimental details
1.1 Catalyst preparation
All the chemicals were analytical grade and were used without further purification.The synthesis of CuFe4Oxcomposite with rod-shape was prepared by an aqueous precipitation method which was in accordance with the preparation of α-Fe2O3nanorods[14].The typical synthesis procedure was described as follows.CuCl2·2H2O(0.68 g),FeCl3·6H2O(4.32 g),NaCl(11.60 g),and PEG(10 mL)were dissolved in 190 mL water and then the solution was gradually heated to 120℃at vigorous stirring and stay at 120℃for 3 h.A Na2CO3aqueous solution (0.2 mol·L-1)was added through a syringe pump at a rate of 1.0 mL·min-1.The mixture was then aged at 120℃for 1 h.The precipitate was washed with water and ethanol,and dried at 60℃for 6 h.Finally,the dried sample was calcined at different temperature.
CuFe4Oxnanoparticles were prepared by coprecipitation method.An aqueous solution of Cu(NO3)2·3H2O(4 mmol)and Fe(NO3)3·9H2O(16 mmol)was rapidly added into 100 mL 0.5 mol·L-1Na2CO3aqueous solution at room temperature.The obtained reddish brown precipitate was collected by filtration,washed with deionized water and ethanol,dried at 80℃overnight and finally calcined at 500℃for 10 h in air condition.
1.2 Characterization of samples
X-ray diffraction(XRD)pattern was performed on aRigakuD/MAX-RB diffractometerwith Cu Kα radiation(λ=0.154 nm)at U=40 kV and I=200 mA.Powder patterns of uncalcined precursor and catalysts obtained by calcined at different conditions were recorded in the 2θ range of 15°~80°and the scan rate was 3°·min-1.In-situ XRD were carried out in the 2θ range of 15°~60°while a catalyst was in a reductive atmosphere(5%H2balanced with 95%He)with a scan rate of 5℃·min-1to track potential evolution of phase in the H2treatment.N2adsorption-desorption of sample was tested by Nova 4200e physicaladsorption instrument.
The HRTEM images were gotten from the JEM-2010 and TEM images were examined using Hitachi 7700 transmission electron microscope.The X-ray photoelectron spectroscopy(XPS)was performed in an ultrahigh-vacuum chamber that has attached a highpressure cell or batch reactor.The sample could be transferred between the reactor and vacuum chamber withoutexposure toair.Temperature-programmed reduction (TPR)experiments were performed in a quartz tube micro-reactor connected to an Auto Chem 2920 instrument.The experiments were performed on 40 mg of catalyst under the flowing 5%H2balanced with N2(30 mL·min-1)with a heating rate of 1℃·min-1.
1.3 Catalytic activity
Catalytic activities of the CuFe4Oxnanoparticles and nanorods were carried out in a continuous flow type reactor with a fixed catalyst bed operated at atmospheric pressure.Isoamylic alcohol serves as reagent was introduced through N2gas.The W/F0is controlled to 41.3 gcatalyst·h·mol-1.Only isoamylic alcohol,isovaleraldehyde and hydrogen were detected in the reaction,other products were found in trace amounts and will be neglected.Analysis of the feed and effluent gas were performed after water condensation by gas chromatograph(GC).
2 Results and discussion
2.1 Controllable synthesis of CuFe4Oxrod
Rod-like CuFe4Oxsample was prepared by the aqueous precipitation method which described detailed in the experiments.It is well known that the shape and phase composition ofsamples were intimately correlated to the synthesis conditions,like pH value,reaction time,calcination temperature.In the process of synthesis of rod-like CuFe4Oxprecursor,Na2CO3precipitant was introduced when the temperature increase to 120℃.It was found that the stirring time at 120℃before Na2CO3was introduced also effect the shape of product.As a result,we focus on the effect of pH value,stirring time at 120℃,calcination temperature on the shape and phase composition of CuFe4Oxsample.
Fig.1showstheTEM imagesofprecursors prepared under various pH values and stirring time at 120℃.In the experiment,yellow-brown precipitants were formed as Fe3+start to hydrolysis when temperature increased to 80~90℃.It is easy to understand the pH value of the system decreases with the hydrolysis of Fe3+.While when the temperature continued rising to 120℃,Fe3+ions were completely hydrolyzed and the pH value of the solution was about 1 which is too low for Cu2+to form precipitant.It is obviously that the pH value was one of the most important factors.The pH value can be easily adjusted by adding different amount of Na2CO3.Fig.1(a,b,c)show the TEM images of precursors prepared at pH=12,10 and 9,respectively.As pointed by the red arrow in Fig.1a and Fig.1b,that some nanoplates or spindle particles were formed besides rods when pH value is high to 10 or 12.While,when pH value was 9,only rod-shaped particles were detected in the products.
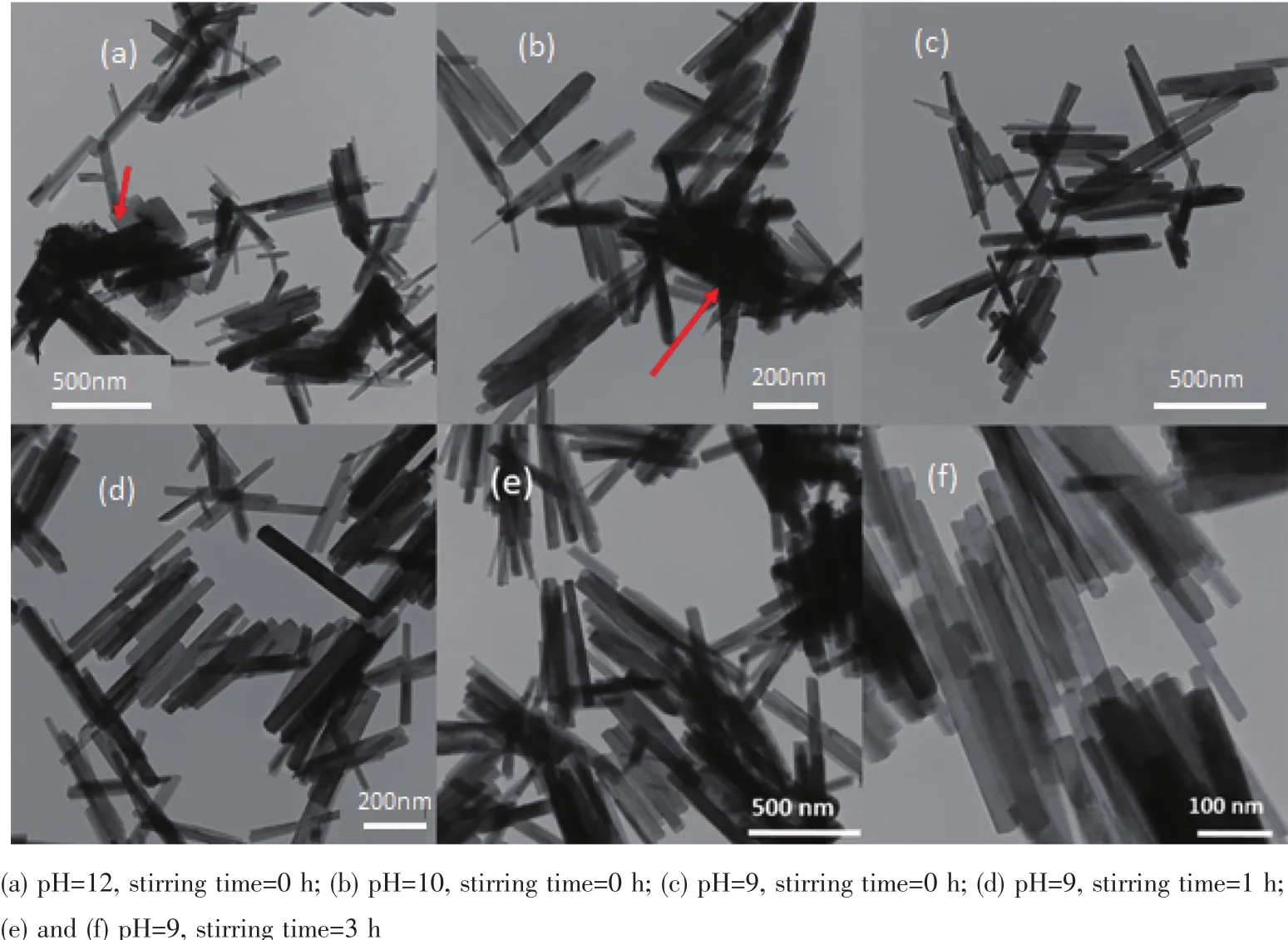
Fig.1 TEM images of precursors prepared at different pH values and at 120℃before Na2CO3solution was introduced
It has been reported that rod-shaped β-FeOOH was formed with the same aqueous precipitant method without Cu2+in the reaction system[14].As a result,particles with plate or spindle shape(as shown in Fig.1a and b)were attributed to the precipitant of Cu2+.While when pH value was low to 9,only rod can be detected which means it is suitable for the preparation of rod-shaped sample when the pH value is 9.But the diameter and length of rods are not very uniform.It was found that the stirring time at 120℃before Na2CO3solution was introduced can also affect the uniform of rod shape.In order to obtain a rod-shaped sample with uniform diameterand length timedependentexperiments were carried out.When prolonging the stirring time at 120℃before Na2CO3solution was introduced,the diameter and length of therod-shaped productbecomemoreand more uniform.When stirring time was extended to 3 h,more uniform rod-shaped precursors can be obtained as shown in Fig.1e and f.The average diameter and length of the rod-shape precursors are 30 and 285 nm,respectively.
Calcination is necessary for the formation of CuFe4Oxoxide composite.Fig.2 showstheXRD patterns of CuFe4Oxprecursor before calcination and calcined at different temperatures for different time.It shows clearly that the uncalcined precursor was composedofβ-FeOOH andCuO phases.When calcined at 500 ℃ for 5 h β-FeOOH transformed to α-Fe2O3with that α-Fe2O3reacted with CuO to form CuFe2O4at the same time.While,CuO phase still can be detected by XRD which means CuO cannot completely reacted with Fe2O3by calcination at 500℃for 5 h.Prolong the calcination time to 10 h at 500℃,CuO phase was almost completely disappeared.However,when improved the calcination temperature to 600℃and calcined for 5 h,CuO phase still can be detected.So,500℃is suitable to form CuFe2O4.Fig.3 shows the TEM images of samples obtained at different calcination conditions.It can be seen clearly that calcined at 500℃for 5 h,sample can retain rod shape while when prolonged the time to 10 h at 500℃or risen the calcination temperature to 600℃,sample was seriously sintered together.In order to obtain uniform rod shape,sample was calcined at 500℃for 5 h.ICP results of CuFe4Oxshow that the ratio of Cu to Fe is consistent with the initial feed ratio which Cu versus Fe equal to 1∶4.The BET surface area of CuFe4Oxwhich was calcined at 500℃for 5 h was 36 m2·g-1.It is relatively high for metal oxide.From the TEM images (Fig.3a),it is clearly that a lot of pores are formed during the calcination.That is the reason why CuFe4Oxshows high BET surface areas.
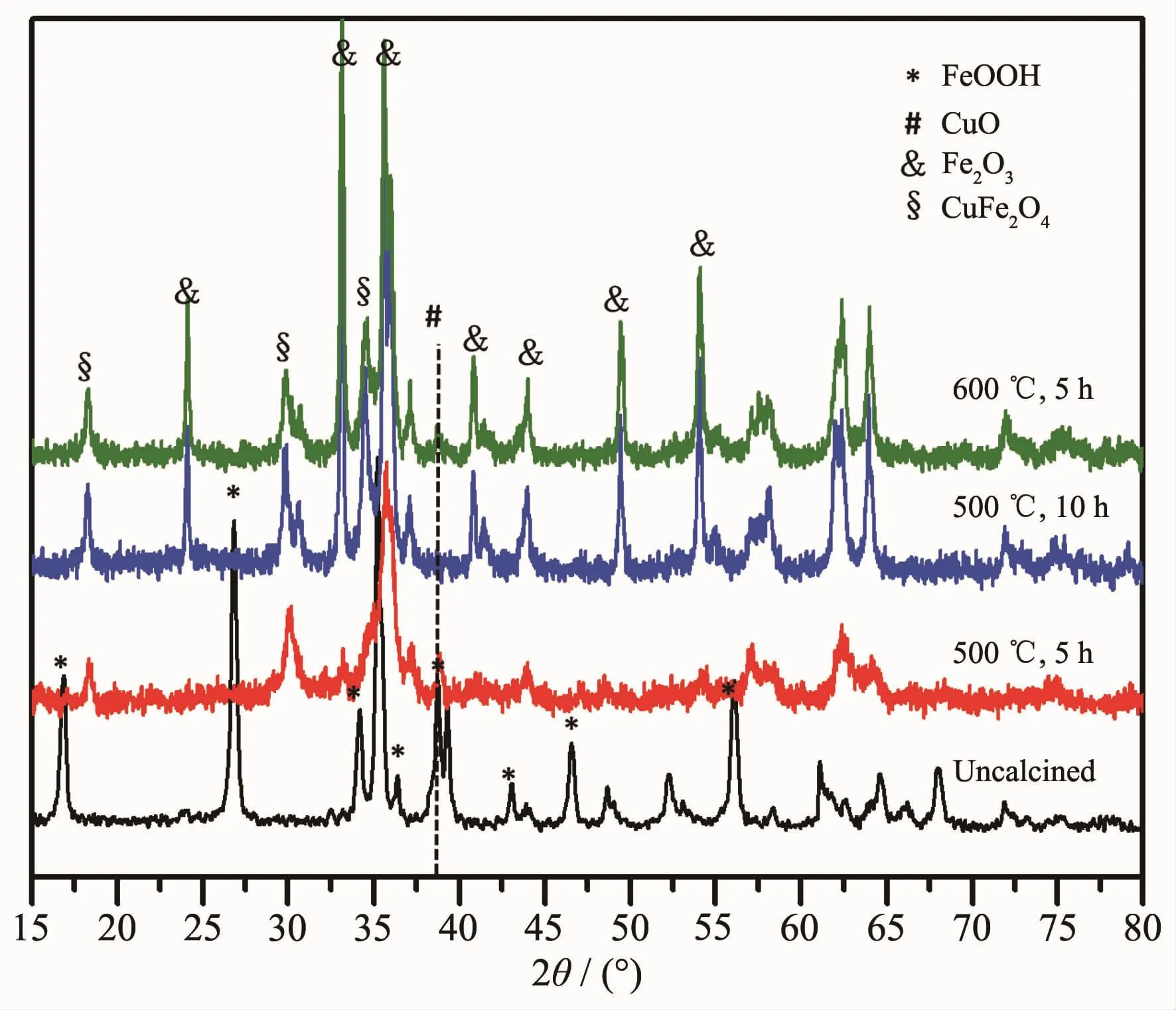
Fig.2 XRD patterns of uncalcined precursor and products obtained by calcined at different conditions
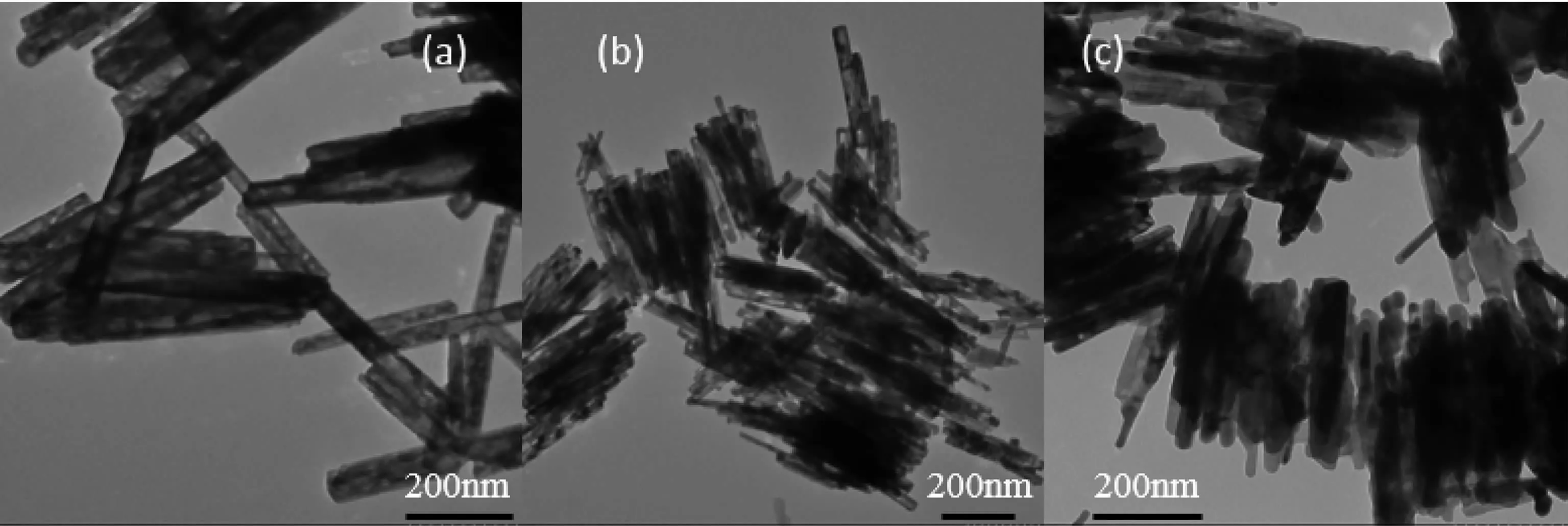
Fig.3 TEM images of CuFe4Oxsamples obtained by calcination at different conditions:(a)500℃for 5 h;(b)500℃for 10 h;(c)600℃for 5 h
2.2 Reducibility of the CuFe4Oxrod sample
In order to elucidate the surface and bulk oxygen reducibility of CuFe4Oxnanorods calcined at 500℃for 5 h,H2-TPR measurement was carried out and the TPR profile was shown in Fig.4.It presented two obviously different reduction regions:the low-temperature regime at 50~353℃and the high-temperature regime at 353~650℃.As well studied that Fe2O3shows one small peak at 420℃for the reduction of Fe2O3to Fe3O4and one higher temperature peak above 650℃ for the reduction of Fe3O4to metal Fe[15-16].While CuFe2O4also presents two distinct reduction peaks with one lower temperature reduction attribute to the reduction of CuFe2O4to Cu and Fe3O4,and the other higher reduction peak assigned to the reduction of Fe3O4to metal Fe[9].It can be concluded that the reductions of Cu species and the reduction of Fe2O3to Fe3O4are much easier than the reduction of Fe3O4to Fe.As a result,the lower peaks below 353℃should be attributed to the reduction of CuFe2O4to Cu0and Fe3O4and the reduction of Fe2O3to Fe3O4.The broad reduction peak around 530℃should be assigned to the reduction of Fe3O4to Fe metal as reported by Kameoka et al.[17]and Faungnawakij et al.[18].It need to note that the small peak at 129℃must be the reduction of surface Cu2+species,as only surface CuO can be reduced at such low temperature.
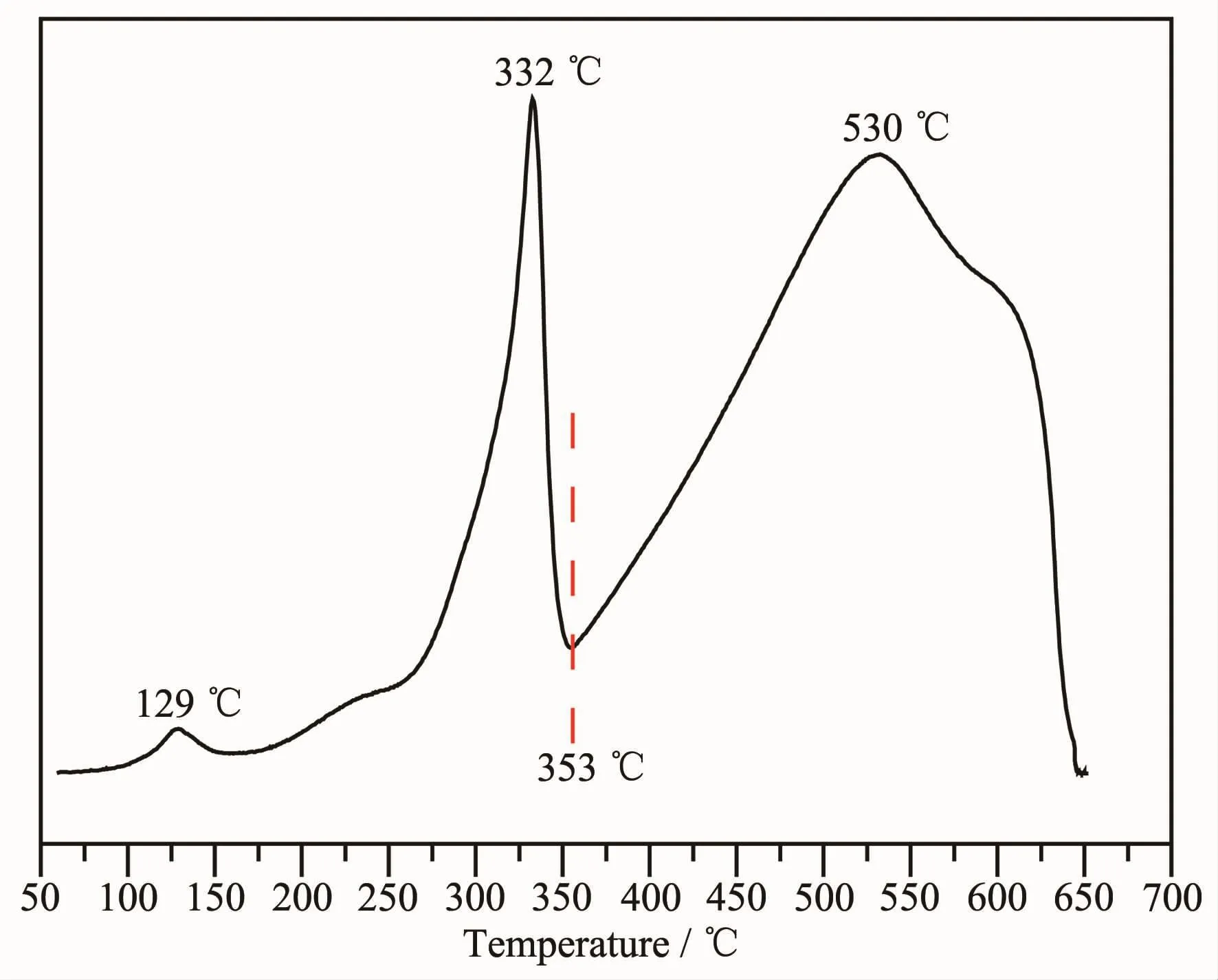
Fig.4 H2-TPR profile of CuFe4Oxnanorods obtained by calcined at 500℃for 5 h
In order to get further insight about the phase transformation in the H2reduction process,the phases of CuFe4Oxwere analyzed via in-situ XRD under 5%H2(He balance).Fig.5 shows the in-situ XRD evolution of CuFe4Oxrods under 5%H2(He balance)at different temperature.As mentioned before,the freshly prepared sample calcined at 500℃for 5 h exhibits a phase mixture of Fe2O3(PDF No.86-0550),CuO(PDF No.80-0076)and CuFe2O4(PDF No.34-0425).It can be observed clearly that CuO and Fe2O3are almost disappeared at 250 and 350℃,respectively,which suggests the reduction of Fe2O3is more difficult than the reduction of CuO.The transformation of CuFe2O4should happened at about 300℃as the XRD reflection of Cu0suddenly increased.So,the spinel phase formed at temperature higher than 300℃should be ascribed to Fe3O4even though it is difficult to distinguish the XRD position of Fe3O4and CuFe2O4.as Cu0was isolated from CuFe2O4.These analysis results support the ascription of TPR peaks at 129℃to the reduction of CuO and the ascription of the following peak at 332℃to the reductions of CuFe2O4and Fe2O3.The broad reduction peak at 552℃should be assigned to the reduction of Fe3O4to Fe0as before.
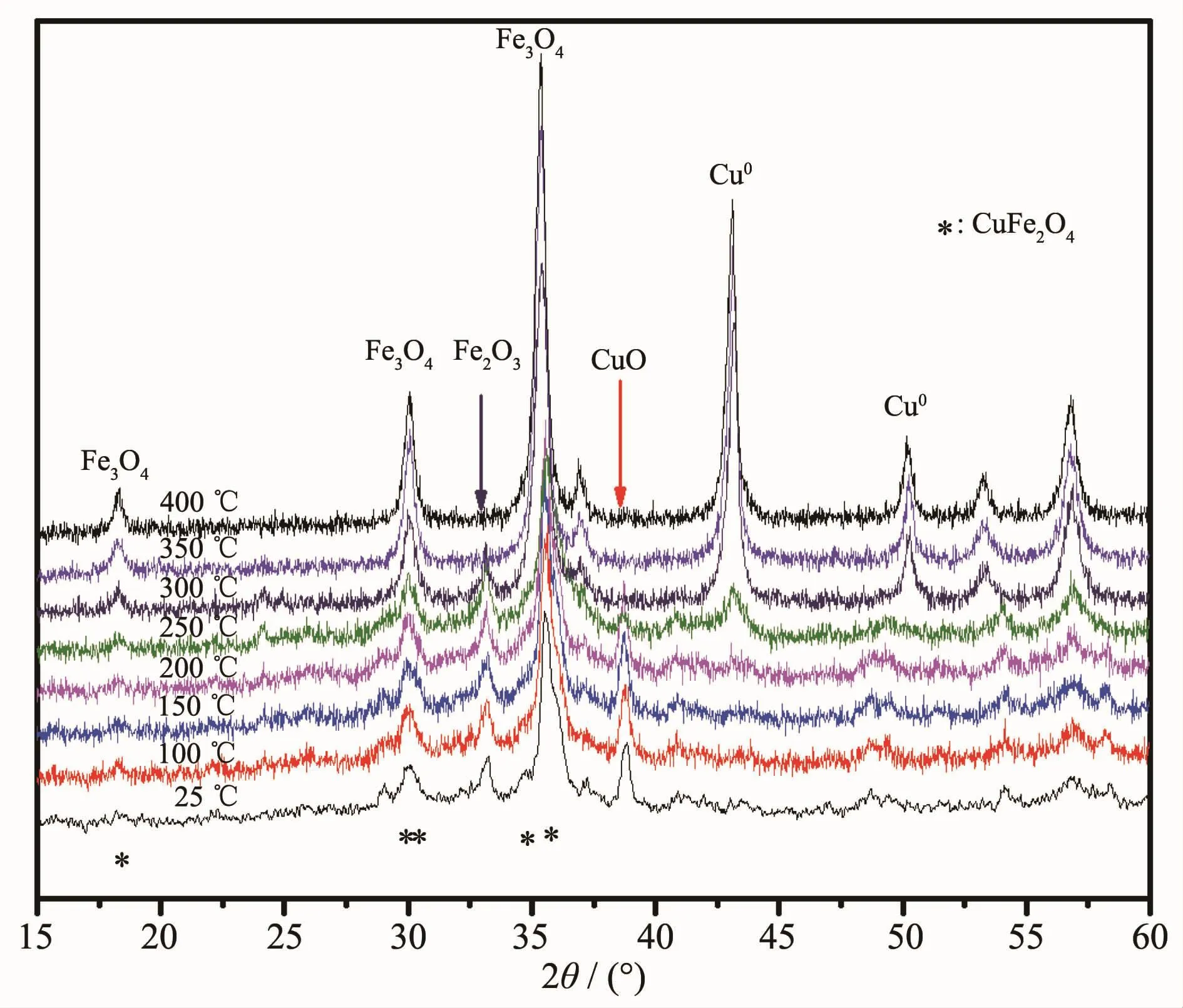
Fig.5 XRD patterns recorded in-situ during CuFe4Ox reduction under 5%H2(He balance)at different temperatures
2.3 Cu species of reduced sample
From the results ofin-situ XRD and TPR analysis,it can be deduced that CuFe4Oxcan be controllably reduced to Cu-Fe3O4.The TEM and HRTEM images of CuFe4Oxsample reduced at 300℃are shown in Fig.6.Fig.6a and b display that Fe3O4can inherit the rod shape of CuFe4Oxsample and a lot of nanoparticles are supported on the rod-particles.HRTEM results are shown in Fig.6c confirm the rodshaped supportsare Fe3O4and supported small nanoparticles are Cu2O.It is well known that freshly formed Cu nanoparticles are very active and can be oxidized when contact with air.As a result,the observed Cu2O nanoparticles must be the production of oxidation of Cu nanoparticles in air conditions.And the conclusion was further confirmed by the in-situ XPS results.
Fig.7 illustrates the XPS profiles of Cu2p and CuL3VV of freshly prepared and in-situ reduced CuFe4Oxsample.For the freshly prepared CuFe4Oxsample,the kinetic energy of 917.5 eV in the spectra of CuL3VV Auger corresponded to Cu2+[19];the binding energy of Cu2p3/2at 932.9 eV,together with the relatively large satellite peak at 938~948 eV with a shake-up structure,further evidenced the presence of Cu2+[20-22].Upon hydrogen reduction at 300℃,the shake-up satellite peak vanished,indicating the reduction of Cu2+species.The kinetic energy of 918.7 eV in CuL3VV Auger spectra indicates the appearance of metallic copper species.Furthermore,the binding energy of Cu2p3/2in Cu2p spectra lowered to 931.9 eV representing metallic copperspecies.Thisresult suggests the presence of Cu0after reduction at 300℃in H2.The combination of the results of in-situ XRD and in-situ XPS and HRTEM confirm that the CuFe4Oxsamples are reduced to Cu0/Fe3O4rods composite by 5%H2at 300℃.
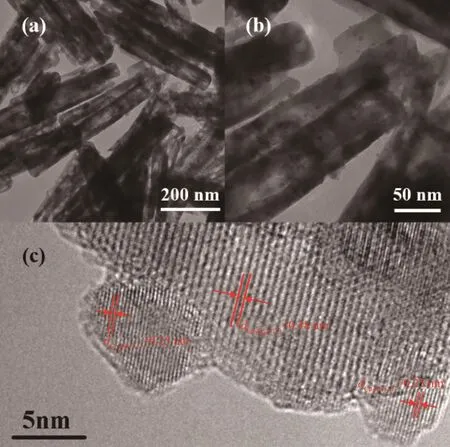
Fig.6 TEM(a,b)and HRTEM(c)images of the reduced CuFe4Ox
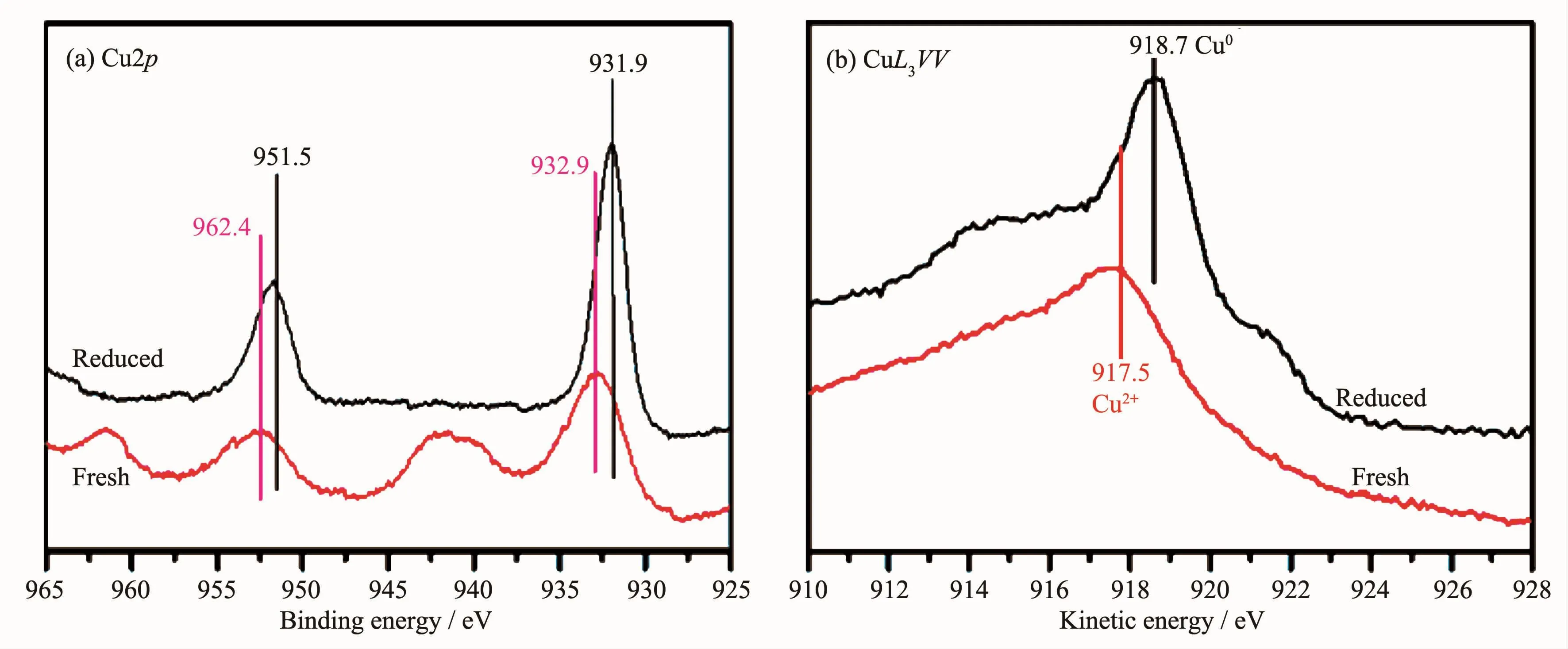
Fig.7 In-situ XPS profiles of reduced and fresh CuFe4Oxnanorods
2.4 Dehydrogenation of isoamylic alcohol activity
It is known that copper based catalyst is used widely in the dehydrogenation of alcohols and copper is indeed an essential component of the active phase of the catalyst for the dehydrogenation of isoamylic alcohol.So,the catalytic activity of CuFe4Oxcalcined at 500℃and hydrogen reduced at 300℃was assessed in the dehydrogenation of isoamylic alcohol to isovaleraldedyde.Co-precipitation method was used to prepare CuFe4Oxcatalyst with same copper contents and its catalytic performance was compared with CuFe4Oxnanorods.ICP results show the molar ratio of copper to iron in both nanorods and nanoparticles are inconsistent with the formula of CuFe4Ox.The TEM imagesand XRD patternsofreduced CuFe4Oxnanoparticles are shown in Fig.6a and 6b.The TEM image showsthe sample wasin the shape of nanoparticles and the XRD profile shows that nanoparticle is composed of well characterized α-Fe2O3and CuFe2O4which shows the similar composition with nanorod samples.
Fig.8 shows the conversion versus time on stream using reduced CuFe4Oxcatalysts with different shapes.It is clear that the initial conversion was similar for both nanoparticlesand nanorodscatalystswhich illustrate that copper is the active phase for this reaction.However,there is a significant difference in their conversion profiles.The conversion of isoamylic alcohol over nanoparticles decreases rapidly to 0%in 500 min.While,the conversion over nanorods decreases to 40%at about 250 min and then the conversion can stable for more than 500 min.According to the catalytic analysis,it can be concluded that rodshaped samples show higher stability than particles.The formation ofthissignificantdifference was thought to be the sintering of Cu particles or the formation of coke.As a result,the rod shape may increase the interaction of Cu0and Fe3O4support and delay the growth of Cu0nanoparticles size.
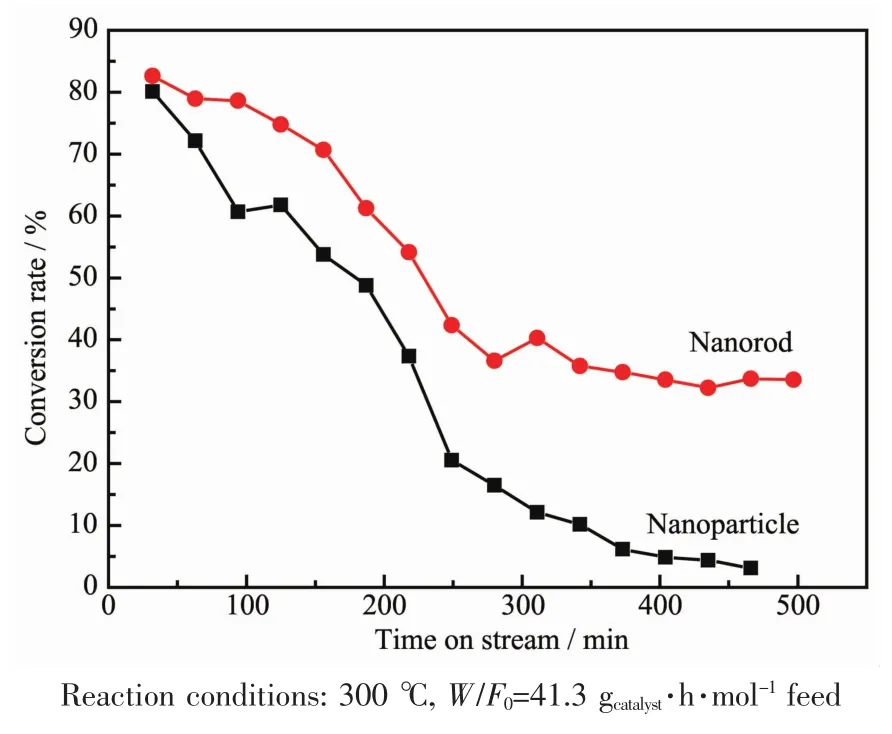
Fig.8 Conversion rate of isoamylic alcohol vs time on stream using reduced CuFe4Oxnanoparticles and nanorods
3 Conclusions
In summary,we have prepared rod-shaped CuFe4Oxcompound by one step liquid-phase precipitation method and the reaction conditionsare carefully controlled.The structuralanalysis and reduction properties of CuFe4Oxhave been performed.In-situ XRD and TPR analysis results show that Cu specie can be reduced at lower temperature than the reduction of Fe2O3phase.When reduced at 300℃by 5%H2,CuFe4Oxcan be reduced to Cu0/Fe3O4while Fe3O4can inherit rod shape.The in-situ XPS and HRTEM results show that Cu0nanoparticles are highly dispersed on the surface ofFe3O4rod.Comparing with Cu/Fe3O4nanoparticles,Cu/Fe3O4nanorods display higher stability in the dehydrogenation of isoamylic alcohol,suggesting that Cu0nanoparticles show better stability on rod-shaped Fe3O4supporter.
Acknowledgements:This work is supported by the National Natural Science Foundation of China (Grant No.21403124)and Natural Science Foundations ofShandong Province(Grants No.ZR2014JL014,ZR2014BM012).
[1]Li Y,Liu Q Y,Shen W J.Dalton Trans.,2011,40(22):5811-5826
[2]CAO Xiao-Feng(曹霄峰),ZHANG Lei(张雷),LI Zhao-Qian(李兆乾),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(11):2373-2378
[3]HU Han-Mei(胡寒梅),DENG Cong-Hai(邓崇海),SUN Feng-Xia(孙凤霞),et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(2):405-410
[4]Ratnasamy C,Wagner J P.Catal.Rev.Sci.Eng.,2009,51(3):325-440
[5]Gawande M B,Goswami A,Felpin F,et al.Chem.Rev.,2016,116(6):3722-3811
[6]DING Ying-Ru(丁莹如),YAO Jian-Hua(姚建华),LIU Qi-Sheng(刘其盛),et al.Chinese J.Inorg.Chem.(无机化学学报),1989,5(1):119-121
[7]Estrella M,Barrio L,Zhou G,et al.J.Phys.Chem.C,2009,113(32):14411-14417
[8]Takeguchi T,Kani Y,Inoue M,et al.Catal.Lett.,2002,83(1/2):49-53
[9]Yang S C,Su W N,Lin S D,et al.Appl.Catal.,B,2011,106(3/4):650-656
[10]Kameoka S,Tanabe T,An P T.Catal.Lett.,2005,100(1/2):89-93
[11]Carotenuto G,Tesser R,Serio M D,et al.Catal.Today,2013,203(203):202-210
[12]Shiau C Y,Chen S,Tsai J C,et al.Appl.Catal.,A,2000,198(1/2):95-102
[13]Crivello M,Pérez C,Fernández J,et al.Appl.Catal.,A,2007,317(1):11-19
[14]Mou X L,Zhang B S,Li Y,et al.Angew.Chem.Int.Ed.,2012,51(12):2989-2993
[15]Khan A,Smirniotis P G.J.Mol.Catal.A:Chem.,2008,280(1/2):43-51
[16]Reddy G K,Gunasekera K,Boolchand P,et al.J.Phys.Chem.C,2011,115(15):7586-7595
[17]Kameoka S,Tanabe T,An P T.Appl.Catal.,A,2010,375(1):163-171
[18]Faungnawakij K,Kikuchi R,Fukunaga T,et al.Catal.Today,2008,138(3/4):157-161
[19]Poulston S,Parlett P M,Stone P,et al.Surf.Interface Anal.,1996,24(12):811-820
[20]Tang X,Zhang B,Yong L,et al.Appl.Catal.,A,2005,288(1/2):116-125
[21]Zeng S H,Liu K W,Zhang L,et al.J.Power Sources,2014,261(5):46-54
[22]Qi L,Yu Q,Dai Y,et al.Appl.Catal.,B,2012,119-120(21):308-320
Rod-like CuFe4OxComposite:Controllable Synthesis and Catalytic Performance in Isoamylic Alcohol Dehydrogenation
MA Hong-Bin MA Ling-Juan*HOU Meng-Ning YUE Ming-Bo
(School of Chemistry and Chemical Engineering,Qufu Normal University,Qufu,Shandong 273165,China)
Uniformly rod-shaped CuFe4Oxcatalysts were controllably fabricated through a liquid-phase precipitation method.The phase structure,morphology and reduction properties of the catalyst were characterized by in-situ powder X-ray diffraction (XRD),high resolution transmission electron microscope (HRTEM)and temperatureprogrammed reduction (TPR).Cu0/Fe3O4-nanorod obtained from the reduction of rod-shaped CuFe4Oxstructure and the surface phase composition of Cu0/Fe3O4was detailed studied by in-situ X-ray photoelectron spectroscopy(XPS).CuFe4Oxcomposites with rod-shape were prepared by an aqueous precipitation method that means after stirring time at 120℃for 3 h,Na2CO3solution was added until pH value equal to 9.In this case,the most uniform CuFe4Oxcomposites with rod-shape were obtained.Cu0/Fe3O4-nanorod catalyst presents higher activity and stability for the dehydrogenation of isoamylic alcohol than Cu/Fe3O4-nanoparticles,due to Cu0nanoparticles supported on the rod-shaped Fe3O4exhibits higher structure stability.
CuFe4Oxcomposite;XPS;dehydrogenation of isoamylic alcohol
O643.32;O614.121
A
1001-4861(2017)12-2193-08
10.11862/CJIC.2017.275
2017-07-12。收修改稿日期:2017-09-22。
国家自然科学基金(No.21403124)和山东省自然科学基金(No.ZR2014JL014,ZR2014BM012)资助项目。
*通信联系人。 E-mail:malingjuan@qfnu.edu.cn
