α-Ti(HPO4)2微球负载型催化剂的合成及其烯烃聚合催化性能
2017-12-13义建军石于坚徐庆红
袁 苑 义建军 石于坚 徐庆红*,
α-Ti(HPO4)2微球负载型催化剂的合成及其烯烃聚合催化性能
袁 苑1,2义建军2石于坚1徐庆红*,1
(1北京化工大学理学院,化工资源有效利用国家重点实验室,北京 100029)
(2中国石油天然气股份有限公司石油化工研究院,北京 102206)
2种茂金属催化剂及1种后过渡金属催化剂分别被固载于经过甲基铝氧烷处理后的α-Ti(HPO4)2微球表面,制备得到3种微球负载型催化剂。在烯烃聚合反应过程中,3种负载型催化剂均表现出比硅胶负载型催化剂更高的催化活性。2种茂金属负载型催化剂在乙烯、丙烯聚合反应中的活性分别高达6.8×107gPE·(molZr·h)-1和5.0×107gPP·(molZr·h)-1,所产生的烯烃聚合产物分子量分布较窄(Mw/Mnlt;2.3),表现出良好的单中心催化特性,而且丙烯聚合产物的等规度高达96.5%。负载型后过渡金属催化剂在乙烯聚合反应中的活性稍低,但也能够达到8.3×106gPE·(molFe·h)-1。3种负载型催化剂催化烯烃聚合产物均成微球型,能够很好地复制载体的形貌。
α-Ti(HPO4)2;球型;茂金属;后过渡金属催化剂;烯烃聚合
0 Introduction
Since pioneering works of Ziegler and Natta in coordination polymerization[1-2],the research on polyolefin catalyst has made great progress and promoted the development of polyolefin industry.Today,Ziegler-Natta catalyst (titanium catalyst)[3]and Phillips catalyst(chromium catalyst)[4]are the most widely used catalysts to produce HDPE,LLDPE,PP and other polyolefin products.Although amount of metallocene catalysts[5]applied in polyolefin industry is relatively small,the demand of metallocene polyolefin grows very fast.
In the last two decades,some non-metallocene transition metal catalysts with high activity were reported,such as Ni α-diimine catalyst[6-7],Fe pyridine-diimine catalyst[8-9],FI catalyst[10-11]and PI catalyst[12-13].These new catalysts can be used to prepare polyolefin with unique properties,such as highly branched polyethylene[14-15],ethylene/polar monomer copolymer[16],multiblock copolymer[17-18], olefin block copolymers(OBCs)[19-20],which are hardly produced by the traditional catalysts.
The homogeneous metallocene catalyst and late transition metal catalyst have stimulated considerable research efforts in the polyolefin area[21-24].In comparison with the conventional Ziegler-Natta catalyst,these homogeneous catalysts exposed two main problems fora large-scale production:difficulty controlling polymer particle morphology and severe reactor fouling.To overcome these drawbacks,homogeneous catalysts are supported on proper carriers[25-26].A variety of materials have been used as carriers for metallocene or non-metallocene catalysts,such as silica[27-28],MgCl2[29-30],Al2O3[31],zeolite[32-33],montmorillonite[34-35],LDHs[36],polystyrene[37-38]and polysiloxane[39].
The acidity in solid supports is found to have benefit in polymerization process,and the existence of Lewis acid sites are found to increase activity of the catalyst[40-41].In our previous studies[42],α-Ti(HPO4)2,a solid acid,was prepared and used to support metallocene catalysts.Fine powdery polyolefin were prepared without obvious bulk and no polymer stuck on the inner wall of polymerizer by using the powder α-Ti(HPO4)2supported metallocene catalysts,and the supported catalysts show higher activities than that of the silica supported catalysts under similar polymerization conditions.α-Ti(HPO4)2can improve efficiency of active sites in metallocene/MAO system and kept the single-site characteristic ofmetallocene.The polyethylene and polypropylene obtained in our previous studies[42]have narrow molecular weight distributions.However,the morphology of polymer particles is irregular which may decrease flow ability and bulk density of the product.
It is known that the morphologies of polyolefin catalysts can be replicated into the polymer particles[43-44],so we designed to use spherical α-Ti(HPO4)2supported catalysts to obtain ball-shaped polyolefin.In our present experiments,α-Ti(HPO4)2was prepared firstly and the spherical carrier was finally prepared by spray drying the slurry of α-Ti(HPO4)2.After being treated with methylaluminoxane (MAO),the new spherical carrier was used to load metallocene or late transition metal catalysts.The supported catalysts were studied and compared with referenced silica supported catalysts in olefin polymerizations.
1 Experimental
1.1 Materials
Methylaluminoxane (MAO)was purchased from Albemarle Corporation(USA)as 10%(w/w)in toluene.H3PO4,TiCl4,Hexane,Toluene were purchased from Sinopharm Chemical Reagent Co.,Ltd (P.R.China).Hexane and Toluene were refluxed over sodium with benzophenone as indicator and distilled under a nitrogen atmosphere prior to use.Silica support 2408d stored under nitrogen protection was purchased from Grace Corporation(USA).(nBuCp)2ZrCl2(98%)and rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2(97%)were purchased from Jamp;K Scientific LTD (USA)and used without further purification.
2,6-Bis(1-(2,6-diisopropyl)phenylimino)ethyl pyridine iron髤chloride was synthesized according to a published procedure[45].
Molecular structures of the above three compounds are shown in Scheme 1.

Scheme 1 Molecular structures of(nBuCp)2ZrCl2(A),rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2(B)and 2,6-Bis(1-(2,6-diisopropyl)phenylimino)ethyl pyridine iron髤(C)
1.2 Preparation of spherical α-Ti(HPO4)2support
600 mL of TiCl4aqueous solution (1.2 mol·L-1)was added slowly to 1 500 mL of H3PO4aqueous solution(1.0 mol·L-1)by stirring at room temperature.The mixing was refluxed for 72 h.The precipitate was separated by centrifuging and washed with deionized waterforseveraltimesuntilpH value ofthe centrifugal liquid was about 6.5.The wet precipitate was stirred at a high speed with deionized water to form white slurry(α-Ti(HPO4)2content in the mixture was about 6%,w/w)and the slurry was spray dried.The wet sample was dried at 160℃under vacuum to remove water and later it was kept under nitrogen protection.
1.3 Preparations of supported catalysts
All manipulations relating to air and/or moisture sensitive compounds were performed in a dry nitrogen glove boxorin schlenk bottlesundernitrogen protection.
α-Ti(HPO4)2/MAO/(nBuCp)2ZrCl2catalyst(cat-1).5 g of the spherical α-Ti(HPO4)2was suspended into 25 mL dry toluene,followed by addition of 15 mL MAO solution (10%in toluene,w/w).The suspension was stirred at 40℃for 1 h under nitrogen protection.70 mg of(nBuCp)2ZrCl2was dissolved in 10 mL dry toluene,followed by addition of 1.0 mL MAO solution(10%in toluene,w/w).The solution was stirred at room temperature for 30 min under nitrogen protection.The(nBuCp)2ZrCl2solution was added to the suspension of spherical α-Ti(HPO4)2support drop by drop by stirring at the same time,and the mixture was stirred and further heated at 40℃for 2 h.The solid product was finally obtained by filtration,washing with toluene and hexane for several times,and dried under vacuum at room temperature.
α-Ti(HPO4)2/MAO/[2,6-bis(1-(2,6-diisopropyl)phenylimino)ethyl pyridine iron髤chloride]catalyst(cat-2).5 g of spherical α-Ti(HPO4)2support was suspended into 25 mL dry toluene,followed by addition of 15 mL MAO solution (10%in toluene,w/w).The suspension was stirred at 40℃for 1 h under nitrogen protection.100 mg of 2,6-Bis(1-(2,6-diisopropyl)phenylimino)ethyl pyridine iron髤chloride was added to the suspension later.The mixture was further heated at 40℃for 2 h.The solid product was finally obtained by filtration,washing with toluene and hexane for several times,and dried under vacuum at room temperature.
α-Ti(HPO4)2/MAO/rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2catalyst(cat-3).5 g of spherical α-Ti(HPO4)2support was suspended into 25 mL dry toluene,followed by addition of 15 mL MAO solution(10%in toluene,w/w).The suspension was stirred at 40℃for 1 h under nitrogen protection.100 mg of rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2was dissolved in 20 mL dry toluene,followed by addition of 10 mL MAO solution(10%in toluene,w/w).The solution was stirred at room temperature for 30 min under nitrogen protection.The rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2solution was later added to the suspension of spherical α-Ti(HPO4)2support drop by drop by stirring at the same time,and the mixture was stirred and further heated at 40℃for 2 h.The solid product was finally obtained by filtration,washing with toluene and hexane for several times,and dried under vacuum at room temperature.
Silica/MAO/(nBuCp)2ZrCl2catalyst(Refcat-1).5 g of silica support was suspended into 25 mL dry toluene,followed by addition of 15 mL MAO solution(10%in toluene,w/w).The suspension was stirred at 40℃for 1 h under nitrogen protection.70 mg of(nBuCp)2ZrCl2was dissolved in 10 mL dry toluene,followed by addition of 1.0 mL MAO solution(10%in toluene,w/w).The solution was stirred at room temperature for 30 min under nitrogen protection.The(nBuCp)2ZrCl2solution was then added to the suspension containing silica support,and the mixture was further heated at 40℃for 2 h.The solid product was finally obtained by filtration,washing with toluene and hexane for several times,and dried under vacuum at room temperature.
Silica/MAO/[2,6-Bis(1-(2,6-diisopropyl)phenylimino)ethyl pyridine iron髤chloride]catalyst(Refcat-2).5 g of silica support was suspended into 25 mL dry toluene,followed by addition of 15 mL MAO solution(10%in toluene,w/w).The suspension was stirred at 40℃for 1 h under nitrogen protection.100 mg of 2,6-Bis(1-(2,6-diisopropyl)phenylimino)ethylpyridine iron髤chloride was added to the above suspension,and the mixture was further heated at 40℃for 2 h.The solid product was finally obtained by filtration,washing with toluene and hexane for several times,and dried under vacuum at room temperature.
Silica/MAO/rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2catalyst(Refcat-3).5 g of silica support was suspended into 25 mL dry toluene,followed by addition of 15 mL MAO solution (10%in toluene,w/w).The suspension was stirred at 40℃for 1 h under nitrogen protection.100 mg of rac-Me2Si (2-Me-4,5-PhInd)2ZrCl2was dissolved in 20 mL dry toluene,followed by addition of 10 mL MAO solution (10%in toluene,w/w),and the solution was stirred at room temperature for 30 min under nitrogen protection.Later,the rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2solution was dropped into the suspension containing silica support by stirring at the same time,and the suspension was further heated at 40℃for 2 h.The solid product was finally obtained by filtration,washing with toluene and hexane for several times,and dried under vacuum at room temperature.
1.4 Ethylene and propylene polymerizations
100 mg of cat-1(or Refcat-1,cat-2,Refcat-2),2.0 mL of 2.4 mol·L-1triethylaluminium (TEAL,in dried hexane)and 1.0 L of dried hexane were added to an autoclave(2.0 L).Ethylene gas was continuously fed into the autoclave at 1.0 MPa during polymerization,and the temperature in autoclave was kept at 60℃.One hour later,the reaction was stopped.The polymer was quickly filtered and dried in a vacuum oven at 60℃overnight.
400 mg of cat-3 (or Refcat-3),1.0 mL of 2.4 mol·L-1TEAL(in dried hexane)and 1.0 kg of purified liquid propylene were added to an autoclave (5.0 L).Polymerization was carried out at 60℃for 2 h.The polymer was finally obtained by rapid filtration and drying in a vacuum oven at 60℃overnight.
1.5 Characterizations
Crystal structure of the relative samples synthesized were determined on powder X-ray diffraction(PXRD),using a Rigaku D/MAX diffractometer(made in Japan)with Cu Kα radiation (λ=0.154 06 nm,scanning speed=10°·min-1)operated at 40 kV and 40 mA.Fourier transform-infrared (FT-IR)spectra of the samples were recorded from KBr pellet(1 mg of sample to 100 mg of KBr)over the range of 400~4 000 cm-1,at 2 cm-1resolution using a Bruker Vector-22 spectrometer(made in Germany).N2sorption isotherms were recorded on a Quantachrome NOVA 2000e sorption analyzer(made in USA)at liquid nitrogen temperature(-196℃).Samples were degassed at 150℃ overnight prior to measurement.Surface areas were determined using the Brunauer-Emmett-Teller (BET)method.Surface acidic strength of the samples were determined by thermo programmed desorption of ammonia(NH3-TPD)on a Thermo TPORO 1100 series(made in USA).Particle sizes of the support and the supported catalyst were measured by MS2000 particle analyzer(made in UK),using hexane as dispersant.Content of Zr and Fe in the supported catalysts were determined by inductively coupled plasma opticalemission spectroscopy (ICP-OES)on a PerkinElmer Optima 2000DV instrument (made in Japan).Electronic micrographs of the samples were observed using a Hitachi S4700 scanning electron microscope(SEM,made in Japan)operated at 15 kV.Mwand polydispersity(Mw/Mn)of the polymers were measured on a PlomerChar GPC-IR(made in Spain)at 140℃,using 1,2,4-trichlorobenzene as the solvent.
2 Results and discussion
2.1 Characterizations of spherical α-Ti(HPO4)2support and the supported catalysts
X-ray powder diffraction of spherical α-Ti(HPO4)2synthesized is shown in Fig.1.Three characteristic diffractions are found at 2θ angles of 11.64°,20.86°and 25.80°,fitting to the standard spectrum of α-Ti(HPO4)2reported(PDF No.38-334)[46].The peak at 11.64°,corresponding to (002),is characteristic of crystalline α-Ti(HPO4)2.Due to the easy occurrence of ionization,a strong stretching vibration absorption contributing to P-O-groups at about 1 030 cm-1is found in IR spectrum (Fig.2),while the stretching vibration absorption of P-OH is commonly found from 2 000 to 2 600 cm-1.The other two absor-ptions at 1 459 and 1 400 cm-1,corresponding to P-O-and P-OH in the figure[47],also prove the existence of free mobile H+ions in interlayer of α-Ti(HPO4)2.
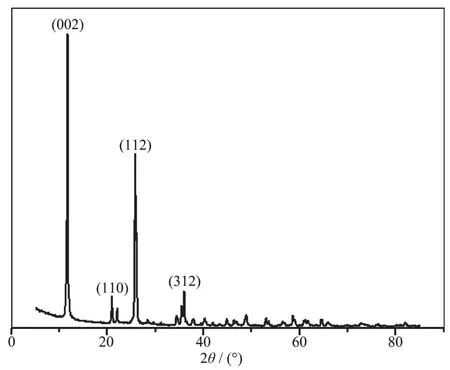
Fig.1 PXRD pattern of spherical α-Ti(HPO4)2
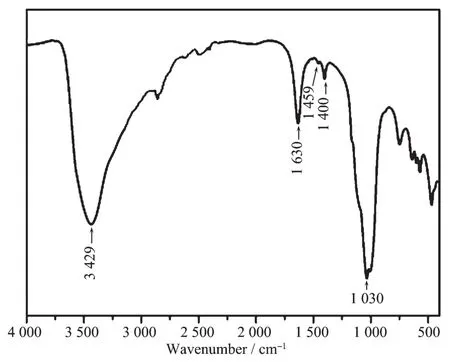
Fig.2 FT-IR spectrum of spherical α-Ti(HPO4)2
Examination of the prepared support with SEM shows that the dried solid support particles are mostly in spherical form (Fig.3A).Average diameter of these particles is about 6.3 μm that was detected by laser particle analyzer.Magnified SEM image (Fig.3B)to one spherical particle indicates that the surface of the support is composited by some sheet-like α-Ti(HPO4)2microcrystallites.SEM image of the spherical α-Ti(HPO4)2supported catalysts (Fig.3C,Fig.3E and Fig.3F)prepared is similar to that of the support(Fig.3A).The majority of catalyst particles are spherical.The α-Ti(HPO4)2microcrystallites can still be found in the surface of catalyst (Fig.3D).The average particle diameter size of the supported catalyst is about 8.7 μm,bigger than that of the pure support(6.3 μm).

Fig.3 SEM images of some samples
Nitrogen adsorption-desorption isotherms of the spherical α-Ti(HPO4)2and a commercial silica support(2408d)are shown in Fig.4.The BET surface area and total pore volume of α-Ti(HPO4)2are about 131 m2·g-1and 0.82 cm3·g-1(Fig.4A),less than those of parameters of silica support (320 m2·g-1and 1.60 cm3·g-1,respectively,Fig.4B).
The Zr(in metallocenes)and Fe(in late transition metal compound)contents of the supported catalysts were list in Table 1.The metal contents of in spherical α-Ti(HPO4)2supported catalysts are in therange of 0.20%~0.30%,which are similar to those in the referenced silica supported catalysts.Therefore,the spherical α-Ti(HPO4)2support shows similar adsorption capacity as silica support in this experiment.
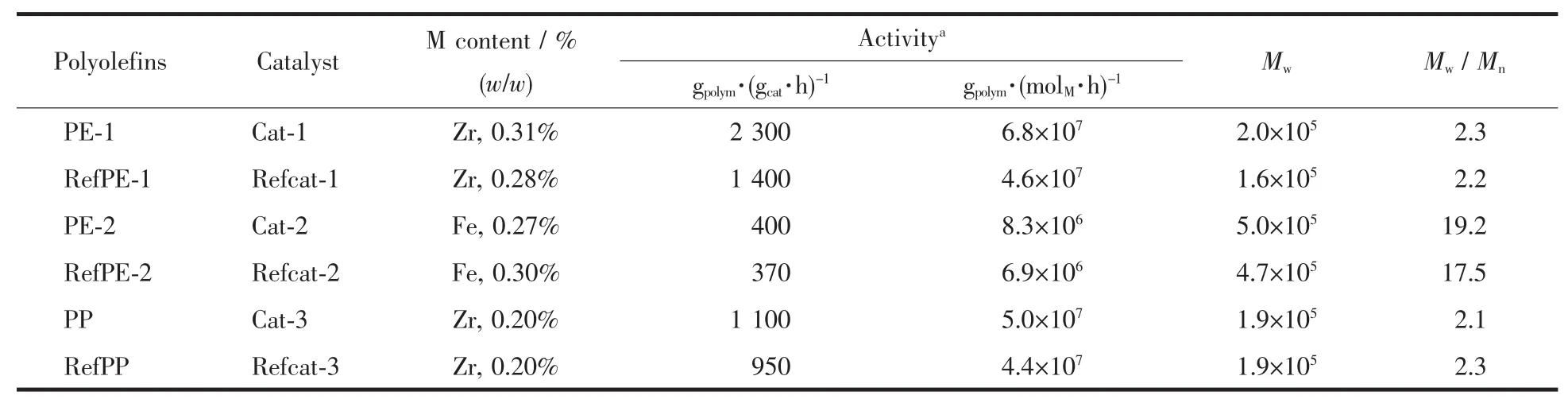
Table 1 Results for olefin polymerization using the supported catalysts
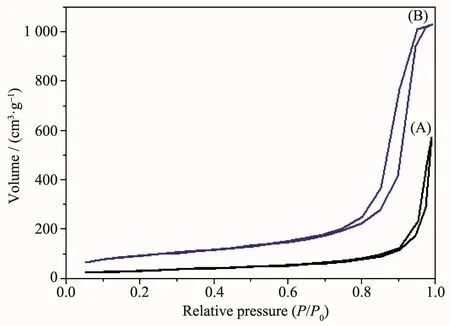
Fig.4 N2adsorption-desorption isotherm of the spherical α-Ti(HPO4)2(A)and silica(B)
2.2 Olefin polymerization results
All the supported catalysts have been tested for their performance in the olefin polymerization with TEAL as cocatalysts.The activities of the supported catalysts and referenced catalysts are listed in Table 1.The polymerization reactions proceeded very smoothly,producing free-flowing polyolefin in high yields.There is no evident reactor fouling or leaching of the catalyst from the support in any experiments.The activities of cat-1 and cat-3 in ethylene and propylene polymerizations are up to 6.8×107gPE·(molZr·h)-1and 5.0 ×107gPP·(molZr·h)-1respectively.The activities of spherical α-Ti(HPO4)2supported catalysts are higherthan the referenced silica supported catalysts under the same polymerization conditions,and even higher than the metallocene catalysts in homogeneous system(4.7×107gPE·(molZr·h)-1and 4.5×107gPP·(molZr·h)-1respectively,using MAO as cocatalyst,nAl/nZr=500).Molecular weight distributions(Mw/Mn)of PE-1 from cat-1 and PP from cat-3 are all no more than 2.3,indicating single-site catalytic behaviors of the composite catalysts.Due to C2symmetry in molecular structure of rac-Me2Si(2-Me-4,5-PhInd)2ZrCl2[48],the isotactic index of polypropylene produced by cat-3 is near to 96.5%.
Although the activity of cat-2(the late transition metal catalyst supported on spherical α-Ti(HPO4)2)is less than the correspondent catalyst in homogeneous system(4.0×107gpolym·(molFe·h)-1,using MAO as cocatalyst,nAl/nFe=2 500)in ethylene polymerization,the supported catalyst exhibits higher activity in ethylene polymerization than that of the catalyst supported on silica(Refcat-2).The broad molecular weight distribution of polyethylene (about 19.2,Fig.5A)produced by cat-2 indicates thatthe supported catalysthas multiple active sites.For comparison,the molecular weight distributions of the polyethylene produced by cat-1 (Fig.5A)and the polypro-pylene produced by cat-3(Fig.5B)are obviously narrow.
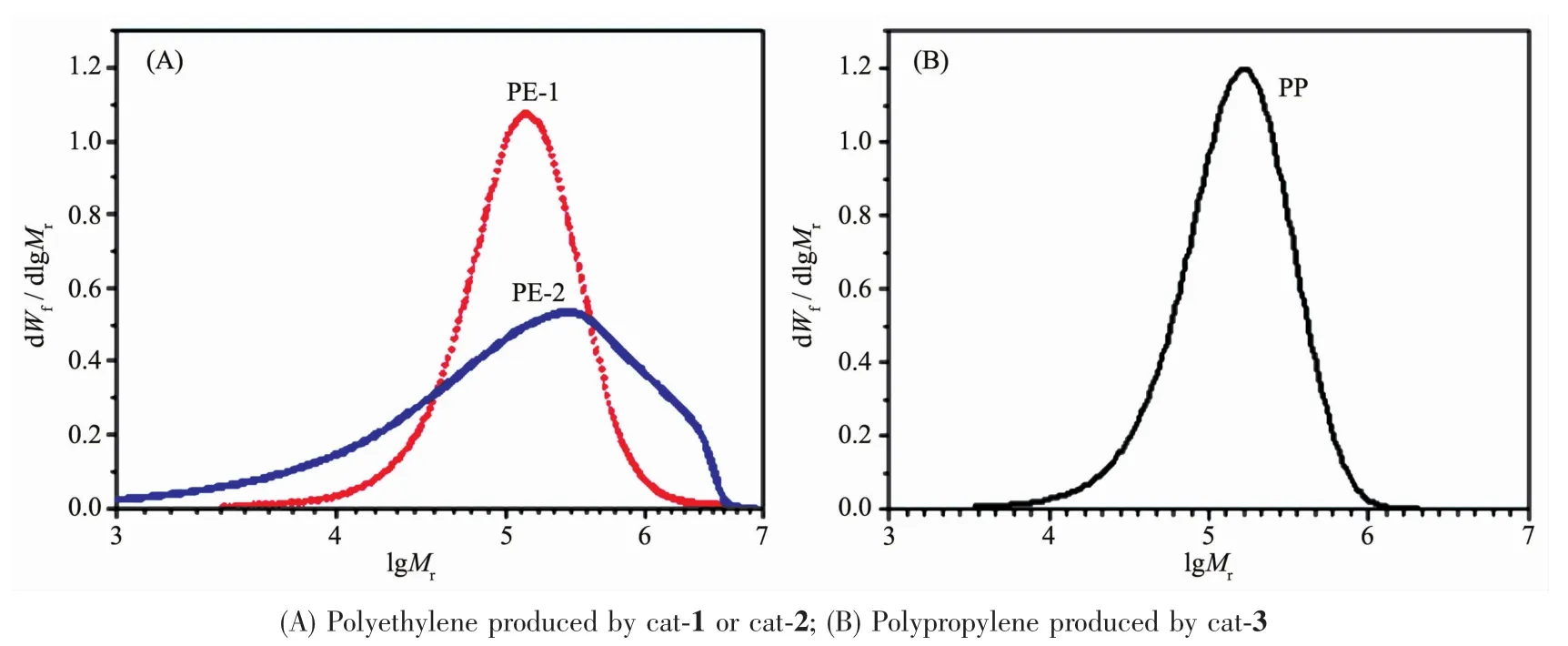
Fig.5 MWD curves of the polyolefin samples
Despite the fact that the spherical α-Ti(HPO4)2support has lower BET surface area and total pore volume than the silica support,spherical α-Ti(HPO4)2supported catalysts show higher activities and produce polyolefin with higher molecular weight.The reason possibly comes from the different acidity in the two materials.The promoting performance of acidic center in support or adding Lewis acid in olefin polymerization has already been proved.Nicholas prepared a kind of super acidic carrier by using concentrated sulfuric acid to deal with γ-Al2O3,and found that the Lewis acidity(coming from the induced effect of sulfate ions)and Br覬nsted acidity on the surface of γ-Al2O3were both increased[49].Cp′Zr(CH3)3loaded on this super acidic support has almost 10 times higher activity than Cp′Zr(CH3)3(Cp′=η5-(CH3)5C5)loaded on dehydroxylated alumina in ethylene homopolymeriza-tion.Damiani found when AlCl3(Lewis acid)was added in an ethylene polymerization system,the activity of EtInd2ZrCl2/MAO increased greatly[40].In his experiment,the activity of EtInd2ZrCl2was about 1.5 times,when cMAO/cAlCl3is about 13 to 14,to no AlCl3added,and the molecular weight and melting point of polyethylene both increased.
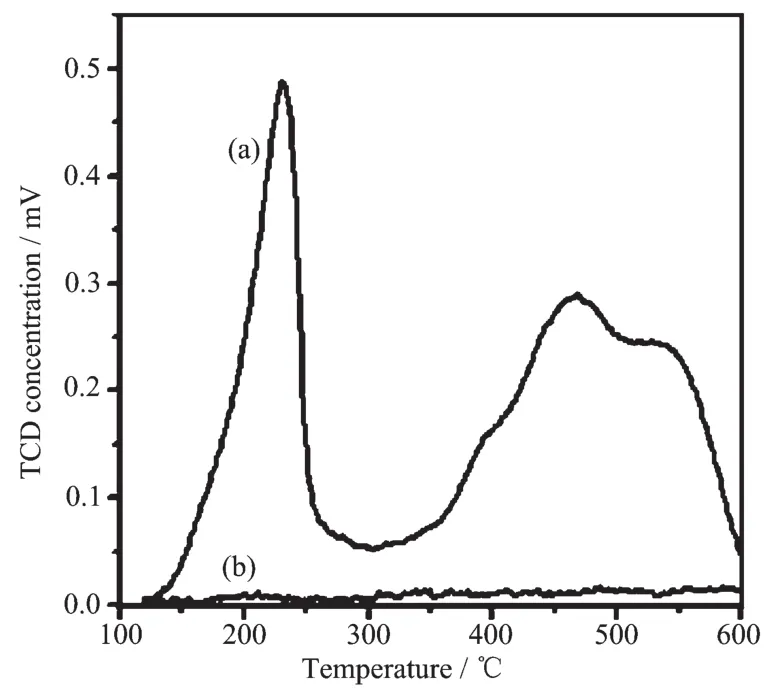
Fig.6 NH3-TPD of α-Ti(HPO4)2(a)and silica(b)
As shown in Fig.6,silica has no response to NH3added,indicating that the silica material close to neutral;however,the acidity of α-Ti(HPO4)2is evident for its strong adsorption to NH3.In this studies,the acidity of α-Ti(HPO4)2plays an important role.As a strong solid acid,α-Ti(HPO4)2acts to MAO to increase Lewis acidity of Al3+in MAO.The α-Ti(HPO4)2modified MAO generates an ionic pair with an increased cationic character on active metal sites,which is shown in the following reaction equations.This system would have an increased olefin insertion constant and decrease chain transfer to MAO.


Fig.7 SEM images of the polyolefin produced by using spherical α-Ti(HPO4)2supported catalysts
Morphologies of the polyolefin produced under existence of the catalysts supported on spherical α-Ti(HPO4)2are shown in Fig.7.It is easily found that the polyethylene-1 (PE-1,Fig.7A)produced by cat-1,polyethylene-2 (PE-2,Fig.7B)produced by cat-2 and polypropylene (PP,Fig.7C)produced by cat-3 all have typical spherical micromorphologies,which well duplicated the spherical shape of original support.Surface study to the magnified image of one particle(Fig.7D) indicates thatthe spherical polyolefin particle is an agglomeration of smaller polymer blocks and fibers,suggesting that the catalyst particles are broke into subparticles during polymerization followed the growth of polymer particles.Although the catalyst particles are broken,the subparticles are separated and covered by the growing polymer to keep regular ball-shape micromorphologies.Due to the growth of the polymer chains from the active sites on the surface of catalyst subparticle,the polymer chains are wrapped and drawn to form the fibersamong microspheres of the broken supported catalyst particles.An illustration of the above course is shown in Scheme 2.

Scheme 2 Particle growth model for olefin polymerization with spherical α-Ti(HPO4)2supported catalyst
3 Conclusions
A new carrier of spherical α-Ti(HPO4)2for metallocenes and late transition metal catalyst in olefin polymerizations was synthesized by spray drying α-Ti(HPO4)2slurry in this paper.Polyolefins,including polyethylene and polypropylene,were prepared under existence of the supported catalysts without any reactor fouling,and the polymer particles could replicate the spherical morphology of α-Ti(HPO4)2support,which is favorable for industrial application.
The activities of the spherical α-Ti(HPO4)2supported catalysts are higher than those of their referenced silica supported catalystundersame polymerization conditions.Molecular weight distributions of the polymers produced by the supported metallocene catalysts are narrow and isotacticity of polypropylene synthesized is high.The broad molecular weight distribution of polyethylene produced by the supported late transition metal catalyst reveals the existence of multiple active sites in the catalyst.According to the results,spherical α-Ti(HPO4)2supported catalysts can keep the advantage ofthe homogeneous catalysts.
Acknowledgements:We are grateful to the financial support from Project of National Natural Science Foundation of China (Grant No.U1362113).And we are also grateful to the financial support from PetroChina Company Ltd.
[1]Ziegler K,Holzkamp E,Breil H,et al.Angew.Chem.,1955,67:541-547
[2]Natta G.Makromol.Chem.,1955,16:213-237
[3]Busico V.Adv.Polym.Sci.,2013,257:37-57
[4]McDaniel M P.Adv.Catal.,2010,53:123-606
[5]Sedov I V,Makhaev V D,Matkovskii P E.Catal.Ind.,2012,4:129-140
[6]Johnson L K,Killian C M,Brookhart M.J.Am.Chem.Soc.,1995,117:6414-6415
[7]Camacho D H,Salo E V,Ziller J W,et al.Angew.Chem.Int.Ed.,2004,43:1821-1825
[8]Britovsek G J P,Gibson V C,Kimberley B S,et al.Chem.Commun.,1998:849-850
[9]Small B L,Brookhart M,Bennett A M A.J.Am.Chem.Soc.,1998,120:4049-4050
[10]Matsui S,Tohi Y,Mitani M,et al.Chem.Lett.,1999,28:1065-1066
[11]Furuyama R,Saito J,Ishii S,et al.J.Organomet.Chem.,2005,690:4398-4413
[12]Yoshida Y,Matsui S,Takagi Y,et al.Organometallics,2001,20:4793-4799
[13]Yoshida Y,Matsui S,Fujita T.J.Organomet.Chem.,2005,690:4382-4397
[14]Guan Z B,Cotts P M,McCord E F,et al.Science,1999,283:2059-2062
[15]Guan Z B.Chem.Eur.J.,2002,8:3086-3092
[16]Terao H,Ishii S,Mitani M,et al.J.Am.Chem.Soc.,2008,130:17636-17637
[17]Mitani M,Nakano T,Fujita T.Chem.Eur.J.,2003,9:2396-2403
[18]Eagan J M,Xu J,Di Girolamo R,et al.Science,2017,355:814-816
[19]Arriola D J,Carnahan E M,Hustad P D,et al.Science,2006,312:714-719
[20]Wang H P,Chum S P,Hiltner A,et al.J.Appl.Polym.Sci.,2009,113:3236-3244
[21]Zhang J,Wang X,Jin G X.Coord.Chem.Rev.,2006,250:95-109
[22]Gibson V C,Spitzmesser S K.Chem.Rev.,2003,103:283-316
[23]Alt H G,Kppl A.Chem.Rev.,2000,100:1205-1222
[24]Gibson V C,Redshaw C,Solan G A.Chem.Rev.,2007,107:1745-1776
[25]Dos Ouros A C,De Souzab M O,Pastore H O.J.Braz.Chem.Soc.,2014,25:2164-2185
[26]Severn J R,Chadwick J C,Duchateau R,et al.Chem.Rev.,2005,105:4073-4147
[27]Fink G,Steinmetz B,Zechlin J,et al.Chem.Rev.,2000,100(4):1377-1390
[28]Ma Z,Sun W H,Zhu N,et al.Polym.Int.,2002,51:349-352
[29]Conte A,Marques M F V.Eur.Polym.J.,2001,37:1887-1893
[30]Xu R W,Liu D B,Wang S B,et al.Macromol.Chem.Phys.,2006,207:779-786
[31]Saga K,Uozumi T,Saitoa M,et al.Macromol.Chem.Phys.,1994,195:1503-1515
[32]Kageyama K,Ng S M,Ichikawa H,et al.Macromol.Symp.,2000,157:137-142
[33]Guo C,Jin G X,Wang F S.J.Polym.Sci.Part A:Polym.Chem.,2004,42:4830-4837
[34]Weiss K,Wirth-Pfeifer C,Hofmanna M,et al.J.Mol.Catal.A:Chem.,2002,182-183:143-149
[35]Choi Y Y,Shin S Y A,Soares J B P.Macromol.Chem.Phys.,2010,211:1026-1034
[36]Buffet J C,Wanna N,Arnold T A Q,et al.Chem.Mater.,2015,27:1495-1501
[37]Nishida H,Uozumi T,Arai T,et al.Macromol.Rapid Commun.,1995,16:821-830
[38]Naundorf C,Matusi S,Saito J,et al.J.Polym.Sci.,Part A:Polym.Chem.,2006,44:3103-3113
[39]Soga K,Ban H T,Arai T,et al.Macromol.Chem.Phys.,1997,198:2779-2787
[40]Belelli P G,Ferreira M L,Damiani D E.Macromol.Chem.Phys.,2000,201:1458-1465
[41]Belelli P G,Ferreira M L,Damiani D E.Macromol.Chem.Phys.,2000,201:1466-1475
[42]Shi Y S,Yuan Y,Xu Q H,et al.Appl.Surf.Sci.,2016,383:126-132
[43]Morrow D R,Richardson G C,Kleinman L,et al.J.Polym.Sci.Part B:Polym.Phys.,1967,5:493-494
[44]Mu觡oz-Escalona A,Hernandez J G,Gallardo J A.J.Appl.Polym.Sci.,1984,29:1187-1202
[45]Small B L,Brookhart M,Bennett A M A.J.Am.Chem.Soc.,1998,120:4049-4050
[46]Slade R C T,Knowles J A,Jones D J,et al.Solid State Ionics,1997,96:9-19
[47]Ortíz-Oliveros H B,Flores-Espinosa R M,Ordoez-Regil E,et al.Chem.Eng.J.,2014,236:398-405
[48]Kaminsky W.Pure Appl.Chem.,1998,70:1229-1235
[49]Nicholas C P,Hongsang A H S,Marks T J.J.Am.Chem.Soc.,2003,125:4325-4331
Spherical α-Ti(HPO4)2Supported Catalysts:Synthesis and Catalytic Properties in Olefin Polymerizations
YUAN Yuan1,2YI Jian-Jun2SHI Yu-Jian1XU Qing-Hong*,1
(1State Key Laboratory of Chemical Resource Engineering,College of Science,Beijing University of Chemical Technology,Beijing 100029,China)
(2Petrochemical Research Institute,PetroChina Company Limited,Beijing 102206,China)
Two metallocene catalysts and one late transition metal catalyst were immobilized respectively on the surface of spherical α-Ti(HPO4)2treated with methylaluminoxane.All the supported catalysts have higher activities than those of their referenced silica supported catalysts in olefin polymerizations.The activities of α-Ti(HPO4)2supported metallocene catalysts in ethylene and propylene polymerizations are up to 6.8 ×107gPE·(molZr·h)-1and 5.0×107gPP·(molZr·h)-1.These supported catalysts can produce narrow molecular weight distribution polyolefins(Mw/Mnlt;2.3)and polypropylene with high isotactic index(96.5%).The late transition metal catalyst supported on α-Ti(HPO4)2has relatively less activity(8.3×106gPE·(molFe·h)-1)in ethylene polymerization but the produced polyethylene has a very broad Mw/Mn(gt;19).The particles of the produced polyolefins are mainly ball-shaped which replicate the morphology of spherical α-Ti(HPO4)2.
α-Ti(HPO4)2;spherical;metallocene;late transition metal catalyst;olefin polymerization
O614 文献识别码:A
1001-4861(2017)12-2329-09
10.11862/CJIC.2017.274
2017-07-21。收修改稿日期:2017-09-06。
国家自然科学基金(No.U1362113)资助项目。
*通信联系人。 E-mail:xuqh@mail.buct.edu.cn
