3,5-二甲基苯溴化镁修饰的ZSM-5催化剂乙苯歧化反应
2017-12-13李军卫武静文许波连
张 英 李军卫 武静文 许波连*,
3,5-二甲基苯溴化镁修饰的ZSM-5催化剂乙苯歧化反应
张 英1李军卫1武静文2许波连*,2
(1贵州电网有限责任公司电力科学研究院,贵阳 550002)
(2南京大学化学化工学院,江苏省机动车尾气污染控制重点实验室,南京 210093)
采用尺寸较大的有机分子格式试剂(3,5-二甲基苯溴化镁)修饰ZSM-5催化剂,用乙苯歧化反应研究了修饰催化剂的择形性,并利用探针分子动力学扩散测试结合探针分子吸附红外等手段研究分子筛孔径和内外表面酸性性质等。结果表明,少量的3,5-二甲基苯溴化镁精确地中和了ZSM-5分子筛外表面的酸性中心,导致乙苯歧化反应中极高的对二乙苯选择性。而探针分子动力学结果表明,这种修饰并未引起分子筛孔道结构的变化。
ZSM-5分子筛;表面酸性;乙苯歧化;择形催化
0 Introduction
Zeolite is a kind of special material with unique pores and channels.It is also called molecular sieve used for selective adsorption and separation[1-3].Since 1940s,many new kinds of zeolite with different pore size,pore structure and acid properties were invented.The varied molecular diffusion rate in these pores,as well as acidic properties,make it widely used in many chemical industrial processes as sorbent,catalyst andcatalyst support[4-7].Particularly,in petroleum chemical process,zeolite catalyst is more and more important used in catalytic cracking,alkyl benzene disproportionation,hysomer,aromatization[8-10],etc.In these processes,surface acid sites are the catalytic active sites.For example,the surface acid sites of ZSM-5 zeolite are the catalytic active sites for ethylbenzene(EB)disproportionation to p-diethylbenzene (p-DEB)reaction.Paparatto et al.concluded that three types of DEB are formed inside the channel of ZSM-5 crystal and then the primary product p-DEB formed under the steric constraints is further isomerized into m-and o-DEB toward the thermodynamic equilibrium on the external surface of ZSM-5[11].In order to obtain high p-DEB selectivity,it is necessary to passivate the acidic sites on the external surface of ZSM-5 for eliminating secondary isomerization of p-DEB.
Therefore proper modification is of practical importance to improve the performance of ZSM-5.Many well known methods to modify external surface of the zeolite have been reported in the past years[12-15].Tetraethoxysilane(TEOS)[16]and other siloxane derived compounds[17]were usually used for their large molecular size which was bigger than zeolite pore diameter.Chemical vapor deposition(CVD)and chemical liquid deposition(CLD)of silica on the external surface of ZSM-5 were reported for ZSM-5 modification[18-22].However,during the deposition process,silica covered all ZSM-5 external surface,not only external acid sites.Thus,in order to neutralize the external acid sites completely,large amount of silica (usually mass loadings over 8%~10%)should be loaded,leading to the result that the modification of ZSM-5 not only eliminates the acidic sites but also narrows the pore sizes[23].Even then,there were residual acidic sites on silica deposited ZSM-5 surface.Because ofthe alkalescence of MgO,modification by loading MgO can also enhance the shape selectivity of ZSM-5[24-25].The normal precursor of MgO,magnesium acetate and magnesium nitrate[23,26]are small enough to enter the micropores.It can both eliminate the external and internal acidic sites[27].The modification just improves shape selectivity a little butdecreased reaction activity more.Usually,MgO is used as additives not the one and only modification reagent.It was reported that ZSM-5 catalyst modified by MgO after the modification of SiO2can further eliminate the residual acidic sites and enhance p-DEB selectivity[23].
During shape selective catalytic reaction,pore size is very sensitive for different molecule size[28-30].To understand the role of pore size and external acid sites in these reactions,we want to develop a zeolite modification method without changing the two factors synchronously.In this work,a new kind of MgO modification precursor,3,5-dimethyl phenylmagnesium bromide,was prepared and used to modify ZSM-5,aimed at eliminating the external acidic sites without changing the pore size.
1 Experimental
1.1 Catalyst preparation
3,5-dimethyl phenylmagnesium bromide was obtained by adding metal magnesium pellets with a partical of I2into 500 mL boiling flask under nitrogen atmosphere,followed by adding 10 mL tetrahydrofuran(Nanjing Chemical Reagent Limited Company)under stirring and adding 3,5-dimethyl benzene bromide(Beijing Ouhe Company)until all metal magnesium pellets were dissolved.
The ZSM-5 catalyst modified by Grignard reagent,named as MB-xMgO/ZSM-5(x is the MgO loadings in weight percentage)was prepared by impregnating the suspension of 6 g ZSM-5(Nankai University,nSiO2/nAl2O3=50,particle size is 5 μm)and 150 mL hexane(Nanjing Chemical Reagent Limited Company)into the 3,5-dimethyl phenylmagnesium bromide solution prepared above,then stirring for 4 h.The solvent was evaporated by heating the flask to 353 K to get the solid sample.The sample was dried at 393 K for 2 h,then calcined at 773 K in air for 8 h.ZSM-5 catalyst modified by magnesium nitrate was prepared by impregnating ZSM-5 with ethanol(Nanjing Chemical Reagent Limited Company)solution of magnesium nitrate (Shanghai Zhenxing Reagent Factory).After 4 h of stirring,the catalyst was obtained by removing solvent,drying and calcining as the same procedure mentioned above.The sample was named as MN-xMgO/ZSM-5(x is the MgO loadings in weight percentage).
1.2 Catalytic test
The ethylbenzene disproportionation reaction was conducted in a continuous flow,fixed-bed reactor(quartz tube:Φ=6 mm,L=40 cm)under the following conditions:0.1 g catalyst,reaction temperature T=573 K,space velocity WHSV=1.5 h-1,pressure=1.0132 5×105Pa.Ethylbenzene was injected into the reactor using a metering pump.Argon was used as carrier gas in a flow rate of 10 mL·min-1.The effluent gas released from the reactor was analyzed by an on-line gas chromatography equipped with a FFAP column(Φ=0.25 mm,L=30 m)and a flame ionization detector.
1.3 Catalyst characterization
X-ray diffraction (XRD)analysis was performed on a Philips X′Pert MPD Pro X-ray diffractometer employing Ni-filtered Cu Kα radiation(λ=0.154 1 nm)in the 2θ range of 10°~80°.The X-ray tube was operated at 40 kV and 30 mA.
The IR spectra of the sample was conducted with a self-supported wafer of sample using Perkin-Elmer 2000 FT-IR spectrometry.The experiment was carried out in order to identify whether the organic groups were completely removed or not.Before and after calcination the IR spectra of ZSM-5 modified by Grignard reagent were recorded.Besides,the IR spectra of parent ZSM-5 and ZSM-5 adsorbed mxylene and hexane were also recorded for comparison.
The IR spectra of adsorbed 2,6-di-tert-butylpyridine(DTBPy-IR)to characterize the acidic sites on the external surface of ZSM-5 was conducted with a selfsupported waferofsample using the same IR spectrometry.Prior to DTBPy adsorption,the samples were evacuated 60 min at 673 K under high vacuum(1×10-2Pa)to clean the surface and eliminate possible impurities.DTBPy adsorption was carried out at room temperature until saturation(equilibration time 30 min)and the excess of physical adsorbed DTBPy was removed under vacuum(1×10-2Pa)at 473 K for 60 min.Then the corresponding spectrum was recorded.Similar process to characterize the total acidic sites was conducted for the IR spectra of adsorbed pyridine(Py-IR).
The experiments of adsorption kinetics of probe molecule (EB,m-DEB,p-DEB)were carried out on self-supporting equipmentby gravimetric method.Before testing,samples were heated to 623 K and evacuated (0.1 Pa)for 2 h to clean the surface and eliminate possible impurities.Then the sample was cooled to 373 K.The weight increase was recorded after the vapor of probe molecule at 273 K was pulled into the vacuum system.
2 Results and discussion
2.1 Modification process analysis
To investigate the MgO modification process,the IR spectra of the modified ZSM-5 with Grignard reagent before and after calcination are shown in Fig.1.The peak at 1 460 cm-1is associated with the vibration of C-H groups to m-xylene and hexane(Fig.1d and e),it can be easily found in Fig.1c.The results indicate that the organic compounds were still on ZSM-5 surface before calcination.After calcination,all organic compounds were removed and only the specific vibration of ZSM-5 at 1 628 and 1 872 cm-1can be observed (Fig.1b),which shows the same IR spectra as the parent ZSM-5(Fig.1a).So the Grignard reagent modification process can be described as schematic route showed in Fig.2.
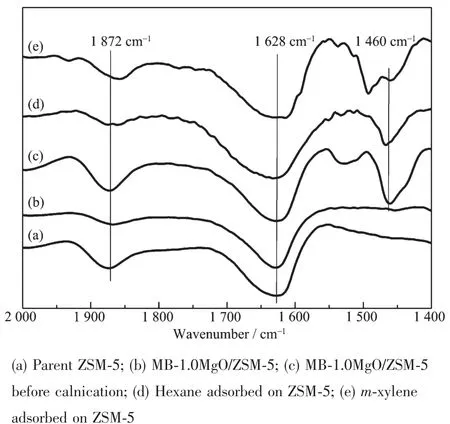
Fig.1 IR spectra of the modified ZSM-5 with Grignard reagent before and after calcinations
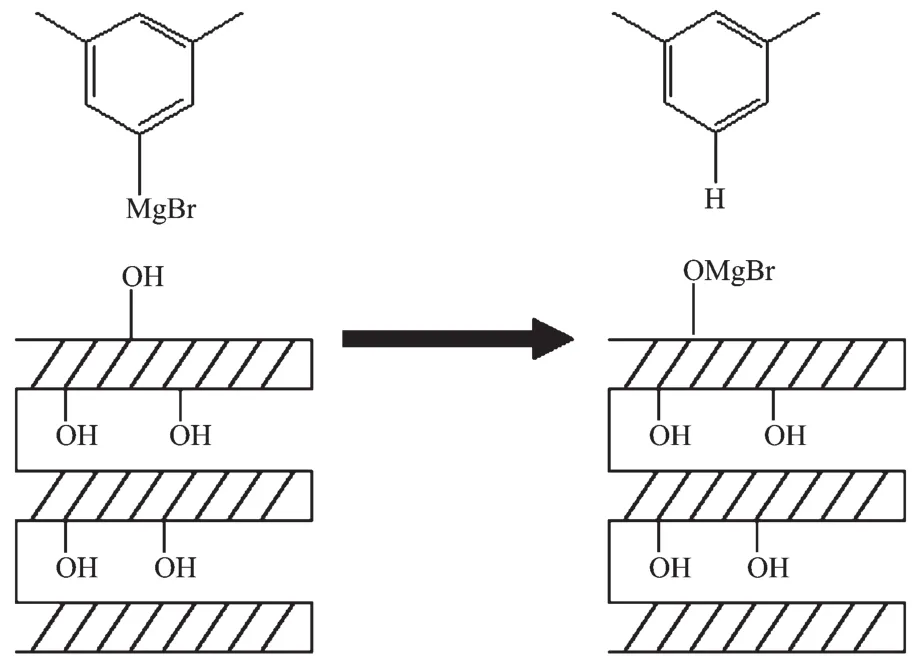
Fig.2 Schematic illustration of Grignard reagent modification process
The XRD results are shown in Fig.3.Not only the samples modified by magnesium nitrate,but also the samples modified by Grignard reagent show the same patterns as pure ZSM-5,which indicate that the crystalstructure ofZSM-5 is keptduring the magnesium oxide modification process.Because the MgO loading amount is very low (≤2%),no magnesium oxide signal was observed.

Fig.3 XRD patterns of the parent ZSM and modified catalysts
2.2 Catalytic properties analysis
Ethylbenzene disproportionation reaction results are shown in Table 1.Parent ZSM-5 catalyst shows the highest EB conversion but the lowest p-DEB selectivity.After MgO modification,EB conversion decreases and p-DEB selectivity increases dramatically.Besides,with the increasing of MgO loading,EB conversion decreases and p-DEB selectivity increases.Compared to the catalysts modified by magnesium nitrate,the catalysts modified by Grignard reagent show similar EB conversion but much higher p-DEB/DEB selectivity under the same MgO loadings.
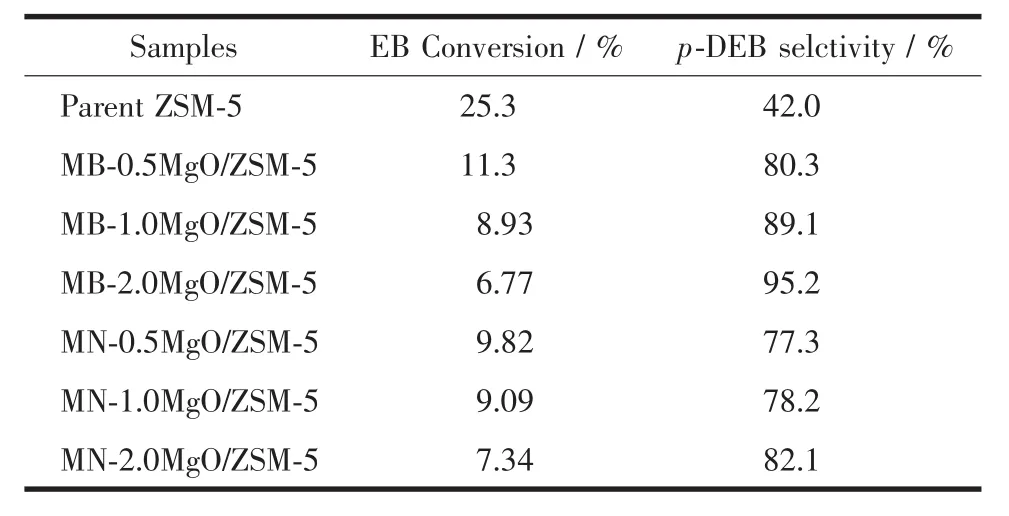
Table 1 EB disproportionation results of parent and MgO modified ZSM-5 catalysts
2.3 Surface acidity analysis

Fig.4 Py-IR spectra of different samples
To investigate the reason of EB disproportionation performances after MgO modification,the surface acidity and pore structure were studied by Py-IR,DTBPy-IR characterization and probe molecular adsorption kinetics experiment.Py-IR characterization in Fig.4 show that MgO modification resulted in the decrease of Br覬nsted acidic sites(1 540 cm-1)due to the coverage of acidic sites with deposited MgO despite of the modification method.It is well understood that Br覬nsted acidic sites are neutralized by basic MgO.On the other hand,Lewis acidic sites(1 450 cm-1)increases with deposited MgO because Mg2+ions creat a new kind of Lewis acid sites on ZSM-5 surface[26,31-32].Compared with adsorption of pyridine both on external surface and in channels of zeolite,the adsorption of 2,6-di-tert-butylpyridine occurs only on the external surface due to its large molecular size.DTBPy-IR results in Fig.5(a~c)show that Grignard reagent modification resulted in the decrease of Br覬nsted acidic sites(1 450 cm-1)while the increase of Lewis acidic sites(1 540 cm-1)due to the coverage of acidic sites with deposited MgO.It indicates that the Grignard reagent modification neutralized the external acid sites and most of the Mg2+was deposited on the external surface.DTBPy-IR characterization results of catalysts modified by magnesium nitrate are show in Fig.5(b′,c′).With the increasing of MgO loading,Br覬nsted acidic sites(1 540 cm-1)decreases slightly and no obvious Lewis acidic sites(1 450 cm-1)increase is observed.This result suggest that only partial deposited Mg2+ions disperse on the external surface.
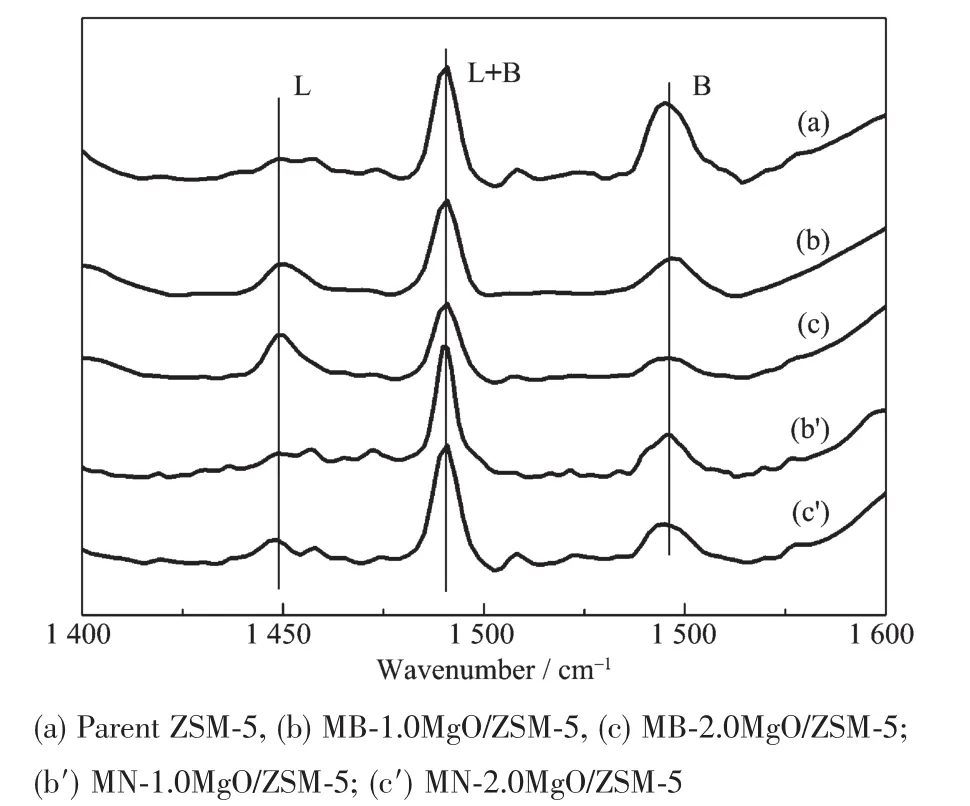
Fig.5 DTBPy-IR spectra of ZSM-5
2.4 Diffusion kinetics analysis
Adsorption curves of EB,p-DEB and m-DEB on parent and modified ZSM-5 are shown in Fig.6 and Fig.7,formodification by Grignard reagentand magnesium nitrate,respectively.For parent ZSM-5 catalyst,EB and p-DEB entered into the ZSM-5 channels easily while the m-DEB hardly entered into it.It is suggested that m-DEB formation can be prohibited in ZSM-5 channels while the pore size is suitable for p-DEB formation.The low p-DEB selectivity of parent ZSM-5 catalyst is caused by the external surface acid sites.After MgO modification,the EB,p-DEB and m-DEB adsorption kinetics follow a similar curve.It is suggested that both the catalysts modified by Grignard reagent and magnesium nitrate keep the same pore size as parent ZSM-5 during MgO modification.The deceasing of EB conversion and increasing of p-DEB selectivity are caused by external acid site removal,not by pore size modification.
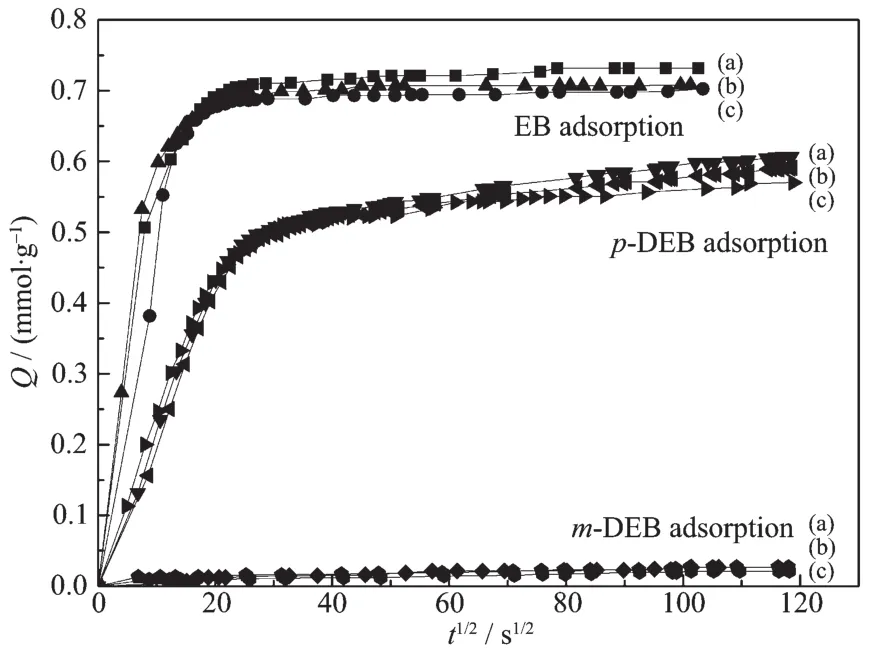
Fig.6 Adsorption kinetics curve of EB,p-DEB,m-DEB on(a)parent ZSM-5,(b)MB-1.0MgO/ZSM-5 and(c)MB-2.0MgO/ZSM-5
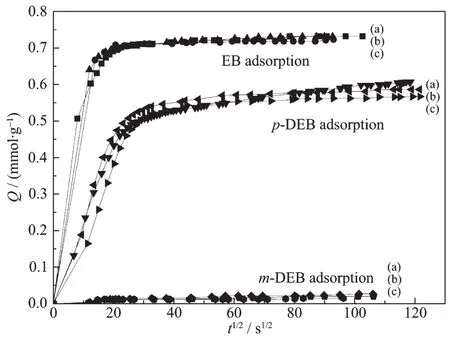
Fig.7 Adsorption kinetics curve of EB,p-DEB,m-DEB on(a)parent ZSM-5,(b)MN-1.0MgO/ZSM-5 and(c)MN-2.0MgO/ZSM-5
3 Conclusions
EB,p-DEB and m-DEB adsorptionkinetics experiment results and XRD results indicate that both MB-MgO/ZSM-5 and MN-MgO/ZSM-5 catalysts have the same crystal structure and pore size.Py-IR show that MgO modification eliminates the surface acid sites and leads to the decrease of EB conversion and the increase of p-DEB selectivity during EB disproportionation reaction.In contrast,Grignard reagent is too large to enter into micropores of ZSM-5 while magnesium nitrate is small enough to enter into it,resulting in the different acidic sites distribution between MB-MgO/ZSM-5 and MN-MgO/ZSM-5 catalysts.DTBPy-IR reveals that the MB-MgO/ZSM-5 catalysts have much more decrease of amount of external acid sites than the MN-MgO/ZSM-5 catalysts.It leads to the higher selectivity of p-DEB for MBMgO/ZSM-5 catalysts than for MN-MgO/ZSM-5 catalysts.It offeres a new method by using small amount of modification reagent to remove the external acid sites of zeolite catalyst and keep its pore size.
Acknowledgment:This work was financially supported by Science and technology project of China Southern Grid Corp(Grant No.GZ2015-2-0048).We are grateful to Prof.CHUN Yuan for adsorption kinetics experiment support and Prof.JI Wei-Jie for Py-IR and DTBPy-IR experiment support.
[1]Keskin S.J.Phys.Chem.C,2011,115(3):800-807
[2]Iwasaki M,Shinjoh H.J.Catal.,2010,273(1):29-38
[3]Chakarova K,Hadjiivanov K.J.Phys.Chem.C,2011,115(11):4806-4817
[4]Poissant R R,Huang Y N,Secco R A.Microporous Mesoporous Mater.,2004,74(1/2/3),231-238
[5]Zhang S B,Zhou Y M,Zhang Y W,et al.Catal.Lett.,2010,135(1/2):76-82
[6]Song Z,Takahashi A,Nakamura I,et al.Appl.Catal.,A,2010,384(1/2):201-205
[7]Zhang Y,Zhou Y,Huang L,et al.Ind.Eng.Chem.Res.,1999,50(13):7896-7902
[8]Rahimi N,Karimzadeh R.Appl.Catal.,A,2011,398(1/2):1-17
[9]Tukur N M,Al-Khattaf S.Catal.Lett.,2009,131(1/2):225-233
[10]Masiero S S,Marcilio N R,Perez-Lopez O W.Catal.Lett.,2009,131(1/2):194-202
[11]Arsenova-Hartel N,Bludau H,Schumacher R,et al.J.Catal.,2000,191(2):326-331
[12]Jin L,Hu H,Wang X,et al.Ind.Eng.Chem.Res.,1993,45(10):3531-3536
[13]Ramesh K,Jie C,Han Y F,et al.Ind.Eng.Chem.Res.,1993,49(9):4080-4090
[14]Ivanova I I,Blom N,Derouane E G.J.Mol.Catal.A:Chem.,1996,109(2):157-168
[15]Choudhary V R,Jana S K.App.Catal.,A,2002,224(1/2):51-62
[16]Berger C,Raichle A,Rakoczy R A,et al.Microporous Mesoporous Mater.,2003,59(1):1-12
[17]Zhu Z,Xie Z,Chen Q,et al.Microporous Mesoporous Mater.,2007,101(1/2):169-175
[18]Niwa M,Katada N,Murakami Y.J.Phys.Chem.,1990,94(16):6441-6445
[19]Gründling C,Eder-Mirth G,Lercher J A.J.Catal.,1996,160(2):299-308
[20]Roger H P,Kramer M,Moller K P,et al.Microporous Mesoporous Mater.,1998,21(4/6):607-614
[21]Weber R W,Moller K P,Unger M,et al.Microporous Mesoporous Mater.,1998,23(3/4):179-187
[22]Weber R W,Mller K P,O′Connor C T.Microporous Mesoporous Mater.,2000,35-36:533-543
[23]Zhu Z,Chen Q,Xie Z,et al.J.Mol.Catal.A,2006,248(1/2):152-158
[24]Uguina M A,Sotelo J L,Serrano D P.Ind.Eng.Chem.Res.,1993,32(1):49-55
[25]Uguina M A,Sotelo J L,Serrano D P,et al.Ind.Eng.Chem.Res.,1994,33(1):26-31
[26]Li X J,Liu S L,Zhu X X,et al.Catal.Lett.,2011,141(10):1498-1505
[27]Zhu Z R,Chen Q L,Xie Z K,et al.J.Mol.Catal.A,2006,248(1/2):152-158
[28]Niwa M,Kato M,Hattori T,et al.J.Phys.Chem.,1986,90(23):6233-6237
[29]Viswanadham N,Dhar G M,Rao T.J.Mol.Catal.A,1997,125(2/3):L87-L90
[30]Ohayon D,Le van Mao R,Ciaravino D,et al.Appl.Catal.,A,2001,217(1/2):241-251
[31]Li Y G,Xie W H,Yong S.Appl.Catal.,A,1997,150(2):231-242
[32]Xue B,Li Y,Deng L.Catal.Commun.,2009,10(12):1609-1614
Ethylbenzene Disproportionation over ZSM-5 Modified by 3,5-Dimethyl Phenylmagnesium Bromide
ZHANG Ying1LI Jun-Wei1WU Jing-Wen2XU Bo-Lian*,2
(1The Electric Power Science Research Institute of Guizhou Power Grid Co.,Ltd,Guiyang 550002,China)
(2Jiangsu Key Laboratory of Vehicle Emissions Control,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,China)
A new kind of ZSM-5 surface modification method was developed by using a large organic molecule Grignard reagent (3,5-dimethyl phenylmagnesium bromide).Their shape selective catalytic properties were studied by ethylbenzene(EB)disproportionation reaction test.The pore structure and surface acid properties were also investigated to understand the shape selectivity enhancement mechanism.Most of the MgO was deposited on external surface due to the big molecule size of 3,5-dimethyl phenylmagnesium bromide.The elimination of external surface acid sites by Grignard reagent is the main reason of its high p-diethylbenzene(p-DEB)selectivity in EB disproportionation reaction.The results of probe molecule adsorption kinetics experiment indicate that this modification method does not change the pore size of ZSM-5.
ZSM-5;surface acidic site;ethylbenzene disproportionation;shape selective catalysis
O647;O643.36
A
1001-4861(2017)12-2351-06
10.11862/CJIC.2017.260
2017-07-09。收修改稿日期:2017-09-27。
中国南方电网公司科技项目(No.GZ2015-2-0048)资助项目。
*通信联系人。 E-mail:xubolian@nju.edu.cn;会员登记号:S06N8035M1406。
