酸性离子液调控下咪唑硫酸四铁簇合物和铁-DMSO配合物的合成
2017-12-13罗倩倩林和春罗春花张媛媛
罗倩倩 林和春*, 罗春花 张媛媛 彭 晖,2
酸性离子液调控下咪唑硫酸四铁簇合物和铁-DMSO配合物的合成
罗倩倩1林和春*,1罗春花1张媛媛1彭 晖1,2
(1华东师范大学信息学院,极化材料与器件教育部重点实验室,上海 200141)
(2山西大学光学协作创新中心,太原 030006)
FeCl2与酸性离子液在乙醇中反应制备得到咪唑硫酸铁簇合物[HMIM]2[Fe2O(SO4)3(DMSO)2]·0.5DMSO(1)和[HPIM]4[Fe4O2(SO4)6(DMSO)4]·2MeCN(2),该类簇合物为四核铁簇Fe4(O)2(SO4)6(DMSO)4结构,4个Fe原子通过中心μ3-O桥、硫酸根上的μ2-O和μ3-O桥连接,形成共平面结构,中间的四核铁簇为-4价,4个质子化的咪唑正离子起电荷平衡作用。该簇合物的晶体结构属于三斜晶系中的P1空间群。变温磁化率测试表明该簇合物具有强的反铁磁性。但在类似的反应条件下,FeCl3在酸性离子液反应没有得到类似的簇合物,而是得到DMSO配合物[FeCl2(DMSO)4][EtSO4]·DMSO(3)。
铁簇合物;开放框架;酸性离子液;反铁磁性
Polynuclear ferric complexes have attracted continuing interest mainly due to their aesthetically fascinating structures and unique properties.They may represent biomimetic models for the iron oxide/hydroxide core of the iron storage protein ferritin[1-4],and can sometimes exhibit unusual and occasionally novel magnetic properties,with some of them even being examples of single molecule magnets(SMMs)[5-13].Polynuclear ferric complexes with primarily oxygen and nitrogen based ligation have been designed and prepared including Fe4,Fe6,Fe7,Fe8,Fe11,Fe14,Fe12,Fe16,Fe19,Fe22and others[13-29].
The open-framework sulfates combining transition metals and organic groups have attracted a great interest due to their fascinating structure diversities and potential applications as functional materials[30-32].Several organically template iron sulphates[33-40]directing by diamines or 1,4-diazabicyclo[2.2.2]octane have been reported with linear and layered structures.In our recent work,we have reported the interreaction of metal salts with ionic liquids to find the anion exchange reactions of BF4-or PF6-based ionic liquids with titanium or zirconium alkoxide to form[Ti(OH)6]2-or[Zr(OH)6]2-complexes[41].Furthermore,imidazole template metal sulfates with zero dimensional monomeric structure or one dimensional metal-organic frameworks were synthesized (HMIM=protonated 1-methylimidazolium)[42].In this article,we investigated the reactions of FeCl2or FeCl3with Brnsted acidic ionic liquids.Two imidazole template Fe4sulfate clusters with interesting magnetic properties were achieved for the reaction of FeCl2.In contrast,the iron DMSO complex[FeCl2(DMSO)4](EtSO4)]·DMSO was obtained with the reaction of FeCl3under the similar reaction conditions.
1 Experimental
1.1 Materials and methods
All reagents were commercially available and used without further purification.Elemental analyses(C,H,N)were carried out with a Vario ELⅢelemental analyzer.FTIR spectra measurements were performed on Nexus 670 IR spectrometer by using KBr pellets in the region of 4 000~400 cm-1.The variable-temperature magnetic susceptibility data were recorded on a Quantum Design PPMS-9 magnetometry in a field of 0.1 T and 3.0~300.0 K.TGA/DTA curves were obtained by using STA449 F3 Jupiter simultaneous thermoanalyzer in N2at a heating rate of 10 K·min-1from room temperature to 800℃.
Single-crystal XRD data of the crystals were collected with a Bruker APEX-ⅡCCD diffractometer with graphite-monochromatic Mo Kα radiation(λ=0.071 073 nm).All the structures were solved by direct methods(SHELXS-97 program[43])and refined by fullmatrix least-squares on F2(SHELXL-97 program[43])using Olex2 software[44].Hydrogen atoms were added geometrically and refined using the riding mode.
CCDC:1495092,1;1495093,2;1495086,3.
1.2 Syntheses of the complexes
Synthesis of [HMIM]2[Fe2O(SO4)3(DMSO)2]·0.5DMSO(1).FeCl2·4H2O (0.50 g,2.5 mmol)was added to a solution of[HMIM][HSO4](0.90 g,5 mmol)in ethanol(20 mL).Green precipitates were generated slowly under the stirring.The reaction mixture was refluxed for 4 h.After cooling to the room temperature,ethanol was removed by rotary evaporation to give rise to dark brown solids.The obtained solidswere dissolved in DMSO (dimethy sulfoxide)at 90℃and filtered.The clear solution in a vessel was kept under MeCN atmosphere to grow crystals.Dark brown flake crystals suitable for SXRD analysis were collected after 2 days.Anal.Calcd.for C13H29Fe2N4O15.55S5.5(%):C,20.06;H,3.76;N,7.20.Found(%):C,20.66;H,4.34;N,8.01.Main IR bands(cm-1):3 423(s),3 416(s),2 963(m),1 636(s),1 585(s),1 551(w),1 420(w),1 280(w),1 224(s),1 139(s),1 076(s),995(s),941(m),832(w),761(w),623(m),601(m),475(w).The product has been identified with single-crystal X-ray diffraction.
Synthesis of [HPIM]4[Fe4O2(SO4)6(DMSO)4]·2MeCN(2).The synthesis procedure was similar to 1.The MeCN solvate molecules probably escaped from crystals during drying under high vacuum.Elemental analysis,TGA and FTIR were based on formula(HPIM)4Fe4O2(SO4)6(DMSO)4.Anal.Calcd.for C32H68Fe4N8O30S10(%):C,24.19;H,4.31 N,7.05.Found(%):C,23.91;H,4.64;N,7.07.Main IR bands(cm-1):3 430(s),2 970(m),1 636(m),1 580(m),1 547(w),1 402(w),1 221(s),1 139(s),1 080(m),990(m),946(m),832(w),599(w),542(m),478(w).The product has been identified with single-crystal X-ray diffraction.
Synthesis of[FeCl2(DMSO)4][EtSO4]·DMSO(3).FeCl3(0.41 g,2.5 mmol)was added to the mixed solution of[HMIM][HSO4](0.90 g,5 mmol)and ethanol(20 mL)under magnetic stirring and refluxed (90℃)for 4 h.The black mixture gradually turned into brown during the reaction.The solvent was removed by rotary evaporation to generate a dark brown liquid.The liquid was resolved in DMSO and filtered to grow crystals in the refrigerator.Yellow flake crystals were collected from the solution after a few days.Anal.Calcd.for C10H29Cl2FeO8S5(%):C,21.28;H,5.18.Found(%):C,20.91;H,5.52.Main IR bands(cm-1):3 434(s),3 114(s),2 834(s),2 517(m),2 135(w),1 794(w),1 635(s),1 579(m),1 541(s),1 400(w),1 239(m),940(w),775(w),688(w),582(w),419(s),400(s).The product has been identified with singlecrystal X-ray diffraction.
2 Results and discussion
2.1 Synthesis
Green precipitates were first generated within a few minutes after the addition of FeCl2·4H2O to an ethanol solution of [HMIM][HSO4].After removing ethanol by rotary evaporation,the colour of precipitates changed from green to dark brown.The obtained solid did not dissolve in most common organic solvent,such as acetone,acetonitrile,DMF or THF,but it did dissolve in DMSO to produce a clear brown solution.The solution was kept under MeCN atmosphere to grow single crystals.Finally,dark brown flake crystalswere obtained,which were identified as[HMIM]2[Fe2O(SO4)3(DMSO)2]·0.5DMSO(1)by the single X-ray diffraction method(Scheme 1).It is compelling to find that Fe髤has been oxidized to Fe髥to produce an imidazole template Fe4sulfate cluster.Under the similar reaction conditions,[HPIM]4[Fe4O2(SO4)6(DMSO)4]·2MeCN(2)was obtained as well.
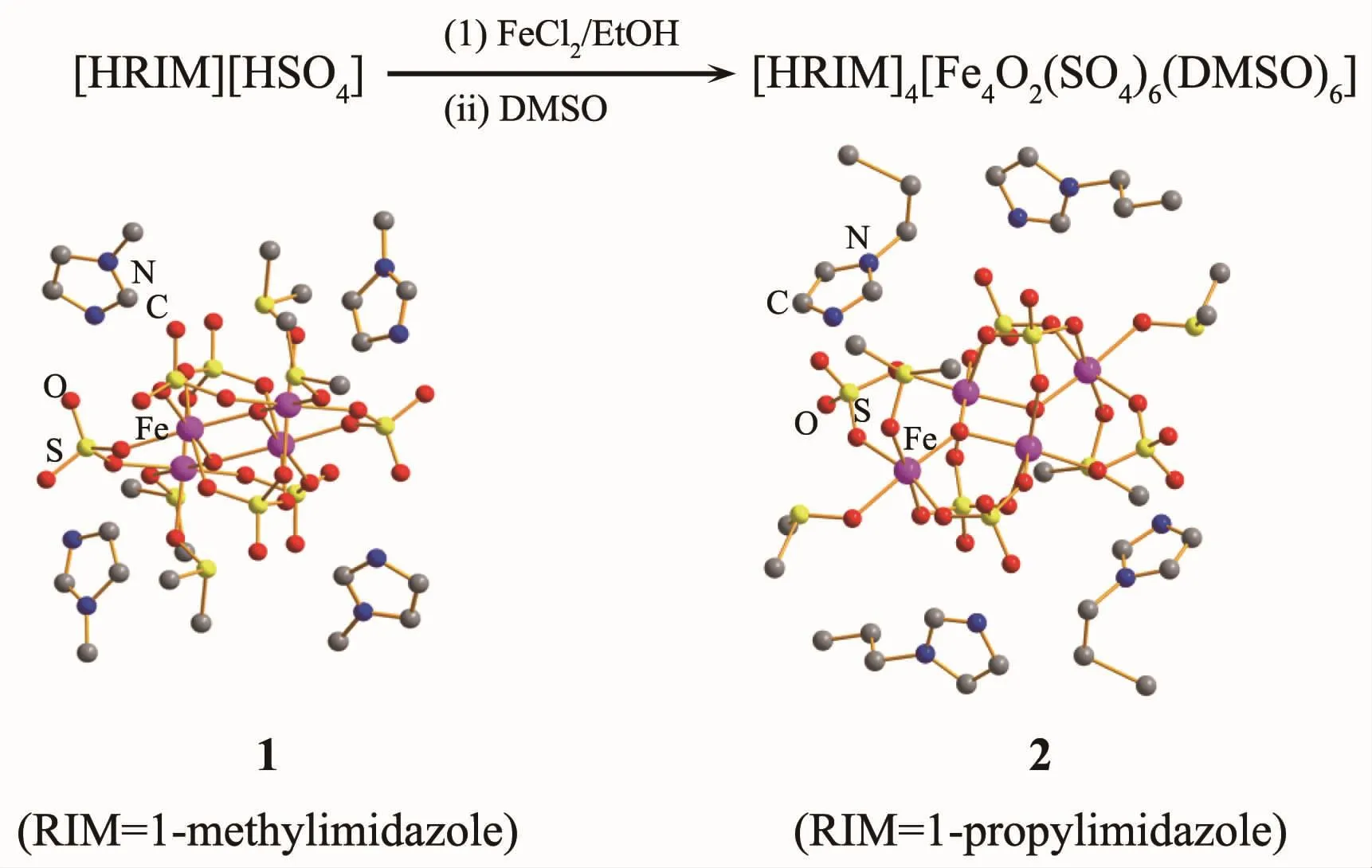
Scheme 1 Reactions of FeCl2with[HRIM][HSO4]in ethanol
To see whether FeCl3can also carry out the similar reaction,we underwent the similar reaction of FeCl3with[HMIM][HSO4]in ethanol.Anhydrous FeCl3was added to the solution of[HMIM][HSO4]in ethanol.The black mixture gradually turned into brown during the reaction.The solvent was removed by rotary evaporation to generate a dark brown liquid.This phenomenon differed from the reaction of FeCl2,where brown solids were obtained.The liquid was then resolved in DMSO to grow crystals.Imidazole template complex was not formed,however,yellow flake crystals[FeCl2(DMSO)4][EtSO4]·DMSO(3) were achieved,where four DMSO molecules coordinate to one iron atom,and one Cl-is substituted by EtSO4-.EtSO4-probably was generated by the reaction of EtOH with HSO4-under this special reaction condition[45].Cations[FeCl2(DMSO)4]+were balanced by the EtSO4-anions.
2.2 Crystal structure
Table 1 summarizes the crystallographic data for complexes 1~3.Complexes 1 and 2 are the products of the reactions of [HRIM][HSO4]with FeCl2in ethanol,which crystallize in triclinic with a space group P1.They have the similar structural fragment and contain the tetranuclear[Fe4(O)2(SO4)6(DMSO)4]core template by imidazole derivatives.The Fe4core structure is shown in Fig.1.The core contains four Fe atoms,two μ3-O,four μ2-SO4,two μ3-SO4,and four DMSO molecules.FourFe atomsare coplanar connected by the central μ3-O bridge and μ2-O and μ3-O bridge of SO4groups.The μ3-oxo-bridged[Fe3(μ3-O)]4fragment presents as a pyramidal geometry.Two SO4groups act as tridentate bridging ligands coordinated to Fe atoms on the two[Fe3(μ3-O)]4fragments,and the other four SO4groups engage in O,Oi-bridging within the tetracluster.DMSO groups act as mono-dentate ligands coordinated to Fe2 and Fe2iatoms.In the asymmetric unit of 1,protonated imidazoliums are the counter cations for the balance of charges of the Fe髥core[Fe2O(SO4)3(DMSO)]2-anion.
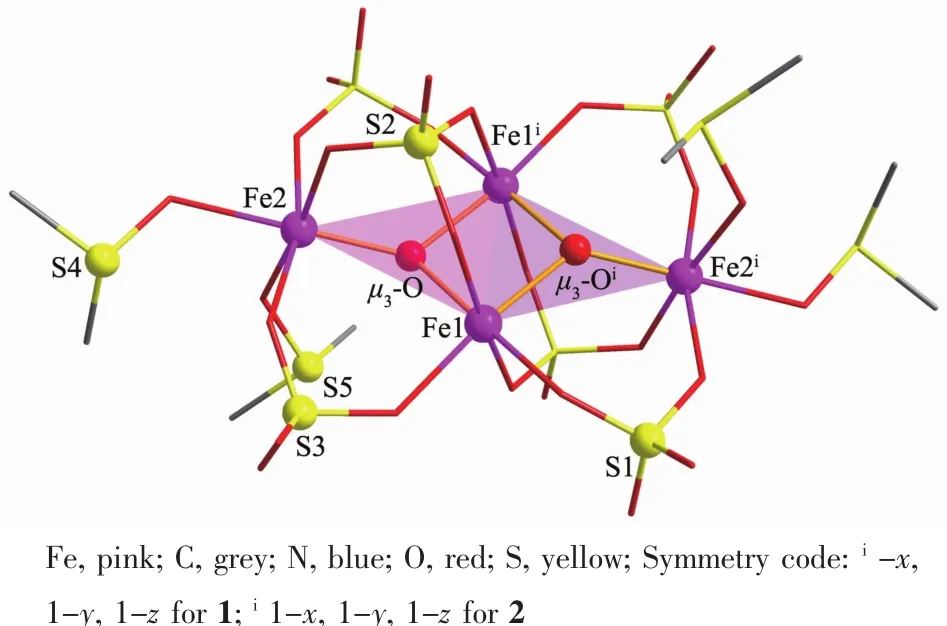
Fig.1 Structure fragment of the coordination clusters
The selected bond distances and angles are listed in Table S1~S3.For complex 1,the distances of Fe1-(μ3-O)and Fe1-(μ3-O)iequal to 0.195 0(5)nm and 0.196 0(4)nm,respecticely,while Fe2-(μ3-O)distances are slight shorter with 0.186 9(5)nm.Fe1-(μ3-O)-Fe1iangles(91.9(2)°)are almost equal to(μ3-O)-Fe1-(μ3-O)iangles(88.1(2)°).They are close to the right angle so that the four atoms Fe1,Fe1i,and two(μ3-O)atoms form a nearly perfect square face which exerts a trans to Fe2 and Fe2iwith respect to the[Fe2O2]plane.Four Fe atoms are coplanar to form a rhombic plane,where Fe1-Fe2-Fe1iand Fe2-Fe1-Fe2iangles are 48.243(30)°and 131.757(44)°,respectively.It is different to the oxygen-based ligation Fe4clusters[25-26,46],where cubic plane is often seen.Two Fe2-ODMSOdistances are separately 0.204 6(5)and 0.211 0(6)nm.Four Fe1-Osulfatedistances vary from 0.198 0(5)to 0.208 8(5)nm while three Fe2-Osulfatedistances fall in the range of 0.200 5(5)~0.203 9(5)nm. The Fe1-Fe1idistance(0.281 0(2)nm)is much shorter than the Fe1-Fe2 distance(0.346 31(11)nm).The coordinated DMSO groups are partly disordered.[HMIM]+cations lay in the lattice without any interaction with the main structure as shown in the crystal packing view of complex 1 (Fig.3).Complex 2 has the almost same core structure parameters with complex 1 except the variation of alkyl groups on imidazole rings and existing different solvent molecules in the lattice as shown in Table S2.The crystal packing views of complexes 2 are presented in Fig.S1.
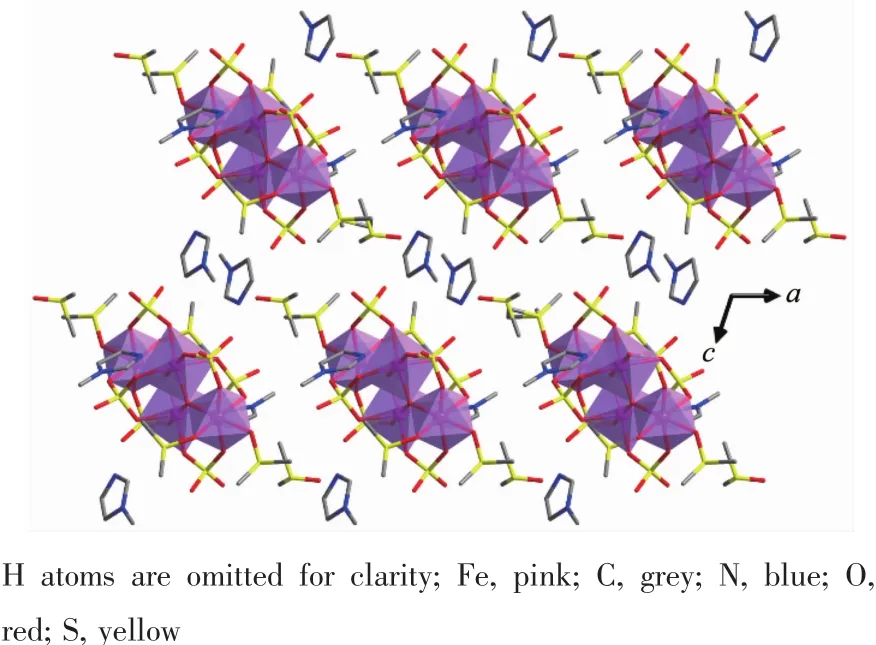
Fig.2 Perspective packing view of the crystal structure of complex 1 along b axis
The reaction of FeCl3in[HMIM][HSO4]led to the formation of[FeCl2(DMSO)4][EtSO4]·DMSO(3).Complex 3 crystallizes in triclinic with a space group P1.Fe atom is bonded to two chlorine atoms and four oxygen atoms from four DMSO leading to a distorted octahedral geometry.Distances of Fe-O bonds fall in the range of 0.200 3(3)~0.202 4(3)nm.Fe-Cl bond lengths are~0.235 nm.DMSO solvent molecules are disordered.Anion [EtSO4]-resides in the side of[FeCl2(DMSO)4]+.[FeCl2(DMSO)4]+is connected to each other with C-H…Cl with 0.283 nm length(Fig.3).
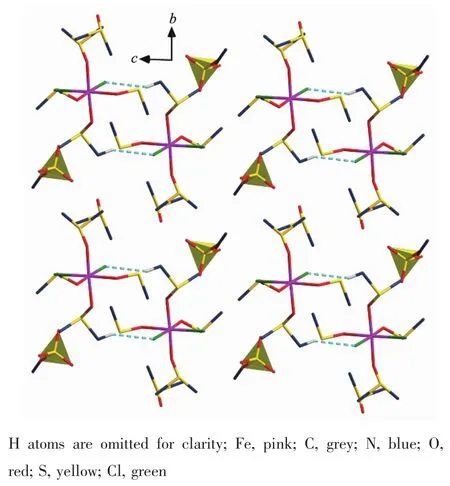
Fig.3 View of the crystal structure of complex 3 along c axis showing hydrogen bonds C-H…Cl
2.3 Thermal behaviour
The TGA curves for the obtained complexes 1~3 are shown in Fig.4.The decomposition of complex 1 undergoes in three steps.DMSO groups are firstly decomposed in the range of 28~300℃(Obsd.62.3%,Calcd.62.1%).The second weight loss process in the 300~540℃range is ascribed to the releasing of four[HMIM]+and three SO3(Obsd.23.3%,Calcd.22.5%).With the increasing of the temperature,the complex is further decomposed and the total weight loss observed is 81.1%.The remaining weight of 18.9%is assigned as Fe2O3(Calcd.20.4%).For complex 2,free solvent molecules are firstly lost in the rage of 28~300℃,and then,four imidazonium cations and four SO42-are further decomposed in the range of 400~700℃to give rise to the remaining weight of Fe2O3.For complex 3,there is a dramatically weight loss in the range of 28~190℃,which is attributed to the losses of four DMSO molecules(Obsd.50.8%,Calcd.51.2%).The second weight loss process occurring in the range of 189~316℃is related to the losses of chloride anions and one DMSO molecule.The third stage in the 316~507℃is due to the loss of anion[EtSO4]-.

Fig.4 TGA curves for complexes 1~3
2.4 Magnetic analysis
Molar magnetic susceptibility(χM) data were collected on powered polycrystalline samples of 1 and 2 in a 0.1 T field in the 3.0~300 K range.The data of 1 and 2 are depicted as χMT versus T in Fig.5.As shown in Fig.5,χMT monotonously decreases with decreasing temperature from 2.15 emu·K·mol-1(for 1)and 3.22 emu·K·mol-1(for 2)at 300 K to 0.13 emu·K·mol-1(for 1)and 0.33 emu·K·mol-1(for 2)at 3 K.The 300 K values are much less than the spin-only(g=2.0)value of 17.5 emu·K·mol-1for four noninteracting Fe髥 ions,indicating the presence of strong antiferromagnetic exchange interactions with the Fe4complexes,leading to a singlet(S=0)ground state.This behaviour is analogous to that of other Fe4clusters with primarily oxygen-based ligatio[25-26,46].
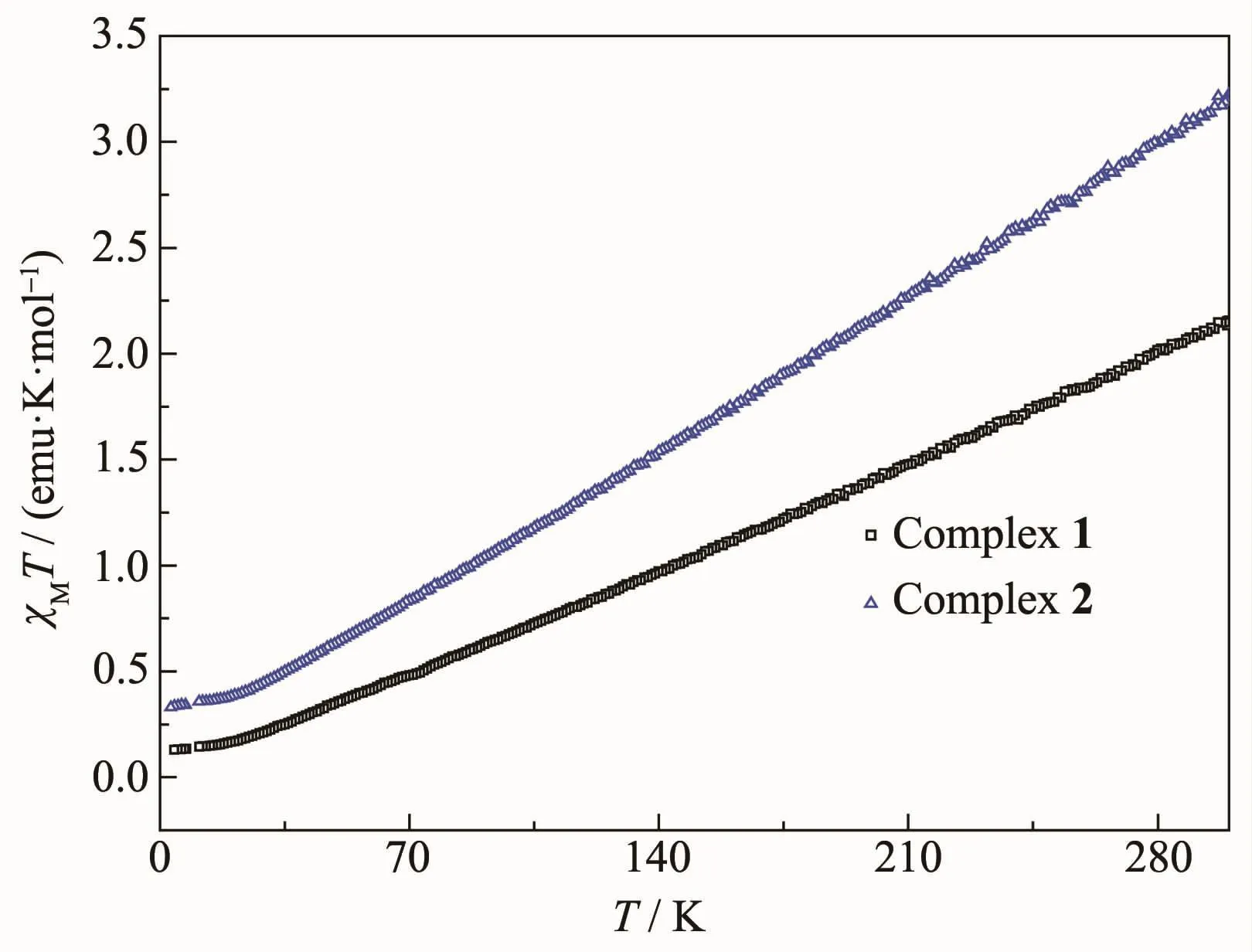
Fig.5 Plots of χMT vs T for complexes 1 and 2
3 Conclusions
In summary,imidazole template Fe4sulfate clusters were synthesized via the reactions of FeCl2with Brnsted acidic ionic liquids [HRIM][HSO4]with ethanol as solvent.In contrast,the reaction of FeCl3under the similar reaction conditions did not lead to the similar cluster structure,and the imidazole free iron complex[FeCl2(DMSO)4][EtSO4]·DMSO(3)was produced.The clusters contain the tetranuclear[Fe4(μ3-O)2(μ2-SO4)6(DMSO)4]core,and protonated imidazoliums are the counter cations for the balance of charges of the core.Variable temperature magnetic susceptibility measurements of the Fe4sulfate clusters reveal that the complexes possess strong antiferromagnetic exchange interactions between the metal centres.
Acknowledgements:This work was supported by the NSF of China(Grants No.61671206,61201071)and Program of Shanghai Subject Chief Scientist(Grant No.12PJ1402600).
Supporting information is available at http://www.wjhxxb.cn
[1]Nair V S,Hagen K S.Inorg.Chem.,1992,31:4048-4050
[2]Taft K L,Papaefthymiou G C,Lippard S J.Inorg.Chem.,1994,33:1510-1520
[3]Frank P,DeTomaso A,Hedman B,et al.Inorg.Chem.,2006,45:3920-3931
[4]Taft K,Papaefthymiou G,Lippard S.Science,1993,259:1302-1305
[5]Gomez-Coca S,Ruiz E,Kortus J.Chem.Commun.,2009:4363-4365
[6]Ruiz E,Cano J,Alvarez S.Chem.Eur.J.,2005,11:4767-4771
[7]Petukhov K,Bahr S,Wernsdorfer W,et al.Phys.Rev.B,2007,75:064408
[8]Benelli C,Cano J,Journaux Y,et al.Inorg.Chem.,2001,40:188-189
[9]Schlegel C,Slageren J,Manoli M,et al.Polyhedron,2009,28:1834-1837
[10]Zhu Y Y,Guo X,Cui C,et al.Chem.Commun.,2011,47:8049-8051
[11]Rigamonti L,Piccioli M,Malavolti L,et al.Inorg.Chem.,2013,52:5897-5905
[12]Jeon I R,Park J G,Xiao D J,et al.J.Am.Chem.Soc.,2013,135:16845-16848
[13]Nachtigall O,Kusserow M,Clérac R,et al.Angew.Chem.Int.Ed.,2015,54:10361-10364
[14]Koumousi E S,Routzomani A,Nguyen T N,et al.Inorg.Chem.,2013,52:1176-1178
[15]Bagai R,Daniels M R,Abboud K A,et al.Inorg.Chem.,2008,47:3318-3327
[16]Alborés P,Rentschler E.Inorg.Chem.,2008,47:7960-7962
[17]Stamatatos T C,Boudalis A K,Sanakis Y,et al.Inorg.Chem.,2006,45:7372-7381
[18]Casey C P,Guan H.J.Am.Chem.Soc.,2009,131:2499-2507
[19]Jones L F,Low D M,Helliwell M,et al.Polyhedron,2006,25:325-333
[20]Ferguson A,Thomas L H,Murrie M.Polyhedron,2013,52:227-233
[21]Ferguson A,McGregor J,Brechin E K,et al.Dalton Trans.,2009:9395-9397
[22]Masello A,Sanakis Y,Boudalis A K,et al.Inorg.Chem.,2011,50:5646-5654
[23]Rajaraman G,Cano J,Brechin E K,et al.Chem.Commun.,2004:1476-1477
[24]Mitchell K J,Abboud K A,Christou G.Inorg.Chem.,2016,55:6597-6608
[25]Taguchi T,Stamatatos T C,Abboud K A,et al.Inorg.Chem.,2008,47:4095-4108
[26]Mulyana Y,Nafady A,Mukherjee A,et al.Inorg.Chem.,2009,48:7765-7781
[27]Murali M,Nayak S,Costa J S,et al.Inorg.Chem.,2010,49:2427-2434
[28]TIAN Ju-Mei(田菊梅),CUI Zheng(崔正),ZHANG Jing-Ping(张景萍).Chinese J.Inorg.Chem.(无机化学学报),2017,33(9):1625-1630
[29]WANG Ming(汪明),PAN Qun-Hui(潘群慧),SHEN Tao(沈涛),et al.Chinese J.Inorg.Chem.(无机化学学报),2005,21(9):1296-1300
[30]Choudhury A,Krishnamoorthy J,Rao C N R.Chem.Commun,2001:2610-2611
[31]Maspoch D,Ruiz-Molina D,Veciana J.Chem.Soc.Rev.,2007,36:770-818
[32]Natarajan S,Mandal S.Angew.Chem.Int.Ed.,2008,47:4798-4828
[33]Yahyaoui S,Rekik W,Nali H,et al.J.Solid State Chem.,2007,180:3560-3570
[34]Behera J N,Rao C N R.Chem.Eur.J.,2006,1:742-750
[35]Paul G,Choudhury A,Sampathkumaran E V,et al.Angew.Chem.Int.Ed.,2002,41:4297-4300
[36]Behera J N,Rao C N R.Inorg.Chem.,2006,45:9475-9479
[37]Fu Y,Xu Z,Ren J,et al.Cryst.Growth Des.,2007,7:1198-1204
[38]Dan M.J.Mol.Struct.,2004,706:127-131
[39]Paul G,Choudhury A,Rao C N R.Chem.Mater.,2003,15:1174-1180
[40]Calligaris M.Coord.Chem.Rev.,2004,248:351-375
[41]Lin H,de Oliveira P W,Huch V,et al.Chem.Mater.,2010,22:6518-6523
[42]Luo Q Q,Lin H C,Zhang Y Y,et al.Inorg.Chim.Acta,2016,447:32-37
[43]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2008,A64:112-122
[44]Dolomanov O V,Bourhis L J,Gildea R J,et al.J.Appl.Crystallogr.,2009,42:339-341
[45]Evans P N,Albertson J M.J.Am.Chem.Soc.,1917,39:456-461
[46]Murali M,Nayak S,Costa J S,et al.Inorg.Chem.,2010,49:2427-2434
Syntheses of Imidazole Template Fe4Sulfate Clusters and Iron-DMSO Complex Mediated by Br覬nsted Acid Ionic Liquids
LUO Qian-Qian1LIN He-Chun*,1LUO Chun-Hua1ZHANG Yuan-Yuan1PENG Hui1,2
(1Key Laboratory of Polar Materials and Devices,Ministry of Education,East China Normal University,Shanghai 200141,China)
(2Collaborative Innovation Center of Extreme Optics,Shanxi University,Taiyuan 030006,China)
Imidazole template iron sulfate clusters,[HMIM]2[Fe2O(SO4)3(DMSO)2]·0.5DMSO(1)and[HPIM]4[Fe4O2(SO4)6(DMSO)4]·2MeCN(2),were synthesized via the reaction of FeCl2with Br覬nsted acidic ionic liquid[HRIM][HSO4]using ethanol as solvent.The clusters crystallize in triclinic with a space group P1.The core contains 4 iron atoms,which are coplanar connected by the central μ3-O bridge, μ2-O and μ3-O bridge of SO4groups.Protonated imidazoliums are the counter cations for the balance of charges of the core.Variable temperature magnetic susceptibility measurements of the Fe4sulfate clusters reveal weak antiferromagnetic exchange interactions between the metal centers.In contrast,the reaction of FeCl3under the similar experimental conditions didn′t produce the similar cluster structure,whereas iron-DMSO complex[FeCl2(DMSO)4][EtSO4]·DMSO(3)was obtained.CCDC:1495092,1;1495093,2;1495086,3.
polynuclear ferric cluster;open-framework;brnsted acidic ionic liquid,antiferromagnetic
O614.81+1
A
1001-4861(2017)12-2186-07
10.11862/CJIC.2017.259
2017-06-11。收修改稿日期:2017-10-13。
国家自然科学基金(No.61671206,61201071)和上海市科委项目(No.12PJ1402600)资助。
*通信联系人。 E-mail:hclin@ee.ecnu.edu.cn
