溶剂对柔性配体 1,3,5-三(三氮唑-1-甲基)-2,4,6-三甲苯的银配合物的影响
2017-12-13倪天军苑强涛杨志军余海红蔡红蕊
倪天军 苑强涛 张 伟 杨志军*, 余海红 蔡红蕊
溶剂对柔性配体 1,3,5-三(三氮唑-1-甲基)-2,4,6-三甲苯的银配合物的影响
倪天军1苑强涛1张 伟1杨志军*,1余海红2蔡红蕊2
(1新乡医学院基础医学院,新乡 453003)
(2新乡医学院药学院,新乡 453003)
通过在不同溶剂中硝酸银与 1,3,5-三(1,2,4-三氮唑-1-甲基)-2,4,6-三甲苯(ttmb)的反应,合成了 2 种新型的银配合物:{[Ag(ttmb)(H2O)]NO3}n(1)、{[Ag(ttmb)]NO3·H2O)}n(2)。研究了室温条件下,2个配合物与相关ttmb配体的固态荧光性质。通过元素分析、粉末和单晶X射线衍射分析、红外光谱分析等对其结构进行表征,结果表明化合物1包含一个高度起伏的二维网状结构,化合物2具有一个二维的(6,3)网状结构。
晶体结构;银配合物;三氮唑配体;配位聚合物
0 Introduction
In recent years,metal-organic frameworks(MOFs)have been rapidly developed and subjected to great attention,not only for their fantastic structures-cage,honeycomb,grid,ladder,multidimensional framework,polyrotaxane and polycatenane[1-5],but also for their potentialapplications to functionalmaterials in magnetism,catalysis and gaseousstorage,ionic/molecularrecognition,ion-exchange and selective guest inclusion[6-11].Tripodal ligands with arene core have been found to be one of the most useful organicbuilding blocks for constructing novel coordination frameworks[12-17].Complexes with specific structures and interesting properties have been obtained by reactions of suitable metal salts with rigid exo-tridentate ligands,such as 2,4,6-tris(4-pyridyl)-1,3,5-triazine[18-20],1,3,5-tricyanobenzene[21-22]and 1,3,5-benzenetribenzoate[23-24].Among them,the rigid ligands have minimal conformational changes upon reacting with metal salts[25].Based on the variable geometric requirements by the metal ions,flexible ligands can adopt a number of conformations and coordination modes,which may offer MOFs with unique structures and useful properties[26]. To further investigate the nature of flexible ligands and their coordination polymers,a triazolyl group containing,not the reported pyridyl and imidazol groups,namely 1,3,5-tris(triazol-1-ylmethyl)-2,4,6-trimethylbenzene (ttmb,Scheme 1),was synthesized.Due to the presence of methylene group between the triazole and benzene ring groups,ttmb is considered as a flexible divergent ligand exhibiting the cis-and trans-configurations.
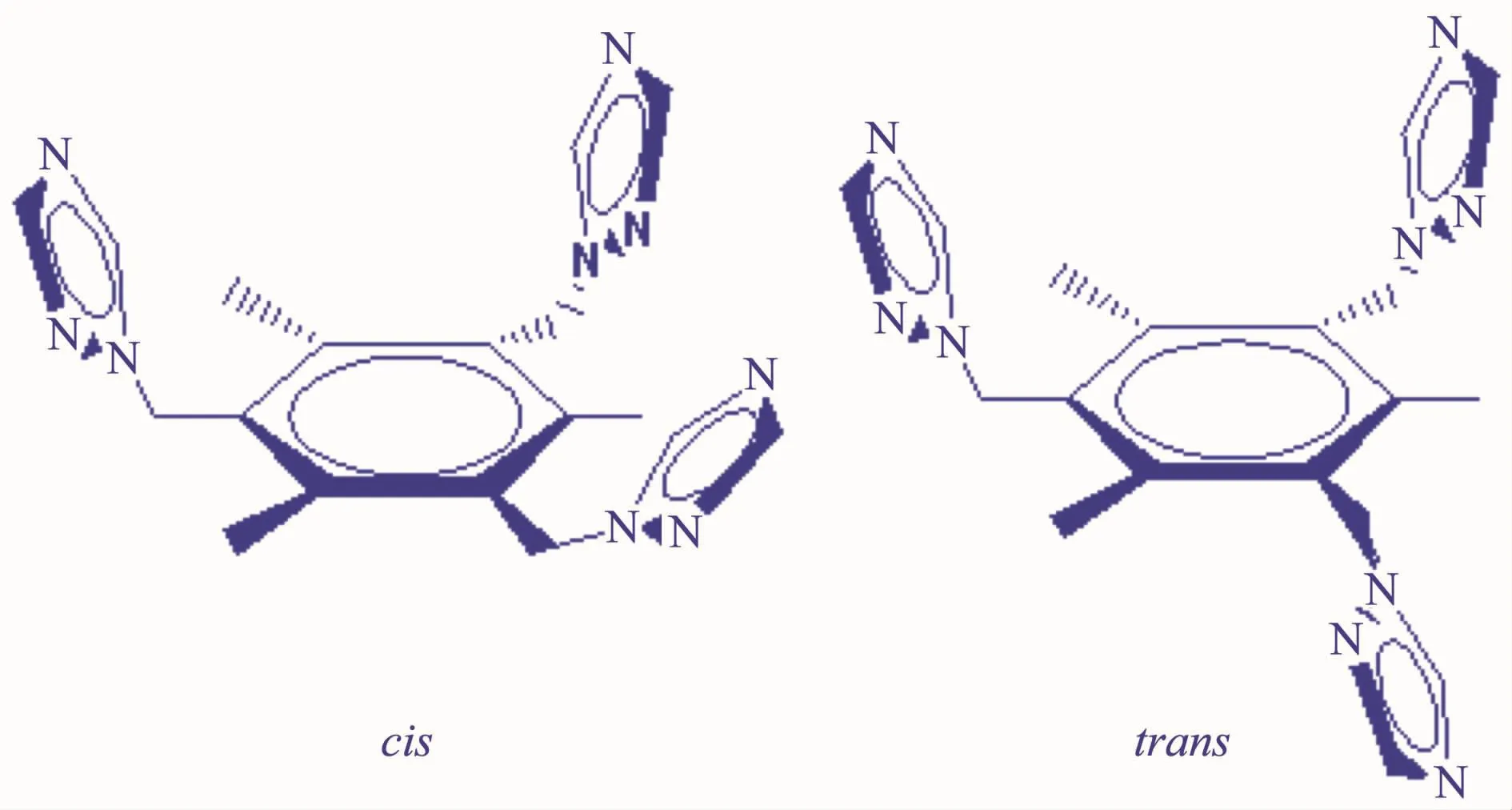
Scheme 1 Cis-and trans-conformations of the flexible tripodal ligand ttmb
As well known,it is very important to control the reaction conditions using flexible ligands during the synthesis of the resulted complexes,such as the counter anions,the ratio of ligand to metal ion,temperature[27-30],because there is a methylene group between the central benzene ring and terminal trizaol group which may change configuration depending on solvents and counter ions.Hence,systematic studies on therelationship between MOF structureand reaction solvents is crucial in understanding MOFs and exploring their applications.
In the present work,two new polymers{[Ag(ttmb)(H2O)]NO3}n(1)and{[Ag(ttmb)]NO3·H2O}n(2)were assembled from flexible tripodal 1,3,5-tris(triazol-1-ylmethyl)-2,4,6-trimethylbenzene (ttmb)and silver nitrate under different conditions.Solvent significantly influences the structures of the coordination polymers(Scheme 2).Asa whole,the synthesis,crystal structures as well as fluorescence properties of the two polymers are reported.
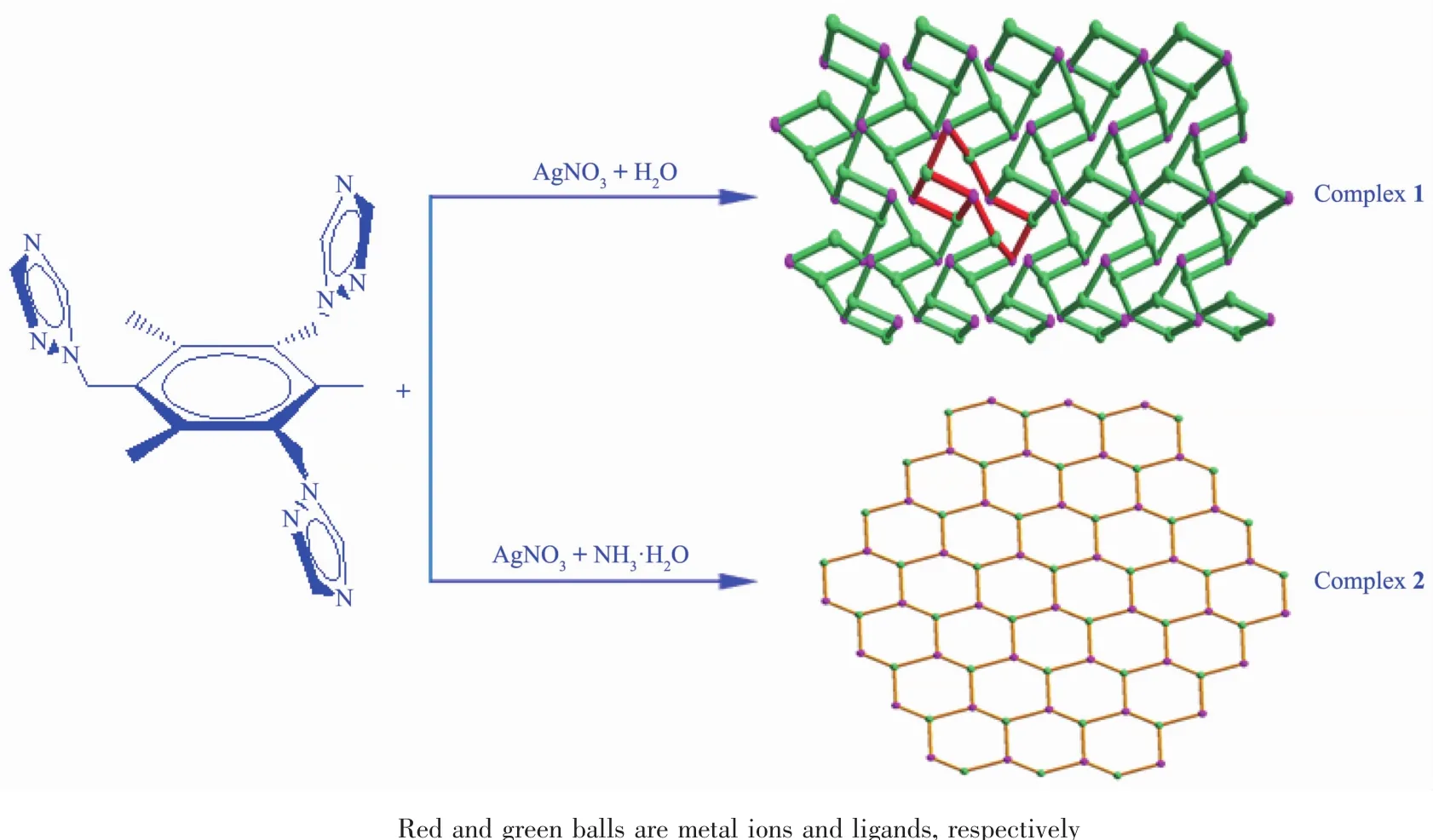
Scheme 2 Solvent control in the synthesis of coordination polymers 1 and 2
1 Experimental
All chemicals were of reagent-grade quality,obtained from commercialsources,and used as received without further purification.Solvents were purified by standard methods prior to usage.1,3,5-tris-(bromomethyl)-2,4,6-trimethylbenzene was synthesized according to the reported procedures[28].Elemental analyses were determined using a Vario ELⅢelemental analyzer.The IR spectra were recorded in the 400~4 000 cm-1region using KBr pellets and a Nicolet AVATAR-370 spectrometer.Excitation and emission spectra were recorded on a Shimadzu F-4500 fluorescence spectrophotometer using slit width of 3.0 nm both for excitation and emission at room temperature for the solid samples.Thermogravimetric(TG)analysis data were collected on a Netzsch STA-409PC under air in the temperature range from 20 to 800℃ with a heating rate of 10℃·min-1.Powder X-ray diffraction (PXRD)patterns were recorded on a Rigaku D/max-2550 X-ray diffractometer with graphite mono-chromatic Cu Kα (λ=0.154 056 nm)radiation at 40 kV and 250 mA and 2θ ranging from 5°to 60°at room temperature.
1.1 Synthesis of{[Ag(L)(H2O)]NO3}n(1)
An aqueous solution(5 mL)of AgNO3(17.0 mg,0.1 mmol)was added slowly to a solution of ttmb(36.3 mg,0.1 mmol)in methanol(10 mL)to give a clear solution.It was stirred for 10 min at room temperature.Colorless block crystals for X-ray diffraction were obtained upon slow evaporation of the solvent after two weeks.Yield:43%based on Ag.Anal.Calcd.for C18H23AgN10O4(%):C 39.21;H 4.20;N 25.40.Found(%):C 39.42;H 4.23;N 25.32.IR(KBr pellet,cm-1):3451br,3110m,1517s,1383s,1 274s,1 202w,1 135s,1 011s,987w,676s,643m.
1.2 Synthesis of{[Ag(L)]NO3·H2O}n(2)
An aqueous solution of ammonia(0.5 mmol·mL-1,2 mL)was dropped to a stirred aqueous solution(5 mL)of AgNO3(17.0 mg,0.1 mmol).The resulting solution was continuously stirred for 30 min and then a solution of ttmb (36.3 mg,0.1 mmol)in methanol(10 mL)was added.Well-shaped colorless block crystals were obtained by slow evaporation at room temperature for one week.They were collected by filtration,washed with a small amount of distilled water and dried in air.Yield:57%based on Ag.Anal.Calcd.for C18H23AgN10O4(%):C 39.21;H 4.20;N 25.40.Found(%):C 39.05;H 4.55;N 25.23.IR(KBr pellet,cm-1):3 448br,3 111m,1 514s,1 363s,1 335s,1 274s,1 208m,1 137s,1 009s,979w,675s,643m.
1.3 X-ray structure determination
Single crystal diffraction data for complexes 1 and 2 were collected on a Bruker Smart Apex-ⅡCCD diffractometer with graphite monochromatic Mo Kα radiation(λ=0.071 073 nm)at 296(2)K.Empirical absorption corrections were performed using SADABS program[31].The structures were extracted by a direct method with SHELXS-97 program[32]and refined by fullmatrix least squares on F2with SHELXL-97 program[33].All non-hydrogen atoms were refined anisotropically and hydrogen atoms were placed geometrically.Molecular graphics were drawn by using XP,and Diamond software.The crystal data and refinement results are given in Table 1.The selected bond distances and angles are listed in Table 2.
CCDC:878517,1;878518,2.
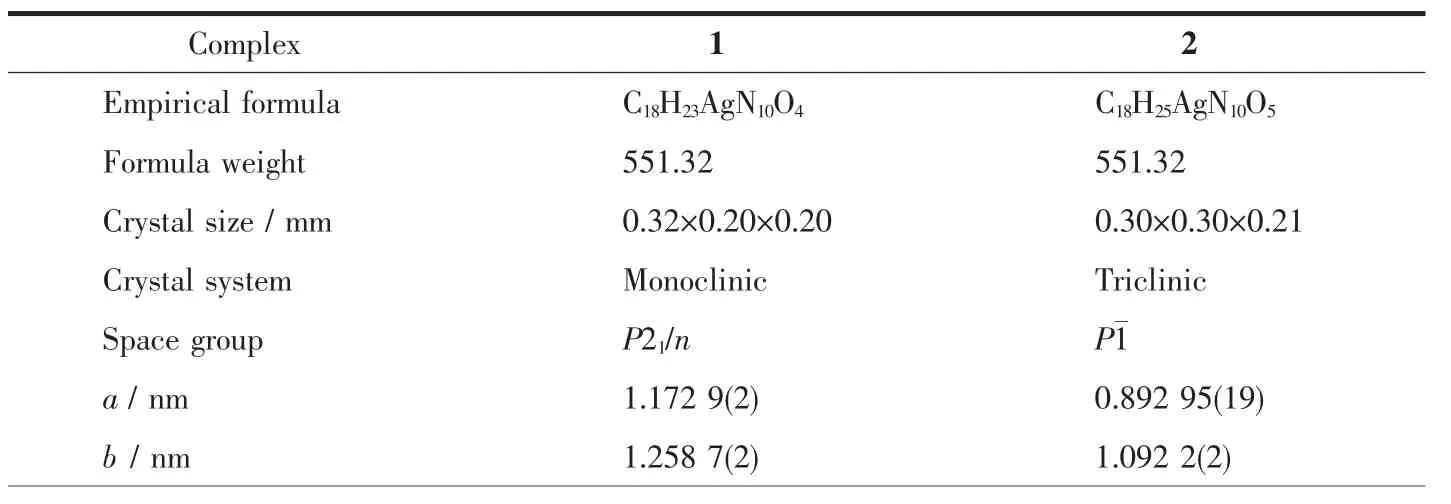
Table 1 Crystallographic data for complexes 1 and 2
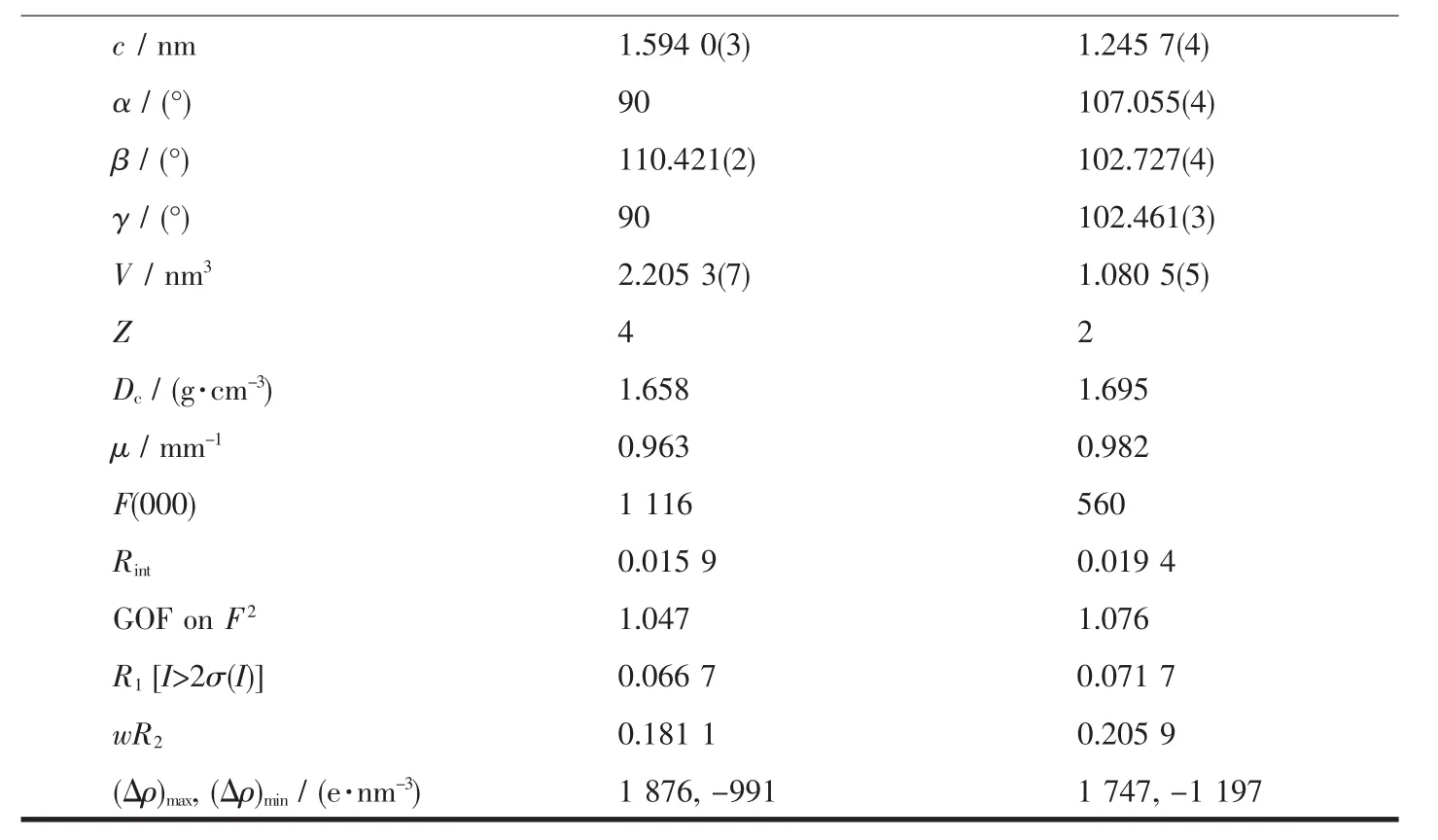
Continued Table 1
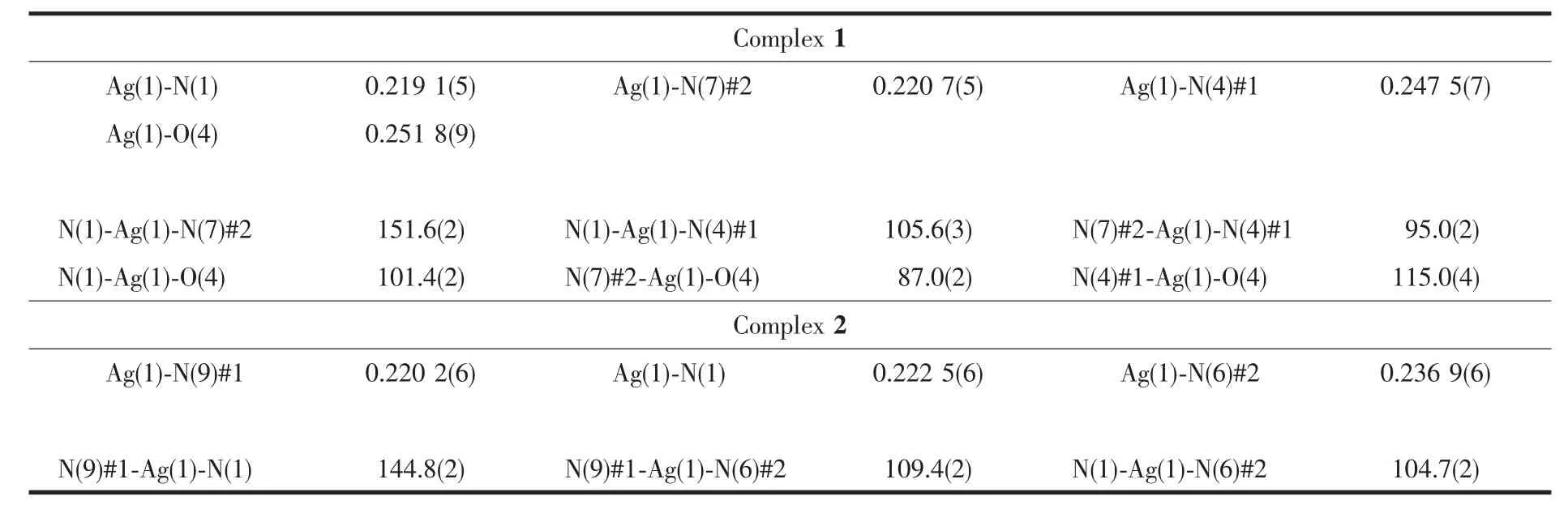
Table 2 Bond lengths(nm)and angles(°)for 1 and 2
2 Results and discussion
2.1 Crystal structure description
The crystal structure of complex 1 is shown in Fig.1 with the atom numbering scheme.Complex 1 crystallizes in the Monoclinic form with P21/n space group.Each asymmetric unit of complex 1 contains one Ag髣center,one ttmb,one water molecule and one nitrate anion.The silver髣ion is coordinated by three nitrogen atomsfrom three individualttmb ligands and one oxygen atom from a water molecule(Fig.1)to form tetrahedral environment.The Ag-N bond lengths are in the range of 0.219 1(5)~0.247 5(7)nm,and Ag-O bond lengths are 0.251 8(9)nm,which are longer than the Ag-N distances.They are in conformity with those found in reported corresponding silver complexes[34].Coordination geometry around Ag髣in complex 1 can be judged as a distorted tetrahedron with a N3O donor set.
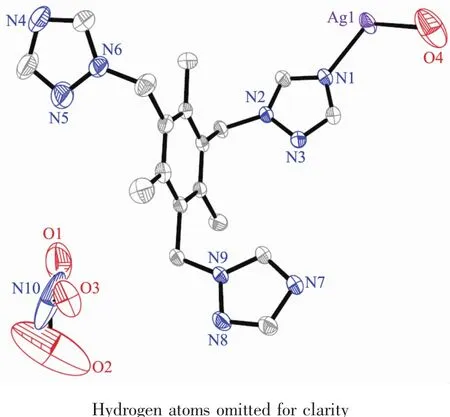
Fig.1 ORTEP drawing of complex 1 with 30%thermal ellipsoids probability
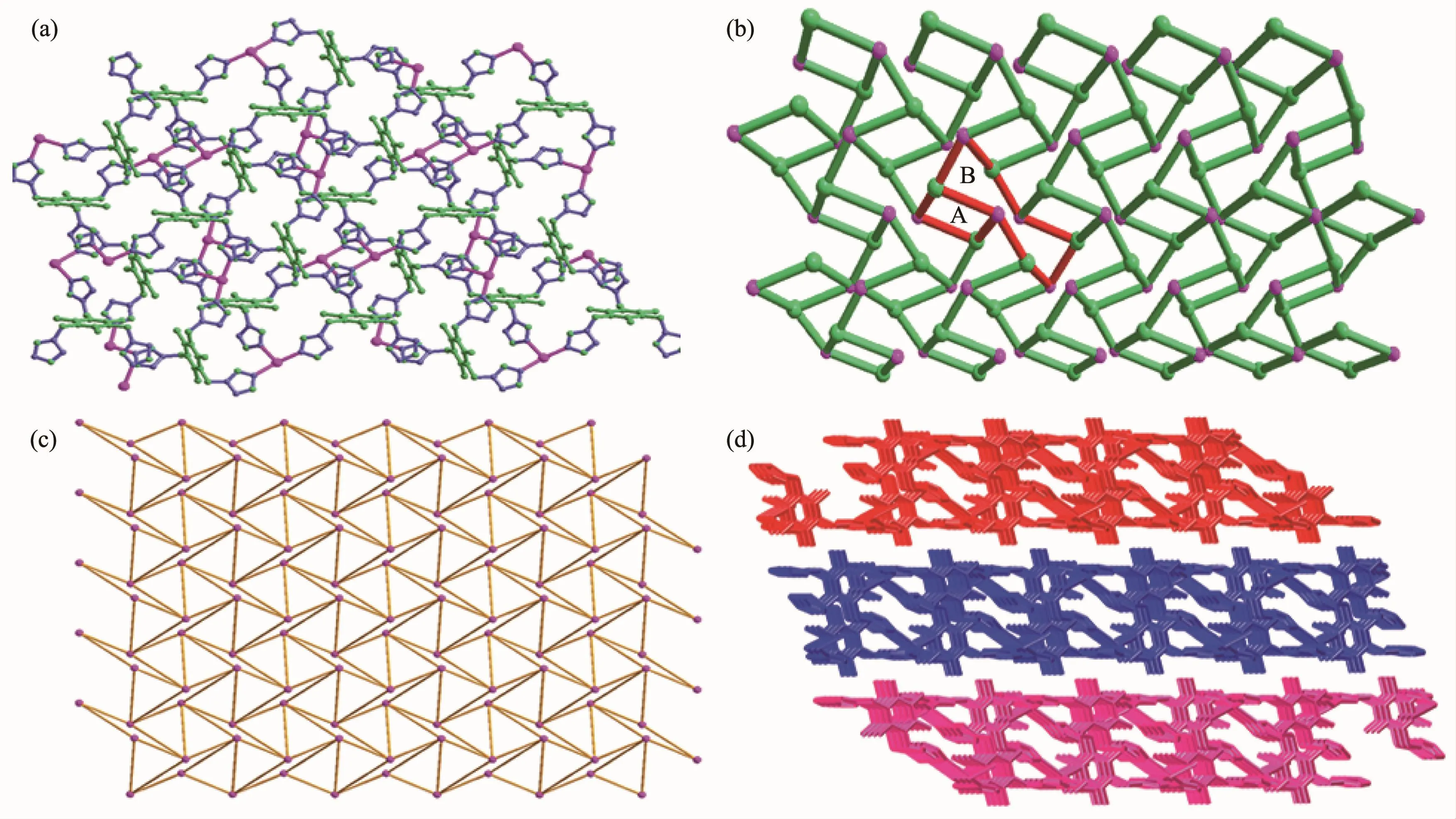
Fig.2 (a)2D network of 1;(b)Simplified structure of the 2D network;(c)Two-dimensional sheet consisting of the four-membered and three membered rings where only the silver髣atoms are present;(d)Crystal packing diagram viewed from the b-axis
Each ttmb shows trans-conformation as trimonodentate linking three different silver髣atoms.The distances between the N1,N4,N7 atoms and the central benzene ring plane are 0.301,0.294 and 0.350 nm,respectively.The dihedral angles between each triazole group and the central benzene ring plane are 78.33°,79.35°and 82.20°,respectively.Three ligands link three Ag髣ions using their three flexible arms to generate an infinite 2D undulated network[35](Fig.2a).
The combination of the flexible ttmb ligands and four-coordinated Ag髣ions leads to the formation of a 2D network with two differentmacrocycles as illustrated in Fig.2b.In macrocyclic ring A,two ttmb ligands,each using two of its three flexible arms,link two Ag髣ions to form a 24-membered ring with a Ag…Ag distance of 1.192 nm,while in ring B,four ttmb ligands are connected by four Ag髣ions to generate a 48-membered ring with intermetallic separations of dAg…Ag=2.224,0.689 nm.
The crystal packing diagram of 1 is displayed in Fig.2d.The 2D networks are further linked together via the interlayer C-H…O (nitrate anion)hydrogen bonds and π-π stacking interactions to generate a 3D framework;the uncoordinated nitrate anionsand coordinated water molecules are located in the voids between the two adjacent layers.
The coordination environment around the Ag髣ion in complex 2 was shown in Fig.3.The X-ray crystallographic analysis indicates that complex 2 crystallizes in the triclinic form with P1 space group.Complex 2 consists of one Ag髣center,one ttmb,one nitrate anion and two uncoordinated water molecules.The Ag髣ion is surrounded by three tridentate bridging ligands,coordinated with three N atoms (N1,N6 and N9)from three different ligands with a N-Ag-N bond angle ranging from 104.7(2)°to 144.8(2)°and Ag-N bond distance ranging from 0.220 2(6)to 0.236 9(6)nm,respectively,which is comparable with those of the reported Ag髣complexes[36].The complex displays a distorted planar triangle geometry around the Ag centre(Table 1)with a N3donor set(N3,N6 and N9).
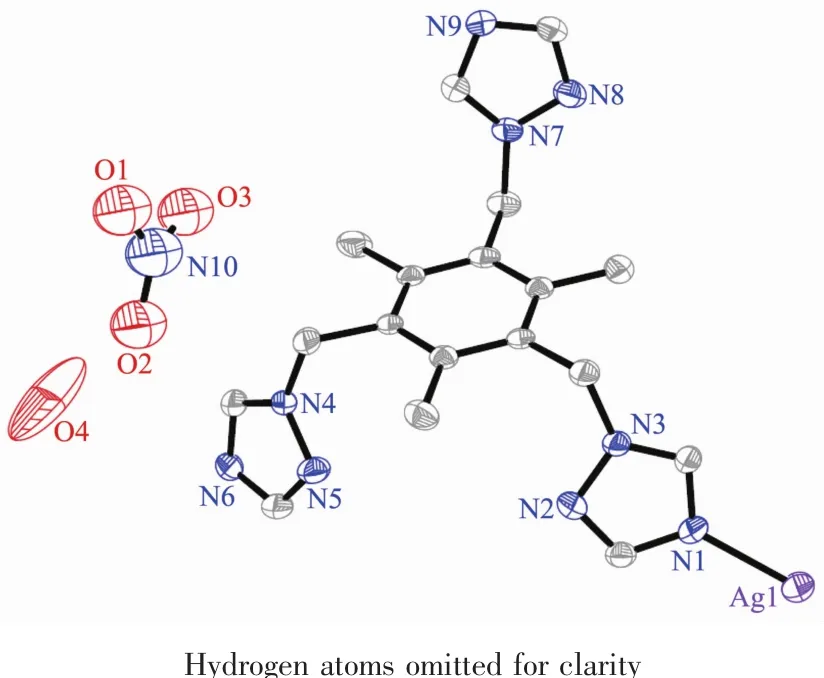
Fig.3 ORTEP drawing of complex 2 with 30%thermal ellipsoids probability
Ligands in complex 2 adopt a cis-,trans-,transconformation and act as a tridentate linker using its three triazole groups to coordinate with three Ag髣ions(Fig.3).The two triazole groups within each ttmb are located in the same side and inclined to the central benzene ring plane with dihedral angles of 10.93°,44.89°,and 72.98°,respectively,and the distancesbetween thecoordinated N atomsand central benzene ring plane are 0.422,0.452,and 0.568 nm,respectively.The polymeric structure of complex 2,generated by such coordination mode and conformation of ligand,is an infinite two-dimensional(2D)stepwise layered network as shown in Fig.4a.Then,a schematic drawing is shown in Fig.4b[37].Fig.4c shows the two dimensional sheet where only the Ag髣 ions are presented.This is a(6,3)sheet-like network consisting of the Ag3unit where the distance between two adjacent Ag髣ions are 0.892,1.192 and 1.384 nm,respectively.These 2D layers are further linked by partial π-π stacking interactions involving the triazol rings to generate a 3D honeycomb network structure in Fig.4d.
Although the complexes 1 and 2 were synthesized by the reaction of AgNO3with ttmb,the structures are actually influenced by the solvent.When the reactant AgNO3was only resolved in H2O,we obtained complex{[Ag(ttmb)(H2O)]NO3}n(1),in which the Ag髣adopts a distorted tetrahedron geometry with a N3O donor set and the coordinated O is from solvent water.When NH3·H2O was added to the aqueous solution of AgNO3,we obtained complex{[Ag(ttmb)]NO3·H2O}n(2),in which the Ag髣results in a planar triangle geometry with a N3donor set and the uncoordinated solvent water.So,it suggests that since the coordination ability of NH3is stronger than H2O,when NH3was added to AgNO3,NH3occupies the coordination site prior to H2O to form[Ag(NH3)2]+firstly,then the N atom of the added ligand ttmb coordinated to Ag髣replaces NH3.As a consequence,the solvent water is unable to coordinate to Ag髣,leading to more crystalline water in complex 2 than that in 1.
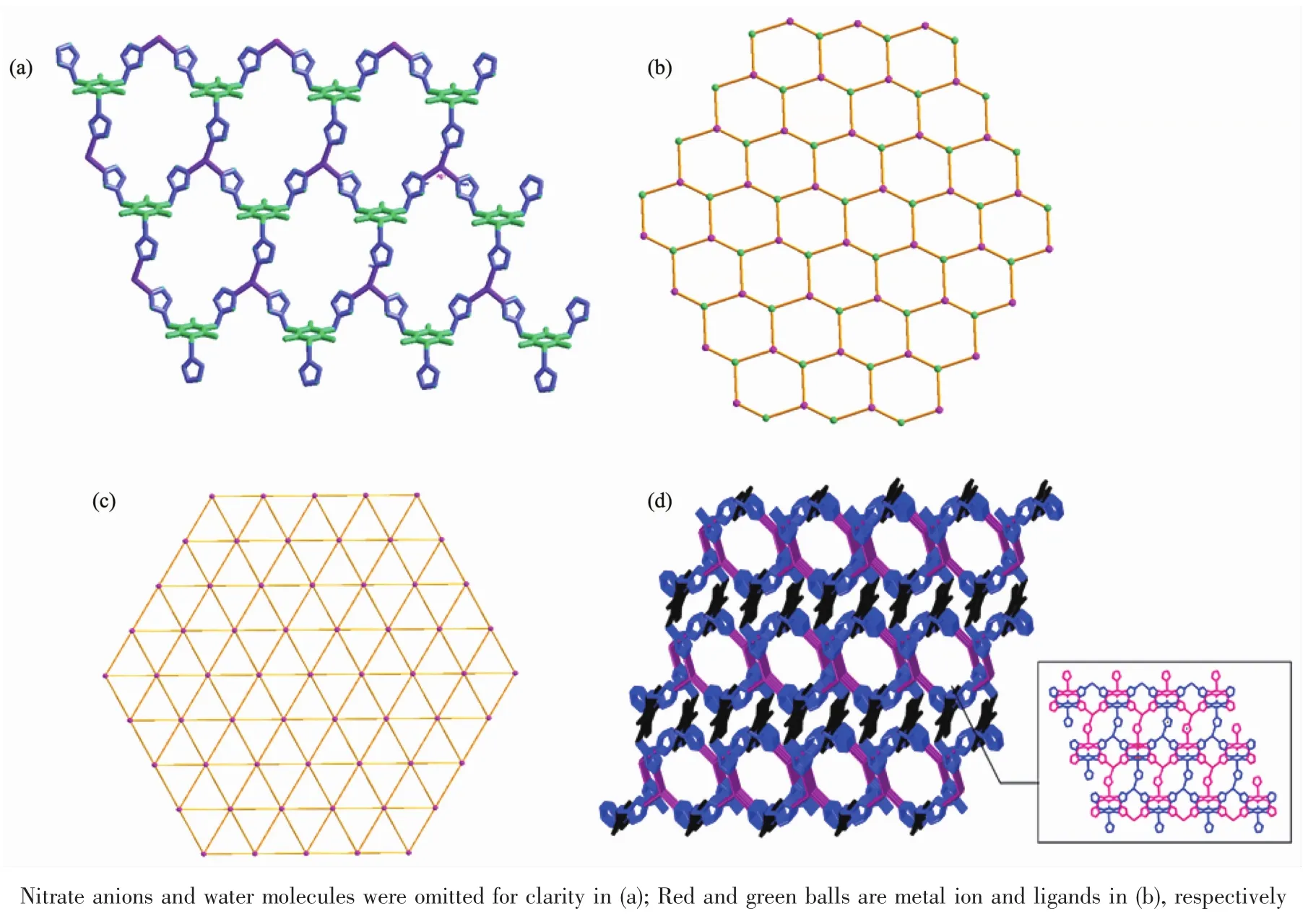
Fig.4 (a)View of the stepwise 2D layered network of 2 in which the;(b)Simplied structure of the 2D coordination polymer;(c)Two-dimensional sheet consisting of the three membered rings where only the silver髣atoms are presented;(d)3D honeycomb network structure by partial π-π stacking interactions involving the triazol rings
Overall,it can be concluded that the structure of the complexes are related to the reaction condition and that the solvent plays an important role.The structural difference of the two complexes may lead to the different properties associated with the complexes,as described below.
2.2 PXRD,IR,thermal analysis and fluorescent spectra
In order to confirm the phase purity of the bulk materials,PXRD was carried out for complexes 1 and 2.The diffraction peaks of simulated (Sim.)and experimental (Exp.)match well in peak positions,indicating that the phase purities of the complexes 1 and 2 are good (Fig.5).The difference in reflection intensities between Sim.and Exp.is due to the different orientation of the crystals in the powder samples.
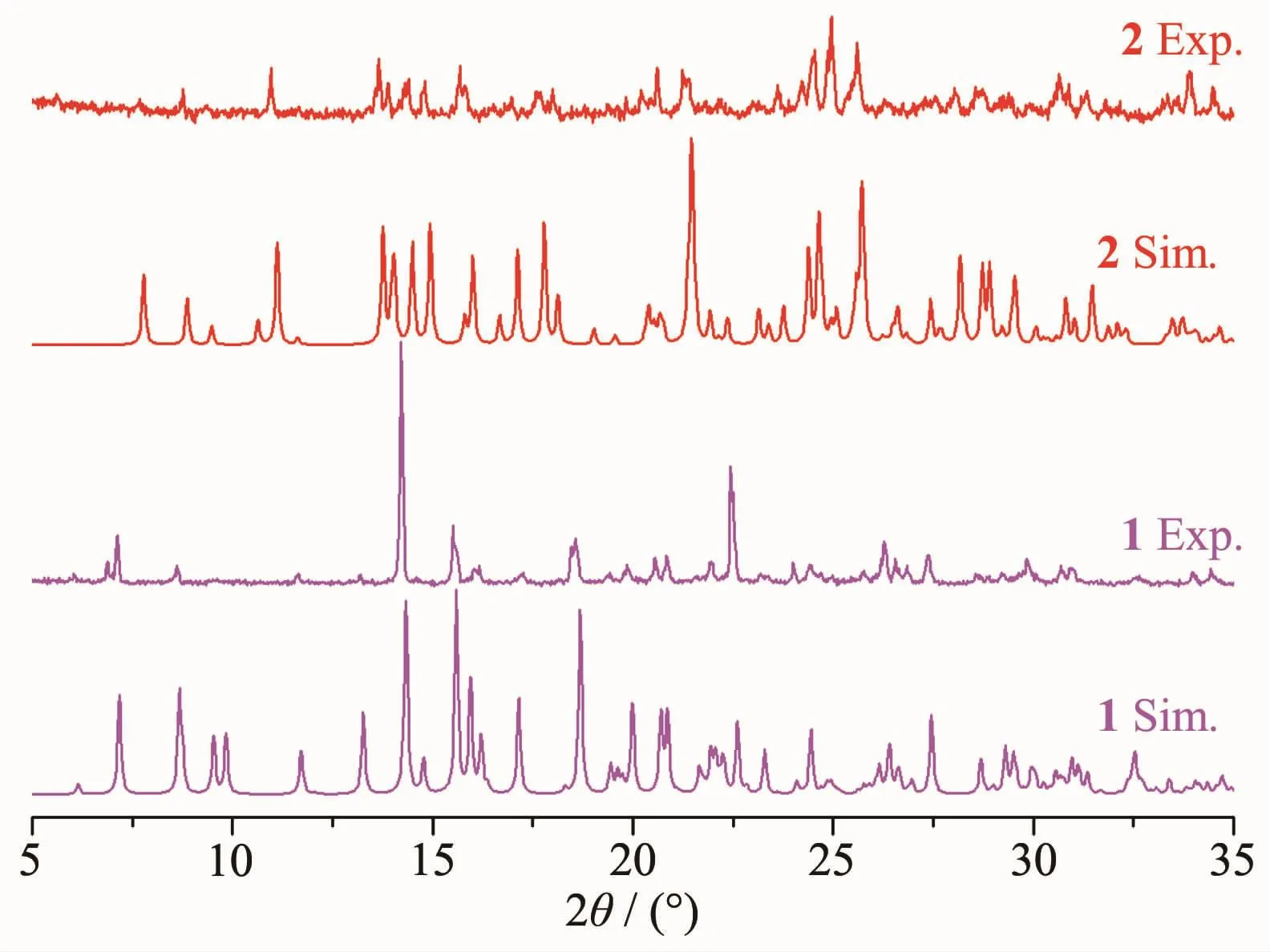
Fig.5 PXRD simulated and experimental patterns for complexes 1 and 2
The IR spectra of the free ligand ttmb and complexes 1 and 2 are shown in Fig.6.Compared with free ttmb,the strong absorption band of the triazole ring at 1 507 cm-1is shifted to 1 520 and 1 510 cm-1in complexes 1 and 2,respectively.The 1 275 cm-1absorption band in ttmb is red-shifted slightly (1 270 cm-1in 1 and 2,Fig.6).The absorption band for the free NO3-anion is both observed at 1 380 cm-1in complexes 1 and 2.The IR data of the complexes agree with their crystal structures.
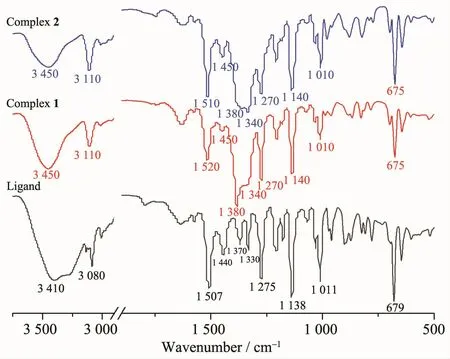
Fig.6 IR spectra of free ttmb ligand and complexes 1 and 2
The thermal stabilities of 1 and 2 were investigated by thermogravimetric analysis(Fig.7).The analysis shows that complex 1 is stable up to 149℃,and subsequently loses one coordinated water molecules(Found 3.38%,Calcd.3.26%)from 149 to 178℃.The complex begins to decompose at 371℃.Complex 2 loses one lattice water molecule(Found 3.53%,Calcd.3.26%)from 26 to 93℃.It suggests that complex 1 is more stable.This can be explained by the fact that the coordinated water and high compactness ofthe crystallattice can stabilize complex 1.
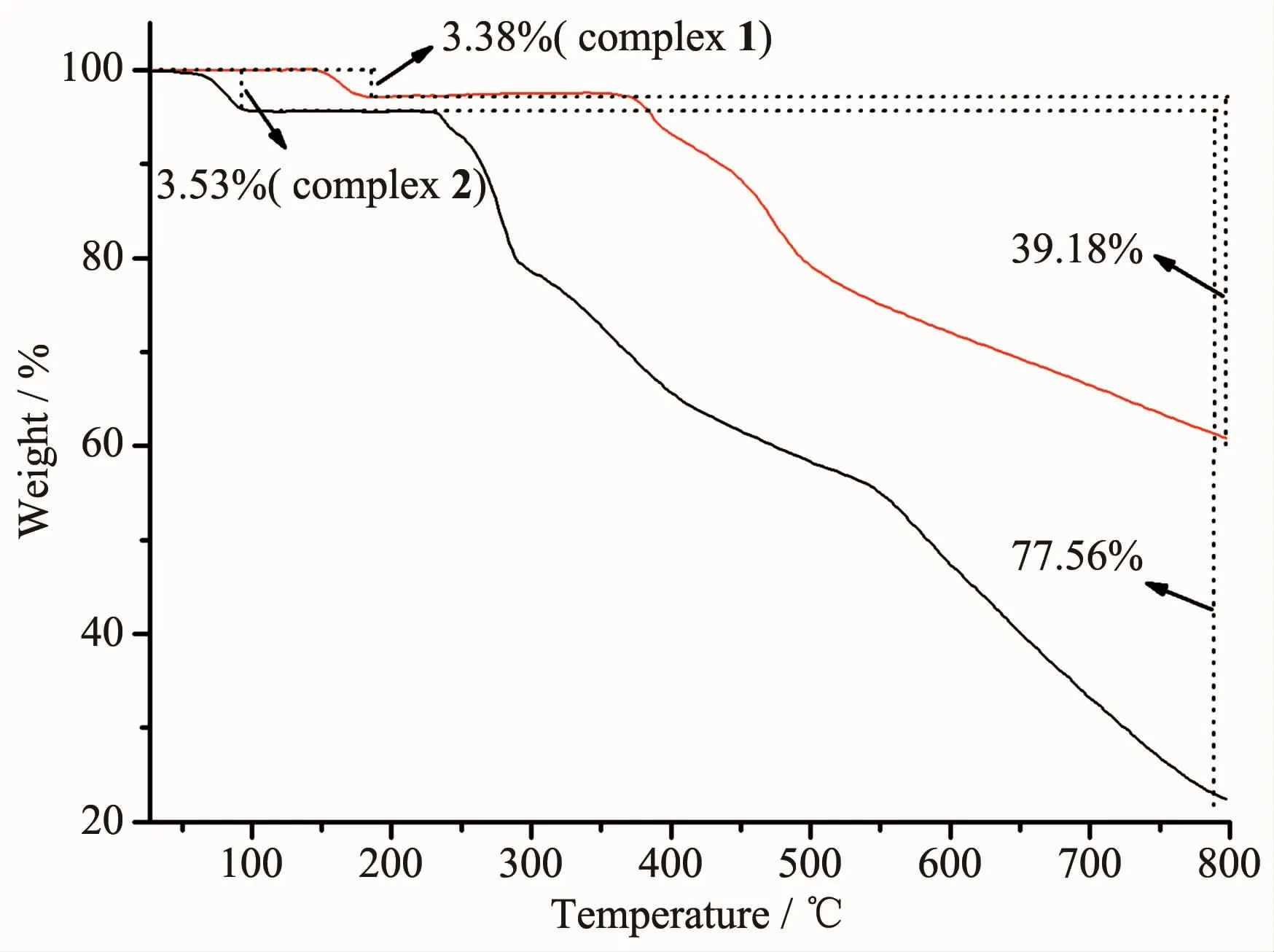
Fig.7 TG curves of complexes 1 and 2
Organic-inorganic coordination polymers,especially those with d10metal centers,have been investigated for their fluorescent properties and potential applications as fluorescent-emitting materials,such as light-emitting diodes[38].Therefore,the emission spectra of complexes 1 and 2 and the free ligand in solid state at room temperature were investigated.Excitation at 360 nm leads to broad fluorescence signals with emission maxima at 388 and 428 nm for free ttmb ligand.Complex 1 and 2 exhibited emission peaks at 386 and 432 nm,387 and 450 nm upon excitation at 360 nm,respectively(Fig.8).In contrast to that of the free ligand ttmb,the emission wavelengths of 1 and 2 are red-shifted by 4 and 22 nm,respectively.The spectrum shapes of 1 and 2 resemble the free ligand ttmb.Probably,the emission either complex 1 or complex 2 ismainly due to a ligand-centered emission-significant charge transfer character induced by the polar cation as reported for Hg髤or other d10metal complexes[39-40]with N-donor ligands based on the position and shape of the emission bands.The difference of red-shifted wavelength can be explained by the fact that the extent of π-π conjugation in 2 is more dramatic than that in 1.
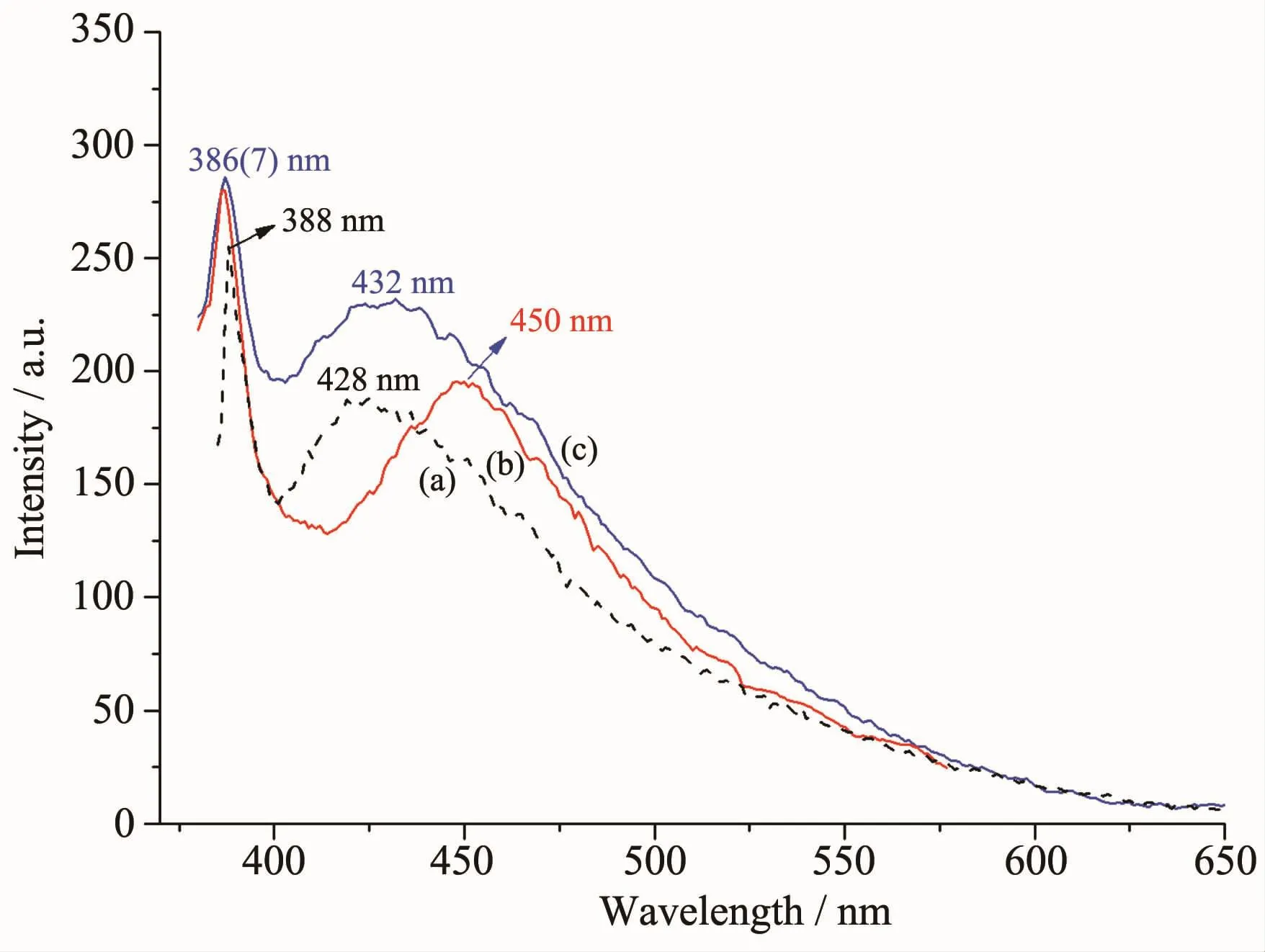
Fig.8 Fluorescence spectra of ligand(a),complex 1(c),complex 2(b)in solid state excited at 360 nm
3 Conclusions
The self-assembly reactions of ttmb with AgNO3yield two 2D network silver髣coordination polymers{[Ag(ttmb)(H2O)]NO3}n(1)in CH3OH-H2O and{[Ag(ttmb)]NO3·H2O}n(2)in CH3OH-NH3·H2O.Complex 1 contains a highly undulated 2D network with Ag髣having a distorted tetrahedron geometry with a N3O donor set and O is from the coordinated solvent H2O.While 2 has a 2D(6,3)network where Ag髣adopts a planar triangle geometry with a N3donor set,and more crystalline water are present than in complex 1.The structure and characterization of the two complexes are influenced by the reaction solvent.Both complexes 1 and 2 show ligand centered emissions but shifted to longer wavelength compared with free ligand.With less crystalline water molecules,the stability of framework of complex 1 is much higher than that of complex 2.In short,two new MOFs clearly demonstrate the subtle interplay of ligand geometry,metal coordination preferences,and solvent control in the formation of framework materials.The structure-function relationships of such components are expected to further guide the synthetic methodologies.
Acknowledgments:This work was financially supported by the National Natural ScienceFoundation (Grant No.81401470),the Education Project of Henan Province(Grants No.13B150216, 14B150023, 18A310020) and the Key Scientific Research Program of Xinxiang City (Grant No.ZG15017).
[1]Li Q,Xu P,Gao W,et al.Adv.Mater.,2014,26:1378-1386
[2]Chen L,Chen Q,Wu M,et al.Acc.Chem.Res.,2015,48:201-210
[3]LIANG Rui(梁 蕊),WANG Yu-Ting(王 玉 停 ),GUO Yong-Kang(郭永康),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(8):1465-1473
[4]Zhang M D,Zheng B H,Chen L,et al.Dalton Trans.,2016,45:3334-3339
[5]Park I H,Medishetty R,Kim J Y,et al.Angew.Chem.Int.Ed.,2014,53:5591-5595
[6]Huang X F,Ma J X,Liu W S.Inorg.Chem.,2014,53:5922-5930
[7]Zhang L,Yang W,Wu X Y,et al.Chemistry,2016,22:11283-11290
[8]Shen X,Yan B.J.Colloid Interface Sci.,2016,468:220-226
[9]Bai L,Wang P,Bose P,et al.ACS Appl.Mater.Interfaces,2015,7:5056-60
[10]Wang Y,Tan C,Sun Z,et al.Chemistry,2014,20:1341-1348
[11]Han M L,Duan Y P,Li D S,et al.Dalton Trans.,2014,43:15450-15456
[12]Weisser F,Hohloch S,Plebst S,et al.Chemistry,2014,20:781-793
[13]Szorcsik A,Matyuska F,Benyei A,et al.Dalton Trans.,2016,45:14998-15012
[14]Stewart C D,Pedraza M,Arman H,et al.J.Inorg.Biochem.,2015,149:25-38
[15]Brown J L,Jones M B,Gaunt A J,et al.Inorg.Chem.,2015,54:4064-75
[16]Haldon E,Delgado-Rebollo M,Prieto A,et al.Inorg.Chem.,2014,53:4192-4201
[17]Martinez-Alanis P R,Sanchez Eguia B N,Ugalde-Saldivar V M,et al.Chemistry,2013,19:6067-6079
[18]Arcis-Castillo Z,Munoz M C,Molnar G,et al.Chemistry,2013,19:6851-6861
[19]Li M X,Miao Z X,Shao M,et al.Inorg.Chem.,2008,47:4481-4489
[20]Zhang L,Kuang X,Wu X,et al.Dalton Trans.,2014,43:7146-7152
[21]Kim H M,Cho B R.J.Mater.Chem.,2009,19:7402-7409
[22]Chepelin O,Ujma J,Wu X,et al.J.Am.Chem.Soc.,2012,134:19334-19337
[23]Fu H R,Wang F,Zhang J.Dalton Trans.,2014,43:4668-4673
[24]Herm Z R,Swisher J A,Smit B,et al.J.Am.Chem.Soc.,2011,133:5664-5667
[25]Zhang M,Chen Y P,Bosch M,et al.Angew.Chem.Int.Ed.,2014,53:815-818
[26]Lin Z J,Lu J,Hong M,et al.Chem.Soc.Rev.,2014,43:5867-5895
[27]Wang Y T,Li S T,Wu S Q,et al.J.Am.Chem.Soc.,2013,135:5942-5945
[28]Ni T,Xing F,Shao M,et al.Cryst.Growth Des.,2011,11:2999-3012
[29]Ni T,Zhao Y,Shao M,et al.Z.Anorg.Allg.Chem.,2011,637:689-697
[30]Stephenson A,Argent S P,Riis-Johannessen T,et al.J.Am.Chem.Soc.,2011,133:858-870
[31]Blessing R H.Acta Crystallogr.Sect.A:Found.Crystallogr.,1995,A51:33-38
[32]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structure,University of G觟ttingen,Germany,1997.
[33]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structure,University of G觟ttingen,Germany,1997.
[34]Connell T U,Schieber C,Silvestri I P,et al.Inorg.Chem.,2014,53:6503-6511
[35]Guo X G,Yang W B,Wu X Y,et al.Dalton Trans.,2013,42:15106-15112
[36]Dutta S,Bucar D K,Elacqua E,et al.Chem.Commun.,2013,49:1064-1066
[37]Saha D,Maity T,Das S,et al.Dalton Trans.,2013,42:13912-13922
[38]Yao L,Zhang S,Wang R,et al.Angew.Chem.Int.Ed.,2014,53:2119-2123
[39]Luo Y H,Wang Y,Zhao J,et al.Spectrochim.Acta,Part A,2014,122:246-251
[40]CHENMan-Sheng(陈满生),HUANGXiu-Yu(黄秀玉),CHEN Xiao-Li(陈小利),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33(6):1090-1096
Influence of Solvents on the Formation of Silver髣Complexes with the Flexible 1,3,5-Tris(triazol-1-ylmethyl)-2,4,6-trimethylbenzene
NI Tian-Jun1YUAN Qiang-Tao1ZHANG Wei1YANG Zhi-Jun*,1YU Hai-Hong2CAI Hong-Rui2
(1School of Basic Medicine,Xinxiang Medical University,Xinxiang,Henan 453003,China)
(2College of Pharmacy,Xinxiang Medical University,Xinxiang,Henan 453003,China)
Two novel silver complexes{[Ag(ttmb)(H2O)]NO3}n(1)and{[Ag(ttmb)]NO3·H2O}n(2)were synthesized by the reaction of AgNO3with 1,3,5-tri(1,2,4-triazol-1-ylmethyl)-2,4,6-trimethylbenzene(ttmb)in different solvents.The solid-state fluorescent properties of these two complexes and related ligand ttmb were then investigated at room temperature.Structural characterizations by elemental analysis,powder and single-crystal X-ray diffraction analysis and infrared spectra shows that complex 1 contains a highly undulated 2D network while complex 2 carries a 2D(6,3)network.CCDC:878517,1;878518,2.
crystal structure;sliver complex;triazole ligand;coordination polymers
O614.122
A
1001-4861(2017)12-2177-09
10.11862/CJIC.2017.269
2017-04-17。收修改稿日期:2017-09-19。
国家自然科学基金(No.81401470)、河南省教育厅(No.13B150216,14B150023,18A310020)和新乡市重点科研计划(No.ZG15017)资助项目。
*通信联系人。 E-mail:zjyang@xxmu.edu.cn
