Characterization and Molecular Docking of Inclusion Complex of Quercetin with Modif i ed Cyclodextrins
2017-12-11LIYunZOUWeiSUNWeiCAIHongyanZHUZhenzhouCHENXuanLIFangDINGWenpingSHENWangyang
LI Yun, ZOU Wei, SUN Wei, CAI Hongyan, ZHU Zhenzhou, CHEN Xuan,LI Fang,, DING Wenping,, SHEN Wangyang,,*
(1. School of Food Science and Technology, Wuhan Polytechnic University, Wuhan 430023, China;2. Key Laboratory of the Deep Processing of Bulk Grain and Oil Authorized by Ministry of Education, Wuhan 430023, China)
Characterization and Molecular Docking of Inclusion Complex of Quercetin with Modif i ed Cyclodextrins
LI Yun1, ZOU Wei2, SUN Wei1, CAI Hongyan1, ZHU Zhenzhou1, CHEN Xuan1,LI Fang1,2, DING Wenping1,2, SHEN Wangyang1,2,*
(1. School of Food Science and Technology, Wuhan Polytechnic University, Wuhan 430023, China;2. Key Laboratory of the Deep Processing of Bulk Grain and Oil Authorized by Ministry of Education, Wuhan 430023, China)
Quercetin is of high interest in pharmaceutical industry due to its antioxidant activity and potential therapeutic effects on airway inflammation and cardiovascular diseases. However, the application of quercetin is limited due to its low water solubility and poor stability. Cyclodextrins (CDs) are macrocyclic molecules able to form inclusion complex with guest molecules, effectively enhancing their solubility, stability and bioavailability. In order to overcome the defect of quercetin, we prepared inclusion complexes of quercetin with three different cyclodextrins such as β-CD, G-β-CD and G2-β-CD. The phase solubility study showed that there was a linear relationship between the solubility of quercetin and the molality of CDs, and G-β-CD performed the best among three cyclodextrins in terms of increasing the solubility of quercetin.In order to confirm the formation of inclusion complex, some analytical techniques were applied, such as ultravioletvisible spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy, X-ray diffraction and thermogravimetric-differential scanning calorimetry. The results revealed that the inclusion complex was formed as expected and the thermal stability of the guest molecule was improved. Furthermore, molecular docking showed that the ring C of quercetin was inserted into the hydrophobic cavity of G-β-CD during the inclusion process.
quercetin; modif i ed cyclodextrins; inclusion complex; characterisation; molecular docking
Quercetin, one of best-known flavonoids, is a class of polyphenolic compounds generally distributing in fruits,vegetables, red wine and black tea[1-2]. Many flavonoids present antioxidant activity and several bioactivities such as anti-inf l ammatory, anti-cancer, immunopotentiating,antithrombotic, antiallergic, hepatoprotective, vasodilatory,anti-ischemic and anti-diabetic[3-9]. In recent years, quercetin has become of high interest for pharmaceuticals owing to its antioxidant and anti-inf l ammatory activity[10-11]. It has been reported that quercetin exerts therapeutic effects on airway inflammation and cardiovascular diseases[12-16]. In addition, quercetin is benef i cial on preventing mitochondrial dysfunctions, neurodegenerative diseases, fluoride-induced hepatotoxicity and cholestatic liver injury[17-20]. However,the application of quercetin in pharmaceutical fi eld has been limited due to its low water-solubility and poor stability.
Cyclodextrins (CDs), produced by the enzymatic degradation of starch, are a family of macrocyclic compounds constituted by sugar molecules[21-22]. Because CDs have a hydrophobic internal cavity and a hydrophilic external surface, they are able to increase water-solubility and stability of drugs via forming complexes with guest molecules. CDs have been applied widely in pharmaceutical, biotechnology,agro and food industry[23-24]. Native CDs mainly include α-,β- and γ-CD, of which β-CD is the most frequently used host molecule to prepare inclusion complex[25]. However, the low water-solubility (1.5 g/mL, 25 ℃) and toxicity to cell have hindered β-CD from being widely used to form inclusion complex[25-26]. Thereby several derivatives of β-CD which exhibit higher water-solubility and lower toxicity have been produced, such as hydroxypropyl-β-CD, methyl-β-CD,6-O-α-D-maltosyl-β-CD, glucosyl-β-CD and so on.
In this paper, the inclusion efficiency of different β-CD derivatives with quercetin has been explored. The physicochemical properties of samples were investigated by ultraviolet (UV) spectroscopy, Fourier transform infrared(FT-IR) spectroscopy, scanning electron microscopy (SEM), X-ray diffraction (XRD) and thermogravimetric-diffraction scanning calorimetry (TG-DSC). Furthermore, molecular mocking was used to clarify the supramolecular structure of the complex.
1 Materials and Methods
1.1 Materials and reagents
Quercetin (purity≥98%); β-CD, 6-O-α-D-maltosylβ-CD Sigma Chemicals Co. (USA); glucosyl-β-CD Seebio Biotech Inc. (Shanghai, China); all other chemicals and solvents belong to analytical grade.
1.2 Instruments and equipments
Multifuge X1R high-speed refrigerated centrifuge Thermo Scientific (USA); 1260 high performance liquid chromatography Agilent (USA); TU-1810PC spectrophotometer Beijing Purkinje General Instrument Co. Ltd. (China); NEXUS670 infrared spectrophotometer Thermo Nicolet Corporation (USA); S-300N SEM Hitachi Ltd. (Japan); 7000 XRD Shimadzu Corporation(Japan); STA449F3 TG/DSC system Netstal Instrument Co.Ltd. (Germany).
1.3 Methods
1.3.1 Phase-solubility measurements
Phase-solubility measurements were performed based on the method of Higuchi et al.[27]. Prior to shaking at 30 ℃,excess amounts of quercetin were added to 5 mL of aqueous solution of β-CD, G-β-CD and G2-β-CD, whose concentrations were 0, 2, 4, 6, 8, 10 mmol/L. The suspensions were filtered through 0.45 μm membrane filters to remove undissolved quercetin after shaking for 72 h, and then measured with high performance liquid chromatograph. The apparent stability constants (K) of the inclusion complexes were calculated from phase-solubility diagrams according to the following equation:
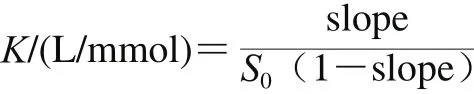
where S0was the solubility of quercetin at 30 ℃ in the absence of cyclodextrins/(mmol/L); slope was the corresponding slope of the phase solubility diagrams.
1.3.2 Preparation of the inclusion complex of quercetin and G-β-CD
Quercetin (0.151 1 g, 0.5 mmol/L) and G-β-CD(3.242 9 g, 2.5 mmol/L) were mixed in 40 mL of 30% methyl alcohol solution. The solution obtained was stirred at 30 ℃for 72 h and then centrifuged at 30 ℃ for 15 min with a rate of 3 000 r/min to obtain the supernatant. The supernatant was freeze-dried and collected.
1.3.3 Preparation of the physical mixture of quercetin and G-β-CD
Quercetin (0.151 1 g, 0.5 mmol/L) and G-β-CD (3.242 9 g,2.5 mmol/L) were mixed and stirred in a mortar until quercetin and G-β-CD was homogenized. The mixture produced was the physical mixture of quercetin and G-β-CD.
1.3.4 UV analysis
The UV spectra of quercetin, G-β-CD, their physical mixture and inclusion complex were collected at a range of 220-400 nm. Prior to measurement, each sample was dissolved respectively in methyl alcohol with a proper concentration.
1.3.5 FT-IR analysis
The FT-IR studies of quercetin, G-β-CD, their physical mixture and inclusion complex were conducted at a range between 4 000-400 cm-1. Each sample was measured with a blank potassium bromide (KBr) disk as control.
1.3.6 SEM analysis
The surface morphologies of quercetin, G-β-CD, their physical mixture and inclusion complex were observed using SEM. Samples evenly dispersed into silicon wafer attached on aluminium circular disks vis double adhesive tapes. In addition, prior to testing, each sample was gold-plated to improve electrical conductivity.
1.3.7 XRD analysis
The XRD patterns of quercetin, G-β-CD, their physical mixture and inclusion complex were obtained from XRD. The samples were measured at the angle 2θ between 10° and 60°.
1.3.8 TG-DSC analysis
The TG-DSC analysis of quercetin, G-β-CD and their complex were conducted on TG-DSC system as the following conditions: keeping dynamic atmosphere of nitrogen at 20 mL/min of nitrogen and heating from 40 to 500 ℃ at a rate of 10 ℃/min.
1.3.9 Molecular docking
The 3D structure of quercetin, which was optimized using the method of molecular mechanics (MM+) and semiempirical quantum chemistry (AM1), was constructed in Hyperchem 8.0 software. The 3D structure of β-CD was obtained from crystallographic databases. The 3D structure of G-β-CD was formed by bringing in a glucosyl group in the narrow rim of β-CD[28]. The molecule docking between G-β-CD and quercetin was conducted via Lamarckian genetic algorithm (LGA) in Auto Dock 4.0 with autogrid box 60 Å ×60 Å × 60 Å and grid spacing 0.375 Å.
2 Results and Analyses
2.1 Phase-solubility analysis

Fig. 1 Phase solubility diagrams of inclusion complexes formed between quercetin and CDs
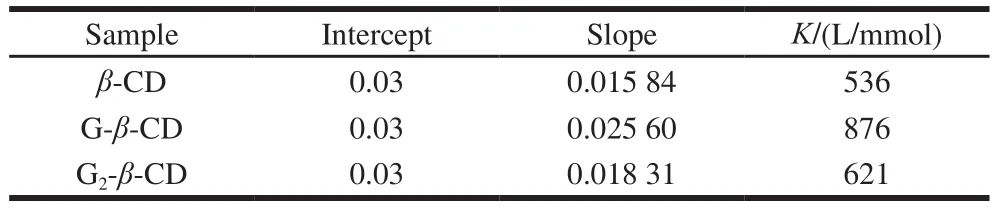
Table 1 Apparent stability constants of the inclusion complexes between quercetin and cyclodextrins
The phase-solubility studies have been widely used to explain the complexation ability of cyclodextrins to guest molecules. The phase-solubility diagrams of quercetin with β-CD, G-β-CD and G2-β-CD all exhibited a typical AL type diagram (Fig. 1), indicating that the solubility of quercetin increased with increasing concentration of CDs and the complexes were formed with a 1:1 stoichiometry[27]. The apparent stability constants of complexes (K) shown in Table 1, were calculated through the slopes of phasesolubility diagrams. The result showed that the stability constant of G-β-CD (876 L/mmol) was higher compared to β-CD (536 L/mmol) and G2-β-CD (621 L/mmol). Therefore,G-β-CD was selected to produce inclusion complex with quercetin for the following studies.
2.2 UV analysis
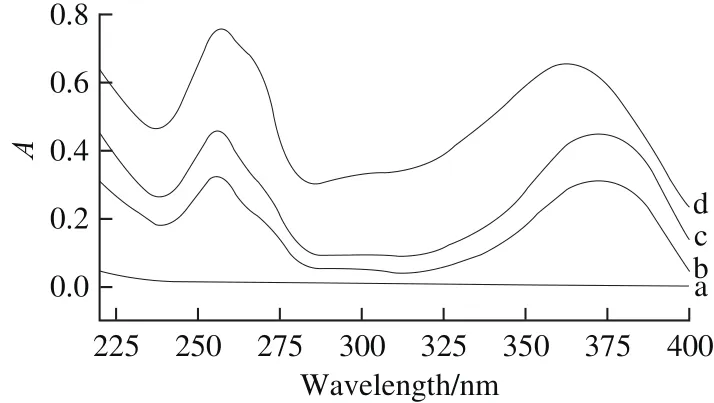
Fig. 2 UV absorption spectra of G-β-CD (a), quercetin (b), their physical mixture (c) and inclusion complex (d)
UV spectroscopy is a useful tool to confirm the formation of complex. The obtained UV spectra of quercetin,G-β-CD, their physical mixture and inclusion complex were showed in Fig. 2. Resulting from the absence of unsaturated bonds, there was no characteristic absorption peak in the UV spectrum of G-β-CD. Quercetin and physical mixture both displayed two characteristic absorption peaks at 256 and 373 nm. Interestingly, the second absorption peak of complex had a blue shift from 373 to 363 nm. It might be caused by the hindrance of G-β-CD to insertion of quercetin into its cavity, which resulted in a change of conjugative structure of quercetin, indicating the formation of complex[29].
2.3 FT-IR analysis
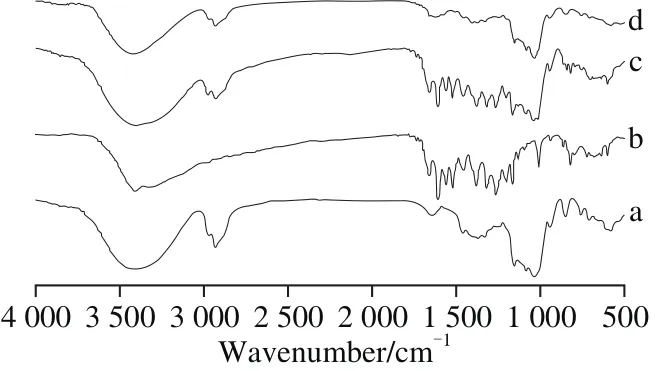
Fig. 3 FT-IR spectra of G-β-CD (a), quercetin (b), their physical mixture (c) and inclusion complex (d)
FT-IR spectroscopy is an insightful technique analyzing the function groups related to the interaction between the host and guest[30]. FT-IR spectrum of quercetin, G-β-CD, their physical mixture and inclusion complex were shown in Fig. 3.The FT-IR spectra of quercetin showed several prominent absorption bands, such as hydroxyl group (3 409 cm-1),carbonyl group (1 662 cm-1) and C—H stretching vibration(1 262 cm-1). In the spectrogram of G-β-CD, prominent absorption bands consisted of O—H stretching vibration(3 399 cm-1), C—H stretching vibration (2 928 cm-1)and C—H and C—O stretching vibration (1 155, 1 084,1 033 cm-1). The FT-IR spectra of physical mixture, a combination of the spectrogram of quercetin and G-β-CD, had no difference from single compound. But the spectrogram of complex was similar to that of G-β-CD with the characteristic peaks of quercetin at 500-1 500 cm-1disappearing, suggesting the complexation of G-β-CD to quercetin.
2.4 SEM analysis
SEM is a visualization method to study the surface morphology of materials. The morphologies of quercetin,G-β-CD, their physical mixture and inclusion complex were illustrated in Fig. 4. The spherical shape of G-β-CD and needle-like crystals of quercetin were displayed. The morphologies of both molecules were present in the image of physical mixture without significant differences to their morphologies before they were mixed. However, there was an obvious change in the morphology of inclusion complex,which exhibited irregular amorphous pieces with disappearing morphologies of both raw materials, indicating the formation of complex[31].
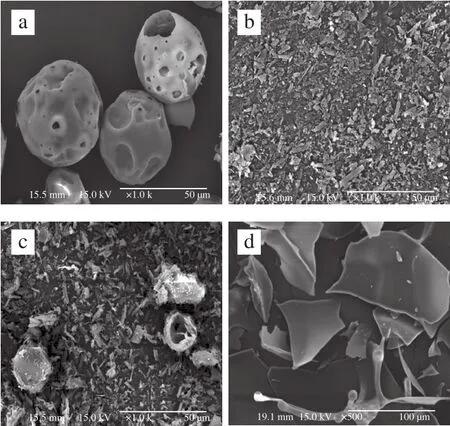
Fig. 4 SEM of G-β-CD (a), quercetin (b), their physical mixture (c) and inclusion complex (d)
2.5 XRD analysis

Fig. 5 XRD patterns of G-β-CD (a), quercetin (b), their physical mixture (c) and inclusion complex (d)
XRD is a useful technique for analyzing the degree of crystallinity of samples. Normally, the crystallinity of guest molecule is decreased after forming inclusion complex with host molecule[32]. As shown in Fig. 5, the XRD patterns of quercetin crystals showed characteristic crystalline peaks in range of 25°-30° which are absent in that of G-β-CD.As a comparison, the X-ray patterns of physical mixture nearly overlapped the patterns of quercetin and G-β-CD,whereas in the X-ray patterns of inclusion complex, the characteristic crystalline peaks of quercetin disappeared,indicating decreased crystallinity of quercetin. This demonstrated that the inclusion complex of quercetin with G-β-CD was formed[33].
2.6 TG-DSC analysis
TG-DSC is a worthwhile method providing both qualitative and quantitative information on the thermal properties of given samples[34]. As shown in Fig. 6, G-β-CD exhibited a shallow endothermic peak starting from 88 ℃with a weight loss of 5%, indicating the evaporation of water. A broad endothermic peak beginning at about 315 ℃was also observed, which led to the degradration of G-β-CD.Quercetin expressed two shape endothermic peak: one beginning with the temperature of about 98 ℃, conf i rmed to the loss of water; the other starting at about 310 ℃ adjacent to the melting point of quercetin (314 ℃), was related to the degradration of quercetin. Yet ahead of 310 ℃, quercetin also presented other mass losses. Hence, quercetin had decomposed partly prior to fusion. As for inclusion complex,the characteristic endothermic peaks of quercetin disappeared and the decomposing onset temperature is shifted to 315 ℃,which is a powerful proof that the inclusion complex was formed and the thermal stability of quercetin was improved.

Fig. 6 TG-DSC curves of G-β-CD (a), quercetin (b) and their inclusion complex (c)
2.7 Molecular docking analysis

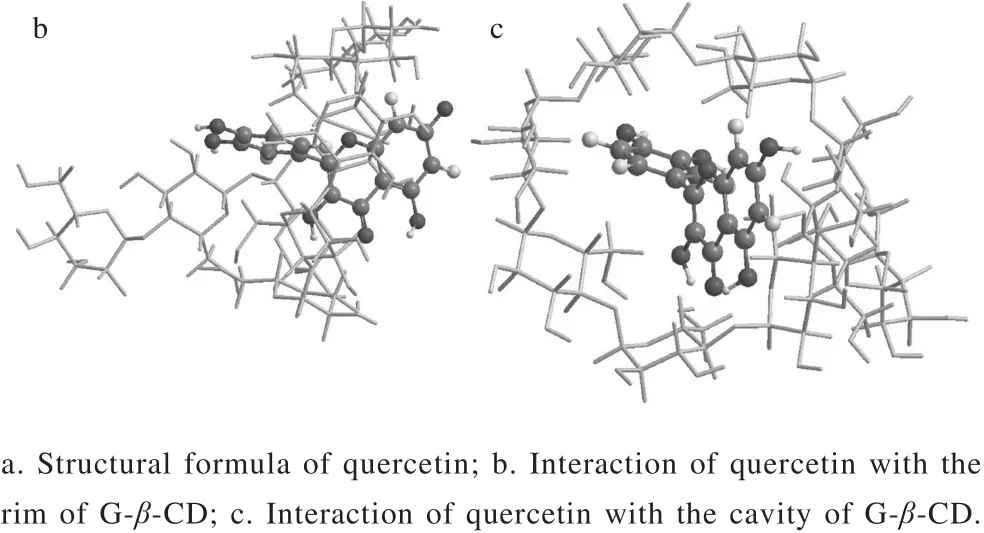
Fig. 7 Schematic representation of inclusion complexation of G-β-CD with quercetin
Molecular docking is a vivid tool to investigate the complexation of the host molecule with guest molecule[35].The structural formula of quercetin was showed in Fig. 7a.The most suitable 3D structure mode (Fig. 7b and c) of the inclusion complex of G-β-CD with quercetin was obtained via molecular simulation. G-β-CD and quercetin were regarded as receptor and ligand respectively. The results showed that the ring C of quercetin was inserted into the cavity of G-β-CD, while the ring A and B were orientated separately toward the narrow and wide rim of G-β-CD. In addition, a plurality of hydrogen bonds had been formed to maintain the supramolecular structure.
3 Conclusions
In this study, we generated inclusion complexs with 1:1 stoichiometry between quercetin and three cyclodextrins(β-CD, G-β-CD, G2-β-CD). Among selected cyclodextrins,our results showed G-β-CD performed the best in terms of increasing solubility of quercetin. UV, FT-IR, SEM and XRD analysis confirmed inclusion complex of G-β-CD and quercetin was formed. Furthermore, TG-DSC results suggested that the thermal stability of quercetin was improved. At last, molecular docking results displayed that the supramolecular complex was most likely formed with the ring C of quercetin inserted into the cavity of G-β-CD.This work successfully improved the solubility, stability and bioavailability of quercetin, broadening its potential application in pharmaceutical industry.
[1] TOUMI M L, MERZOUG S, BAUDIN B, et al. Quercetin alleviates predator stress-induced anxiety-like and brain oxidative signs in pregnant rats and immune count disturbance in their offspring[J].Pharmacology, Biochemistry and Behavior, 2013, 107: 1-10.DOI:10.1016/j.pbb.2013.03.009.
[2] DE PAZ E, MARTÍNB A, EVERYA H, et al. Production of watersoluble quercetin formulations by antisolvent precipitation and supercritical drying[J]. The Journal of Supercritical Fluids, 2015, 104:281-290. DOI:10.1016/j.supf l u.2015.07.006.
[3] HEGDE A H, PRASHANTH S N, SEETHARAMAPPA J.Interaction of antioxidant fl avonoids with calf thymus DNA analyzed by spectroscopic and electrochemical methods[J]. Journal of Pharmaceutical and Biomedical Analysis, 2012, 63: 40-46.DOI:10.1016/j.jpba.2012.01.034.
[4] PROCHÁZKOVÁ D, BOUŠOVÁ I, WILHELMOVÁ N. Antioxidant and prooxidant properties of flavonoids[J]. Fitoterapia, 2011, 82(4):513-523. DOI:10.1016/j.f i tote.2011.01.018.
[5] GARCIA-LAFUENTE A, GUILLAMÓN E, VILLARES A, et al.Flavonoids as anti-inf l ammatory agents: implications in cancer and cardiovascular disease[J]. Inf l ammation Research, 2009, 58(9): 537-552. DOI:10.1007/s00011-009-0037-3.
[6] DMITRIENKO S G, APYARI V V, KUDRINSKAYA V A, et al.Preconcentration of fl avonoids on polyurethane foam and their direct determination by diffuse reflectance spectroscopy[J]. Talanta, 2012,102: 132-136. DOI:10.1016/j. Talanta. 2012.08.017.
[7] DOS SANTOS M C D, GONÇALVES C F L, VAISMAN M, et al.Impact of flavonoids on thyroid function[J]. Food and Chemical Toxicology, 2011, 49(10): 2495-2502. DOI:10.1016/j.fct.2011.06.074.
[8] CALDERONE V, CHERICONI S, MARTINELLI C, et al.Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels[J]. Naunyn-Schmiedebergs Archives of Pharmacology, 2004, 370(4): 290-298. DOI:10.1007/s00210-004-0964-z.
[9] BABU P V A, LIU D M, GILBERT E R. Recent advances in understanding the anti-diabetic actions of dietary fl avonoids[J]. Journal of Nutritional Biochemistry, 2013, 24(11): 1777-1789. DOI:10.1016/j.jnutbio.2013.06.003.
[10] ROEDIG-PENMAN A, GORDON M H. Antioxidant properties of myricetin and quercetin in oil and emulsions[J]. Journal of the American Oil Chemists’Society, 1998, 75(2): 169-180. DOI:10.1007/s11746-998-0029-4.
[11] ROGERIO A P, KANASHIRO A, FONTANARI C, et al. Antiinf l ammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma[J]. Inf l ammation Research, 2007, 56(10): 402-408. DOI:10.1007/s00011-007-7005-6.
[12] YANG T, LUO F, SHEN Y C, et al. Quercetin attenuates airway inf l ammation and mucus production induced by cigarette smoke in rats[J]. International Immunopharmacology, 2012, 13(1): 73-81.DOI:10.1016/j.intimp.2012.03.006.
[13] LI N, LI Q, ZHOU X D, et al. The effect of quercetin on human neutrophil elastase-induced mucin5AC expression in human air way epithelial cells[J]. International Immunopharmacology, 2012, 14(2):195-201. DOI:10.1016/j.intimp.2012.07.008.
[14] ZAAFAN M A, ZAKI H F, EL-BRAIRY A I, et al. Protective effects of atorvastatin and quercetin on isoprenaline-induced myocardial infarction in rats[J]. Bulletin of Faculty of Pharmacy, Cairo University,2012, 51(1): 35-41. DOI:10.1016/j.bfopcu.2013.03.001.
[15] SHEN Y, WARD N C, HODGSON J M, et al. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: a critical role for heme oxygenase-1[J]. Free Radical Biology and Medicine, 2013, 65:908-915. DOI:10.1016/j.freeradbiomed.2013.08.185.
[16] LIU H, GUO X L, CHU Y, et al. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats[J]. Gene, 2014, 545(1): 149-155.DOI:10.1016/j.gene.2014.04.043.
[17] SANDHIR R, MEHROTRA A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington’s disease[J].Biochimica et Biophysica Acta, 2013, 1832(3): 421-430.DOI:10.1016/j.bbadis.2012.11.018.
[18] CHOI G N, KIM J H, KWAK J H, et al. Effect of quercetin on learning and memory performance in ICR mice under neurotoxic trimethyltin exposure[J]. Food Chemistry, 2012, 132(2): 1019-1024. DOI:10.1016/j.foodchem.2011.11.089.
[19] NABAVI S M, NABAVI S F, ESLAMI S, et al. In vivo protective effects of quercetin against sodium fl uoride-induced oxidative stress in the hepatic tissue[J]. Food Chemistry, 2012, 132(2): 931-935.DOI:10.1016/j.Foodchem.2011.11.070.
[20] LIN S Y, WANG Y Y, CHEN W Y, et al. Benef i cial effect of quercetin on cholestatic liver injury[J]. Journal of Nutritional Biochemistry,2014, 25(11): 1183-1195. DOI:10.1016/j.jnutbio.2014.06.003.
[21] GRANDELLI H E, HASSLER J C, WHITTINGTON A, et al.Melting point depression of Piroxicam in carbon dioxide + co-solvent mixtures and inclusion complex formation with β-cyclodextrin[J].The Journal of Supercritical Fluids, 2012, 71: 19-25. DOI:10.1016/j.supf l u.2012.07.001.
[22] FLOARE C G, PIRNAU A, BOGDAN M.1H NMR spectroscopic characterization of inclusion complexes of tolfenamic and fl ufenamic acids with β-cyclodextrin[J]. Journal of Molecular Structure, 2013,1044: 72-78. DOI:10.1016/j.molstruc.2012.11.021.
[23] SRINIVASAN K, STALIN T, SHANMUGAPRIYA A, et al.Spectroscopic and electrochemical studies on the interaction of an inclusion complex of β-cyclodextrin with 2,6-dinitrophenol in aqueous and solid phases[J]. Journal of Molecular Structure, 2013, 1036: 494-504. DOI:10.1016/j. molstruc.2012.10.018.
[24] HO B T, JOYCE D C, BHANDARI B R. Encapsulation of ethylene gas into α-cyclodextrin and characterisation of the inclusion complexes[J]. Food Chemistry, 2011, 127(2): 572-580. DOI:10.1016/j.foodchem.2011.01.043.
[25] BENGHODBANE S, KHATMI D. A theoretical study on the inclusion complexation of doxycycline with Crysmeb[J]. Comptes Rendus Chimie, 2012, 15(5): 371-377. DOI:10.1016/j.crci.2011.11.007.
[26] BELLRINGER M E, SMITH T G, READ R, et al. β-Cyclodextrin:52-week toxicity studies in the rat and dog[J]. Food and Chemical Toxicology, 1995, 33(5): 367-376. DOI:10.1016/0278-6915(94)00149-I.
[27] HIGUCHI T, CONNORS K A. Phase solubility techniques[J]. Advances in Analytical Chemistry and Instrumentation, 1965, 4: 117-212.
[28] EID E E M, ABDUL A B, SULIMAN F E O, et al. Characterization of the inclusion complex of zerumbone with hydroxypropylβ-cyclodextrin[J]. Food Chemistry, 2011, 83(4): 1707-1714.DOI:10.1016/j. carbpol.2010.10.033.
[29] ZHAO M M, WANG H Y, YANG B, et al. Identif i cation of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity[J]. Food Chemistry, 2010, 120(4): 1138-1142.DOI:10.1016/j.foodchem. 2009.11.044.
[30] ZHANG J Q, LI K, CONG Y W, et al. Preparation, characterisation and bioactivity evaluation of the inclusion complex formed between picoplatin and γ-cyclodextrin[J]. Carbohydrate Research, 2014, 396:54-61. DOI:10.1016/j.carres.2014.07.015.
[31] RAJAMOHAN R, KOTHAINAYAKI S, SWAMINATAN M.Spectrofluorimetric study on inclusion complexation of 2-amino-6-fluorobenzothiazole with β-Cyclodextrin[J]. Collection of Czechoslovak Chemical Communications, 2008, 73(2): 147-160.DOI:10.1135/cccc 20080147.
[32] PRABU S, SWAMINATHAN M, SIVAKUMAR K, et al. Preparation,characterization and molecular modeling studies of the inclusion complex of caffeine with beta-cyclodextrin[J]. Journal of Molecular Structure, 2015, 1099: 616-624. DOI:10.1016/j.molstruc.2015.07.018.
[33] WANG J, CAO Y P, SUN B G, et al. Characterisation of inclusion complex of trans-ferulic acid and hydroxypropyl-β-cyclodextrin[J].Food Chemistry, 2011, 124(3): 1069-1075. DOI:10.1016/j.foodchem.2010.07.080.
[34] PERIASAMY R, KOTHAINAYAKI S, SIVAKUMAR K.Preparation, physicochemical analysis and molecular modeling investigation of 2,2’-bipyridine: β-cyclodextrin inclusion complex in solution and solid state[J]. Journal of Molecular Structure, 2015, 1100:59-69. DOI:10.1016/j.molstruc.2015.07.026.
[35] MISIUK W, JOZEFOWICZ M. Study on a host-guest interaction of hydroxypropyl-β-cyclodextrin with of l oxacin[J]. Journal of Molecular Liquids, 2015, 202: 101-106. DOI:10.1016/j.molliq.2014.12.029.
槲皮素与环糊精衍生物包合物的理化性质和分子对接研究
李 赟1,邹 伟2,孙 威1,蔡红燕1,祝振洲1,陈 轩1,李 芳1,2,丁文平1,2,沈汪洋1,2,*
(1.武汉轻工大学食品科学与工程学院,湖北 武汉 430023;2.大宗粮油精深加工省部共建教育部重点实验室,湖北 武汉 430023)
槲皮素具有较强的抗氧化活性,对呼吸道炎症和心血管疾病具有一定疗效,在医药行业中受到广泛关注。但是,槲皮素的水溶性和热稳定性较低,使其在医药行业的应用受到限制。环糊精(cyclodextrins,CDs)是一种大环分子,能够与客体分子包合形成包合物,从而有效提高客体分子的溶解性、稳定性和生物利用率。本实验制备槲皮素与β-CD、G-β-CD及G2-β-CD的包合物,利用相溶解度法研究环糊精与槲皮素的包合效果,利用紫外光谱、傅里叶变换红外光谱、扫描电子显微镜、X射线衍射、热重及差示扫描量热技术表征槲皮素与G-β-CD包合物,借助分子对接研究槲皮素与G-β-CD包合物的超分子结构。结果显示:槲皮素的溶解度与环糊精的浓度呈线性关系,G-β-CD与槲皮素的包合效果最好,且包合后槲皮素的热稳定性提高。分子对接结果表明槲皮素的C环嵌入G-β-CD的空腔中形成包合物。
槲皮素;环糊精衍生物;包合物;理化性质;分子对接
TS201.2
A
1002-6630(2017)23-0045-06
2016-09-18
国家自然科学基金青年科学基金项目(31301415)
李赟(1990—),女,硕士研究生,研究方向为食品生物化学。E-mail:shuang7061234@163.com
10.7506/spkx1002-6630-201723008
LI Yun, ZOU Wei, SUN Wei, et al. Characterization and molecular docking of inclusion complex of quercetin with modif i ed cyclodextrins[J]. 食品科学, 2017, 38(23): 45-50.
10.7506/spkx1002-6630-201723008. http://www.spkx.net.cn
*通信作者:沈汪洋(1978—),男,副教授,博士,研究方向为食品生物化学。E-mail:whwangyangshen@126.com
LI Yun, ZOU Wei, SUN Wei, et al. Characterization and molecular docking of inclusion complex of quercetin with modif i ed cyclodextrins[J]. Food Science, 2017, 38(23): 45-50. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201723008. http://www.spkx.net.cn
