白藜芦醇对糖尿病大鼠视网膜病变的影响
2017-11-14刘俊辉李春江李玉涛
刘俊辉,李春江,李玉涛,3*
(1.唐山爱尔眼科医院眼科,河北 唐山 063000;2.冀东眼科医院眼科,河北 唐山 063000;3.华北理工大学附属医院眼科,河北 唐山 063000)
白藜芦醇对糖尿病大鼠视网膜病变的影响
刘俊辉1,2,李春江2,李玉涛2,3*
(1.唐山爱尔眼科医院眼科,河北 唐山 063000;2.冀东眼科医院眼科,河北 唐山 063000;3.华北理工大学附属医院眼科,河北 唐山 063000)
目的观察白藜芦醇(Resveratrol,RES)对糖尿病大鼠视网膜病变的影响并探讨相关机制。方法清洁级健康雄性SD大鼠45只随机分为对照组、模型组和RES组各15只。应用链脲佐菌素腹腔注射制作糖尿病大鼠模型。造模后1周,RES组大鼠给予白藜芦醇40 mg/kg(10 mL/kg)腹腔注射治疗,1次/d,连续治疗6周。治疗结束后,测量各组大鼠血糖、体质量,采用酶联免疫吸附测定法检测大鼠视网膜中肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)和白细胞介素1β(interleukin-1β,IL-1β)含量,检测大鼠血清中血管内皮生长因子(vascular endothelial growth factor,VEGF)、缺氧诱导因子1α(hypoxia inducible factor-1α,HIF-1α)、 丙二醛(methane dicarboxylic aldehyde,MDA)和超氧化物歧化酶(superoxide dismutase,SOD)含量。结果模型组和RES组血糖高于对照组,体质量低于对照组(P<0.05);模型组与RES组血糖、体质量差异均无统计学意义(P>0.05)。模型组和RES组大鼠TNF-α、IL-1β、VEGF、HIF-1α和MDA表达明显高于对照组,RES组大鼠TNF-α、IL-1β、VEGF、HIF-1α和MDA表达低于模型组,差异均有统计学意义(P<0.05)。模型组和RES组大鼠SOD表达明显低于对照组,RES组大鼠SOD表达高于模型组,差异均有统计学意义(P<0.05)。结论RES可抑制炎症反应和HIF-1α/VEGF通路,减轻氧化应激损伤,从而改善糖尿病视网膜的血管病变。
糖尿病视网膜病变;白藜芦醇;大鼠;链脲佐菌素
10.3969/j.issn.1007-3205.2017.11.016
糖尿病视网膜病变(diabetic retinopathy,DR)是糖尿病患者眼部最重要的并发症,是糖尿病患者常见的微血管病变之一[1-2]。随着人们生活水平的逐渐提高和生活方式的改变,DR发病率逐年增长[3],同时伴随眼部并发症的病程逐渐缩短。病程20年以上的糖尿病患者大多伴有不同程度的DR。DR导致患者视力急剧下降,严重时会出现失明,给患者带来极大痛苦[4]。目前,DR具体发病机制仍不十分清楚,而越来越多的研究表明早期DR特征表现为血管的神经炎症、血-视网膜屏障发生损害、出现氧化应激损伤和细胞凋亡[5]。由此可见,早期对糖尿病患者进行抗炎治疗可能是预防DR的一种行之有效的干预措施。白藜芦醇(Resveratrol,RES)具有抗炎、抗氧化应激以及抗凋亡等特性,但是RES对DR抗炎方面的相关研究未见系统报道。本研究探讨RES对糖尿病大鼠视网膜病变的抗炎作用及相关机制,旨在为临床治疗DR提供重要的理论依据。报告如下。
1 材 料 与 方 法
1.1 动物模型及分组 清洁级健康雄性SD大鼠45只,体质量200~250 g,由天津市实验动物中心提供。所有SD大鼠随机分为正常对照组15只、糖尿病模型组15只和RES组15只,所有大鼠进行适应性喂养7 d。模型组和RES组大鼠接受链脲佐菌素左下腹腹腔注射建立糖尿病模型(链脲佐菌素溶于生理盐水中,按60 mg/kg进行注射),对照组注射等量的生理盐水。链脲佐菌素注射72 h后尾静脉测量血糖(血糖仪测定),若血糖>16.7 mmol/L视为糖尿病模型制作成功。造模后1周,RES组大鼠给予40 mg/kg(10 mL/kg)白藜芦醇单侧腹腔注射治疗,1次/d,连续治疗6周。对照组和模型组大鼠每日给予等量生理盐水腹腔注射。
1.2 试剂 白藜芦醇、链脲佐菌素购自美国sigma公司。白细胞介素1β(interleukin-1β,IL-1β)及肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)酶联免疫吸附测定(enzyme linked immunosorbent assay,ELISA)试剂盒购自Abcam公司。其他试剂均购自北京博奥森生物技术有限公司。
1.3 实验方法 RES治疗结束后每只大鼠称体质量,尾静脉取血,血糖仪测量空腹血糖。大鼠腹腔注射10%水合氯醛(3 mL/kg)进行腹腔麻醉,去双侧眼球,显微镜下分离视网膜,-80 ℃冰箱保存备用。各组大鼠摘除眼球后,暴露心脏,用真空采血管从腹主动脉抽吸血液,离心后-80 ℃冰箱保存备用。
1.4 TNF-α、IL-1β、血管内皮生长因子(vascular endothelial growth factor,VEGF)、缺氧诱导因子1α(hypoxia inducible factor-1α,HIF-1α)、丙二醛(methane dicarboxylic aldehyde,MDA)和超氧化物歧化酶(superoxide dismutase,SOD)含量检测 采用随机数字表法将每组15只动物分成3小组,每小组5只,5只进行TNF-α和IL-1β检测,5只行VEGF和HIF-1α检测,另5只行MDA和SOD检测。①视网膜加入组织裂解液,超声粉碎后离心,取上清液蛋白定量,按ELISA试剂盒说明书检测视网膜组织中TNF-α和IL-1β含量。②取大鼠血清,按ELISA试剂盒说明书检测VEGF、HIF-1α、MDA和SOD含量。
1.5 统计学方法 应用SPSS 17.0统计软件分析数据。计量资料比较分别采用单因素方差分析和LSD-t检验。P<0.05为差异有统计学意义。
2 结 果
2.1 各组大鼠血糖和体质量比较 模型组和RES组血糖高于对照组,差异均有统计学意义(P<0.05);模型组与RES组血糖差异无统计学意义(P>0.05)。模型组和RES组体质量低于对照组,差异均有统计学意义(P<0.05);模型组与RES组体质量差异无统计学意义(P>0.05)。见表1。

组别血糖(mmol/L)体质量(g)对照组6.74±0.76362.47±25.86模型组21.97±3.66∗241.91±20.75∗RES组22.68±2.69∗248.29±18.43∗F87.532168.605P0.0000.000
*P<0.05与对照组比较(LSD-t检验)
2.2 各组大鼠视网膜组织中IL-1β和TNF-α含量比较 模型组和RES组大鼠视网膜中IL-1β和TNF-α表达明显高于对照组,RES组IL-1β和TNF-α表达低于模型组,差异均有统计学意义(P<0.05),见表2。


组别IL⁃1βTNF⁃α对照组46.03±2.7425.48±2.03模型组72.65±2.28∗61.32±4.67∗RES组59.13±3.41∗#42.85±5.26∗#F104.533125.294P0.0000.000
*P<0.05与对照组比较 #P<0.05与模型组比较(LSD-t检验)
2.3 各组大鼠血清中VEGF和HIF-1α含量比较 模型组和RES组大鼠血清中VEGF和HIF-1α表达明显高于对照组,RES组VEGF和HIF-1α表达明显低于模型组,差异均有统计学意义(P<0.05),见表3。
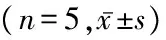
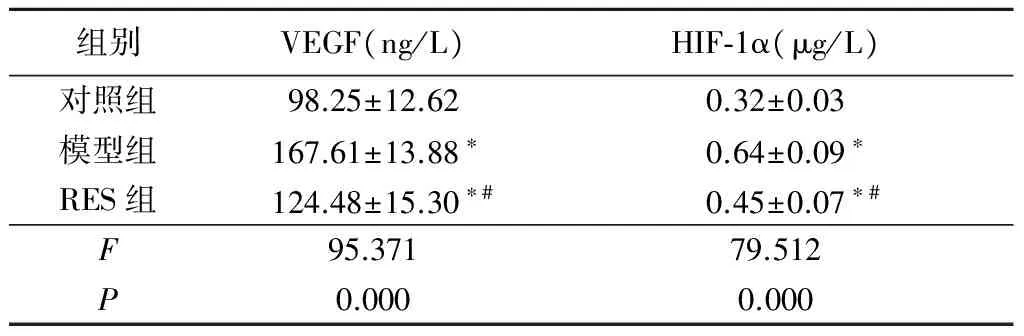
组别VEGF(ng/L)HIF⁃1α(μg/L)对照组98.25±12.620.32±0.03模型组167.61±13.88∗0.64±0.09∗RES组124.48±15.30∗#0.45±0.07∗#F95.37179.512P0.0000.000
*P<0.05与对照组比较 #P<0.05与模型组比较(LSD-t检验)
2.4 各组大鼠血清中MDA和SOD含量比较 模型组和RES组大鼠血清中MDA表达明显高于对照组,RES组MDA表达低于模型组,差异均有统计学意义(P<0.05)。模型组和RES组大鼠SOD表达明显低于对照组,RES组SOD表达高于模型组,差异均有统计学意义(P<0.05)。见表4。


组别MDASOD对照组6.72±1.53713.26±92.08模型组24.19±5.67∗435.61±87.89∗RES组14.28±3.07∗#583.45±79.62∗#F148.337120.106P0.0000.000
*P<0.05与对照组比较 #P<0.05与模型组比较(LSD-t检验)
3 讨 论
DR是糖尿病最为常见的一种微血管并发症,同时也是失明的一个主要原因[6],其对患者自尊心有极大影响并给社会和家庭造成严重的负担。目前DR的主要治疗方法是疾病早期行激光光凝术以及疾病晚期应用玻璃体切割手术,该方法对患者的视力造成损害并带来不适感觉。因此,治疗DR有效药物的研究就显得至关重要。
近年来,越来越多的研究证实DR是一种与炎症密切相关的疾病,炎症过程导致DR结构和功能改变[7-8]。炎性细胞因子在DR的新生血管形成过程中起到了重要作用,在DR的发展过程中,视网膜炎症和白细胞黏膜微血管(白细胞淤滞)促进血-视网膜屏障的破坏[9]。炎性细胞因子的过度表达形成的炎性反应可造成糖尿病个体视网膜出现损伤,其中炎性因子TNF-α和IL-1β[10-12]与DR的关系密切,参与了DR的发展进程。TNF-α是由巨噬细胞或单核细胞活化后产生的一种多功能的炎症因子,与血-视网膜屏障的破坏和血管细胞死亡呈正相关,被认为是炎症反应的触发器[13],其增多可用来监测DR的严重程度。IL-1β是IL-1家族的一员,在DR的进程中发挥关键的作用。DR可导致IL-1β的增多,IL-1β及其他炎性因子可通过影响细胞黏附分子的表达、钙超载、凋亡途径等,进一步加重糖尿病患者的视网膜病变[14-15]。慢性炎症是DR发病机制中关键的一环,有研究报道减轻炎症反应可以减缓DR的进展[16]。控制DR的炎症反应是治疗DR的重要策略之一。
RES是广泛存在于葡萄、虎杖等植物中的一种酚类植物抗毒素。大量研究表明其具有抗炎、心血管保护、抗凋亡、抗氧化、提高免疫活性、减肥降脂、抗癌、抗突变等特性[17-20]。RES可抑制前列腺癌中的IL-6信号通路,发挥抗肿瘤的作用[21]。Xu 等[22]证实RES可通过抑制细胞凋亡和炎症反应改善肾脏缺血和再灌注损伤。有研究应用RES治疗糖尿病大鼠的视网膜病变,发现其可抑制高血糖造成的谷氨酸摄取、谷氨酰胺合成酶的活性和表达的减少[23]。本研究观察了RES对糖尿病大鼠视网膜内TNF-α和IL-1β表达的影响,结果显示模型组大鼠视网膜中TNF-α和IL-1β表达增多,RES有效抑制了DR大鼠视网膜中TNF-α和IL-1β的表达。因此,推断抑制炎症反应可能是RES治疗DR的分子生物学机制之一。
VEGF是调节视网膜血管渗漏和新血管形成主要的一种生长因子[24]。慢性高血糖刺激VEGF的合成和分泌[25],炎性因子同时可诱导VEGF的分泌[26],促进视网膜新生血管的形成。VEGF由HIF-1α 转录调控,HIF-1α是影响新生血管形成的主要蛋白,其是调节高血糖状态下VEGF表达的一种转录因子[24]。本研究结果显示,模型组VEGF和HIF-1α的表达增多,而RES组两者的含量下降。表明RES治疗降低了VEGF和HIF-1α的表达。因此,推断RES通过抑制HIF-1α/VEGF信号通路降低血管内皮细胞的增殖,从而改善了糖尿病视网膜的血管病变。有研究发现,HIF-1α在DR中增多,抑制HIF-1α的表达可抑制DR中促炎因子IL-6和TNF-α的表达[27]。本研究显示RES对糖尿病大鼠的血糖无明显影响。故认为RES治疗后血清中VEGF和HIF-1α的减少是抑制炎症因子过度表达的重要机制。
氧化应激同样也是影响DR过程中的重要因素,大量的动物和临床研究证实了DR出现了氧化应激损伤[28]。MDA和SOD在许多研究中均被用作氧化应激的相关指标。MDA是氧化应激状态下脂质过氧化反应的产物,与氧化应激和脂质过氧化程度呈正相关[29],通常作为氧化应激损伤的常用标志物,MDA的升高被认为是氧化损伤中脂质过氧化的结果。氧化应激损伤同时会造成抗氧化的防御系统出现损害,影响抗氧化物酶[30]。DR中MDA表达增多、SOD活性下降[31]。RES具有抗氧化应激特性,可通过SIRT1/FOXO3a通路减轻高血糖导致的肾小管氧化应激损伤,同时可影响甲氨喋呤引起的大鼠回肠组织的氧化应激。本研究结果显示,糖尿病导致了氧化应激指标MDA表达增多和SOD活性下降,提示DR大鼠处于氧化应激状态,而RES治疗减轻了高血糖造成的氧化应激损伤。说明抗氧化应激作用是RES治疗DR的又一重要机制。
综上所述,RES可降低糖尿病大鼠视网膜中TNF-α、IL-1β的表达,通过抗炎作用起到对视网膜的保护作用。同时,RES抑制了HIF-1α/VEGF信号通路,可能通过此机制影响视网膜新生血管的形成,从而改善糖尿病视网膜的血管病变。RES治疗同样减轻了高血糖造成的氧化应激损伤。在今后的工作中将会继续深层次探讨RES的药理作用,尤其是在眼科疾病中的基础和临床研究,从而为DR乃至其他眼部疾病患者的治疗提供理论依据。
[1] 尹艳华,孙海燕,赵立,等.老年2型糖尿病住院患者糖脂代谢、慢性并发症及临床用药的现状分析[J].中国糖尿病杂志,2015,23(5):390-393.
[2] Madonna R,Balistreri CR,Geng YJ,et al. Diabetic microangiopathy:pathogenetic insights and novel therapeutic approaches[J]. Vascul Pharmacol,2017,90:1-7.
[3] 陈淑惠,孟倩丽,张敏,等.2型糖尿病视网膜病变与糖尿病其他并发症的相关性[J].国际眼科杂志,2016,16(2):309-312.
[4] Trento M,Charrier L,Salassa M,et al. Cognitive function may be a predictor of retinopathy progression in patients with type 2 diabetes[J]. Eur J Ophthalmol,2017,27(3):278-280.
[5] Mozetic V,Freitas CG,Riera R. Statins and fibrates for diabetic retinopathy:protocol for a systematic review[J]. JMIR Res Protoc,2017,6(2):e30.
[6] Cheung N,Mitchell P,Wong TY. Diabetic retinopathy[J]. Lancet,2010,376(9735):124-136.
[7] Du M,Martin A,Hays F,et al. Serum retinol-binding protein-induced endothelial inflammation is mediated through the activation of toll-like receptor 4[J]. Mol Vis,2017,23:185-197.
[8] Wang LL,Chen H,Huang K,et al. Elevated histone acetylations in Müller cell contribute to inflammation:a novel inhibitory effect of minocycline[J]. Glia,2012,60(12):1896-1905.
[9] Rangasamy S,Mcguire PG,Das A. Diabetic retinopathy and inflammation:novel therapeutic targets[J]. Middle East Afr J Ophthalmol,2012,19(1):52-59.
[10] Koleva-Georgieva DN,Sivkova NP,Terzieva D. Serum inflammatory cytokines IL-1beta,IL-6,TNF-alpha and VEGF have influence on the development of diabetic retinopathy[J]. Folia Med(Plovdiv),2011,53(2):44-50.
[11] Zhang ZH,Chen QZ,Jiang F,et al. Changes in TL1A levels and associated cytokines during pathogenesis of diabetic retinopathy[J]. Mol Med Rep,2017,15(2):573-580.
[12] Portillo JC,Lopez Corcino Y,Miao Y,et al. CD40 in retinal Müller cells induces P2X7-dependent cytokine expression in macrophages/microglia in diabetic mice and development of early experimental diabetic retinopathy[J]. Diabetes,2017,66(2):483-493.
[13] Sharma S,Purohit S,Sharma A,et al. Elevated Serum Levels of Soluble TNF Receptors and Adhesion Molecules Are Associated with Diabetic Retinopathy in Patients with Type-1 Diabetes[J]. Mediators Inflamm,2015,2015:279393.
[14] Zhu X,Xie M,Wang K,et al. The effect of puerarin against IL-1β-mediated leukostasis and apoptosis in retinal capillary endothelial cells(TR-iBRB2)[J]. Mol Vis,2014,20:1815-1823.
[15] Rajamani U,Jialal I. Hyperglycemia induces toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells:implications for diabetic retinopathy[J]. J Diabetes Res,2014,2014:790902.
[16] Tarr JM,Kaul K,Chopra M,et al. Pathophysiology of diabetic retinopathy[J]. ISRN Ophthalmol,2013,2013:343560.
[17] Cho S,Namkoong K,Shin M,et al. Cardiovascular protective effects and clinical applications of resveratrol[J]. J Med Food,2017,20(4):323-334.
[18] Ahmet I,Tae HJ,Lakatta EG,et al. Long-term low dose dietary resveratrol supplement reduces cardiovascular structural and functional deterioration in chronic heart failure in rats[J]. Can J Physiol Pharmacol,2017,95(3):268-274.
[19] Opydo-Chanek M,Rak A,Cierniak A,et al. Combination of ABT-737 and resveratrol enhances DNA damage and apoptosis in human T-cell acute lymphoblastic leukemia MOLT-4 cells[J]. Toxicol In Vitro,2017,42:38-46.
[20] Rai G,Suman S,Mishra S,et al. Evaluation of growth inhibitory response of Resveratrol and Salinomycin combinations against triple negative breast cancer cells[J]. Biomed Pharmacother,2017,89:1142-1151.
[21] Al Aameri RFH,Sheth S,Alanisi EMA,et al. Tonic suppression of PCAT29 by the IL-6 signaling pathway in prostate cancer:Reversal by resveratrol[J]. PloS One,2017,12(5):e0177198.
[22] Xu Y,Zhang B,Xie D,et al. Nanoparticle-mediated dual delivery of resveratrol and DAP5 ameliorates kidney ischemia/reperfusion injury by inhibiting cell apoptosis and inflammation[J]. Oncotarget,2017,8(24):39547-39558.
[23] Zeng K,Yang N,Wang D,et al. Resveratrol prevents retinal dysfunction by regulating glutamate transporters,glutamine synthetase expression and activity in diabetic retina[J]. Neurochem Res,2016,41(5):1050-1064.
[24] Yan HT,Su GF. Expression and significance of HIF-1α and VEGF in rats with diabetic retinopathy[J]. Asian Pac J Trop Med,2014,7(3):237-240.
[25] Cehofski LJ,Honoré B,Vorum H. A review:proteomics in retinal artery occlusion,retinal vein occlusion,diabetic retinopathy and acquired macular disorders[J]. Int J Mol Sci,2017,18(5):E907.
[26] Makabe T,Koga K,Miyashita M,et al. Drospirenone reduces inflammatory cytokines,vascular endothelial growth factor(VEGF) and nerve growth factor(NGF) expression in human endometriotic stromal cells[J]. J Reprod Immunol,2017,119:44-48.
[27] Tsapournioti S,Mylonis,Hatziefthimiou A,et al. TNFα induces expression of HIF-1α mRNA and protein but inhibits hypoxic stimulation of HIF-1 transcriptional activity in airway smooth muscle cells[J]. J Cell Physiol,2013,228(8):1745-1753.
[28] Goharinia M,Zareei A,Rahimi M,et al. Can allopurinol improve retinopathy in diabetic rats? Oxidative stress or uric acid; which one is the culprit?[J]. Res Pharm Sci,2017,12(5):401-408.
[29] Liu H,Zhao M,Yang S,et al. (2R,3S)-Pinobanksin-3-cinnamate improves cognition and reduces oxidative stress in rats with vascular dementia[J]. J Nat Med,2015,69(3):358-365.
[30] Ghorbanzadeh V,Mohammadi M,Mohaddes G,et al. Protective effect of crocin and voluntary exercise against oxidative stress in the heart of high-fat diet-induced type 2 diabetic rats[J]. Physiol Int,2016,103(4):459-468.
[31] Chen WP,Wang YD,Ma Y,et al. Danhong Huayu Koufuye combined with metformin attenuated diabetic retinopathy in Zucker diabetic fatty rats[J]. Int J Ophthalmol,2015,8(6):1094-1100.
TheeffectsofResveratrolondiabeticratswithretinopathy
LIU Jun-hui1,2, LI Chun-jiang2, LI Yu-tao2,3*
(1.DepartmentofOphthalmology,TangshanAierEyehospital,HebeiProvince,Tangshan063000,China; 2.DepartmentofOphthalmology,JidongOphthalmologyHospital,HebeiProvince,Tangshan063000,China; 3.DepartmentofOphthalmology,NorthChinaUniversityofScienceandTechnologyAffiliatedHospital,HebeiProvince,Tangshan063000,China)
ObjectiveTo observe the effects of Resveratrol(RES) on diabetic rats with retinopathy, and to explore its mechanism.MethodsForty-five cleaning degree healthy male SD rats were randomly divided into 3 groups, control group,model group and RES group(n=15/group). The diabetic rats model were established by streptozotocin intraperitoneal injection. One week after modeling, rats in RES group was administrated intraperitoneally with RES(40 mg/kg, 10 mL/kg) once daily for consecutive 6 weeks. After treatment, the blood glucose and body weight of different groups were measured. The expression of tumor necrosis factor alpha(TNF-α) and interleukin-1β(IL-1β) in retina of different groups were detected by enzyme linked immunosorbent assay(ELISA). The expression of vascular endothelial growth factor(VEGF), hypoxia inducible factor-1α(HIF-1α), methane dicarboxylic aldehyde(MDA) and superoxide dismutase(SOD) in serum of different groups were detected by ELISA.ResultsThe blood glucose of model group and RES group was higher than control group, body weight of model group and RES group was lower than control group(P<0.05), there were no significantly difference between model group and RES group(P>0.05). The expressions of TNF-α, IL-1β, VEGF, HIF-1α and MDA in model group and RES group were higher than that of control group. The e expressions of TNF-α, IL-1β, VEGF, HIF-1α and MDA in RES group were lower than that of model group(P<0.05). The expression of SOD in model group and RES group was lower than that of control group. The expression of SOD in RES group was higher than that of model group(P<0.05).ConclusionRES could inhibit inflammatory reaction, suppresses HIF-1α/VEGF pathway and reduces oxidative stress injury, then ameliorates vascular lesions in diabetic retina.
diabetic retinopathy; Resveratrol; rats; streptozotocin
·论著·
2017-05-22;
2017-06-23
刘俊辉(1978-),女,河北唐山人,唐山爱尔眼科医院主治医师,医学学士,从事眼科疾病诊治研究。
*通讯作者。E-mail:Liyutao@jidongeye.com
R587.26
A
1007-3205(2017)11-1310-05
(本文编辑:许卓文)
