棉铃虫复眼中Clock生物钟基因的昼夜表达模式
2017-11-07闫硕刘彦君张馨方秦萌刘慧朱家林李贞张青文刘小侠
闫硕,刘彦君,张馨方,秦萌,刘慧,朱家林,李贞,张青文,刘小侠
棉铃虫复眼中生物钟基因的昼夜表达模式
闫硕1,2,刘彦君1,张馨方3,秦萌2,刘慧2,朱家林4,李贞1,张青文1,刘小侠1
(1中国农业大学植物保护学院,北京100193;2全国农业技术推广服务中心,北京100125;3河北省农林科学院昌黎果树研究所,河北昌黎066600;4北京出入境检验检疫局,北京100026)
【】克隆并分析棉铃虫()复眼()生物钟基因的cDNA序列,探讨棉铃虫复眼中生物钟基因的昼夜表达模式及其表达水平的影响因子,以确认其在复眼中是否起着调节生物节律的功能,为理解复眼中生物钟基因网络提供理论参考。【】以2日龄棉铃虫复眼为试验材料,采用RT-PCR和RACE末端扩增技术克隆棉铃虫生物钟基因。利用生物信息学软件对得到的棉铃虫CLK氨基酸序列进行生物信息学分析。采用实时荧光定量PCR(qRT-PCR)技术,检测棉铃虫成虫不同器官(头、胸、腹、足、翅、脑、触角、复眼)中生物钟基因的表达水平;通过设置不同的光周期环境,检测复眼中生物钟基因的昼夜表达模式;通过在暗期设置6 h不同波段光 (UV、蓝光和绿光)照射,检测复眼中生物钟基因的表达水平;通过设置棉铃虫雌雄蛾交配处理,检测交配结束0 h和3 h复眼中生物钟基因的表达水平;通过饥饿处理棉铃虫雌雄蛾,检测复眼中生物钟基因的表达水平。【】克隆得到棉铃虫生物钟基因的cDNA序列,命名为(GenBank登录号为KM233158),开放读码框1 860 bp,编码619个氨基酸组成的多肽,理论推测分子量(Mw)为69.32 kD,等电点(pI)为5.71。推导得到的氨基酸序列具有3个跨膜拓扑结构,包含多个昆虫CLK蛋白的保守区域(PAS和HLH),其与甜菜夜蛾()和黑脉金斑蝶()的同源性较高,分别为97%和74%。与点蜂缘蝽()和马铃薯甲虫()的同源性较低,分别为53%和52%。qRT-PCR结果表明在检测的成虫器官中,在复眼中表达水平最低,触角中表达水平最高。在14L﹕10D光周期下,复眼中的表达量在白天增高,夜晚下降。生物钟基因的昼夜表达模式在1 d黑暗下可以持续,而在持续黑暗下固有表达节律消失。复眼中的表达水平在6 h光照后上调,但不同波段光照射无显著性差异。复眼中的表达水平在交配后有下调趋势,在雄蛾交配后表达水平显著性下降。复眼中的表达水平在饥饿处理后无显著性变化。【】成功从夜蛾棉铃虫的复眼中克隆得到生物钟基因,由生物钟基因推导得到的氨基酸序列具有典型的CLK蛋白特征,且与昆虫CLK蛋白同源性较高。在检测的棉铃虫成虫器官中,在复眼中的表达水平最低。在外周组织复眼中的表达水平受蛾类自身节律、光照和蛾类生理状态的影响,证实棉铃虫复眼中在调节生物节律方面具有重要作用,但生物钟基因网络在复眼与中枢神经中是否类似有待进一步深入研究。
棉铃虫;生物钟;复眼;光感受器;外周组织;节律
0 引言
【研究意义】作为最普遍的节律现象,昼夜节律是调节昆虫生命活动以24 h为周期的内源性振荡,其参与昆虫的许多生物学行为,如产卵、羽化、交配、滞育、迁徙等[1-5]。昆虫的昼夜节律受到自身生物钟基因的调控,前人对于生物钟基因的研究主要集中在昆虫中枢神经系统[6-9],外周组织中生物钟基因的昼夜表达研究相对较少,其表达是否存在固有节律有待进一步验证。外周组织复眼是夜蛾感光的重要器官,阐明复眼中生物钟基因的表达模式有利于理解外周组织中生物钟基因的功能和夜蛾感受光调节自身节律的机制。【前人研究进展】鳞翅目昆虫的生物钟基因网络比较清楚,()和()生物钟基因转录的CLK和CYC蛋白在细胞核中结合形成异二聚体,结合在()、()、()生物钟基因和控制某些行为和生理的基因的E-box上启动它们的转录,PER、TIM和CYR2蛋白在细胞质中形成三聚体。果蝇没有CRY2蛋白,鳞翅目昆虫CRY2蛋白作为转录负调控因子,进入细胞核作用于CLK/CYC二聚体,从而抑制自身的转录[2,4,10-14]。鳞翅目昆虫CRYPTOCHROME1(CRY1)蛋白与果蝇的CRY1蛋白功能相似,均作为感光受体,当感受到光刺激后导致TIM蛋白降解[15-18]。昆虫生物钟基因的表达受到内源性和外源性因子的影响,包括光照、温度和生理状态[19-23]。现有的研究表明,光感受器中生物钟基因的表达可能受到自身节律调控[24]。【本研究切入点】复眼作为夜蛾光感受的直接器官,复眼生物钟基因的功能研究相对较少。有关棉铃虫()生物钟基因的研究尚无报道,其在复眼中的表达模式尚不清楚,其重要的生物学功能有待阐明。【拟解决的关键问题】利用RT-PCR和RACE技术克隆棉铃虫生物钟基因全长,并检测其在外周组织复眼中的表达模式,阐明夜蛾复眼中生物钟基因的作用机制,理解复眼中生物钟基因网络。
1 材料与方法
试验于2014年9月至2016年5月在中国农业大学完成。
1.1 试虫
供试棉铃虫幼虫采自河北邯郸棉田,长期饲养于中国农业大学有害生物综合治理实验室。室内饲养温度(27±1)℃,相对湿度(75±10)%,光周期14L﹕10D,在ZT0(CT0)进入光期,ZT14(CT14)进入暗期。幼虫用人工饲料饲养[25],初孵幼虫群体饲养,3 龄后单管饲养以防自相残杀。蛹期分雌雄,分别置于养虫笼(20 cm×25 cm×30 cm)中等待羽化,暗期羽化的成虫记为0日龄蛾[26-27],成虫羽化后饲喂10%蜂蜜水。
1.2 Clock生物钟基因的克隆
以棉铃虫2日龄蛾复眼为材料,参照RNA提取试剂盒(RNeasy Mini Kit购自Qiagen公司)提取复眼总RNA。经NanoDrop2000测定的RNA样本进行反转录生成cDNA(反转录酶Omniscript RT Kit购自Qiagen公司),可于-20℃保存备用。
通过NCBI在线比对设计简并引物(表1,引物由上海生工生物工程公司合成),对目的基因 (包括起始密码子)进行PCR扩增(DNA聚合酶Trans Taq-T购自北京全式金生物技术有限公司),PCR扩增程序:94℃预变性3 min;94℃45 s,55℃30 s,72℃60 s,35个循环;72℃延伸10 min。PCR产物经琼脂糖凝胶电泳检测后,将回收的目的条带(DNA胶回收试剂盒Gel Extraction Kit购自Omega公司)与克隆载体(pEASY-T1 Cloning Vector购自北京全式金生物技术有限公司)连接,转化到大肠杆菌内,37℃培养过夜。菌落进行蓝白斑筛选(IPTG和Amp购自Takara公司;X-gal购自北京拜尔迪生物有限公司),随机选取阳性克隆送至北京擎科生物技术有限公司测序。
参照RACE试剂盒说明,在RNA水平上做3′RACE处理(First Choice RLM-RACE购自Ambion公司)。根据扩增得到的棉铃虫生物钟基因的5′端序列设计引物(表1),结合试剂盒提供的 2 个下游引物进行巢式PCR。PCR扩增程序:94℃预变性3 min;94℃45 s,56℃30 s,72℃90 s,35个循环;72℃延伸10 min。PCR产物经电泳检测后测序。

表1 基因克隆和荧光定量PCR所用引物
1.3 序列分析
序列的拼接借助DNAMAN v.5.2.2软件完成;序列同源性比对和保守区分析通过NCBI(http://www. ncbi.nlm.nih.gov/)完成;分子量和等电点预测通过Computepl/Mw tool(http://web.expasy.org/compute _pi/)完成;功能位点分析通过PROSITE SCAN (http://npsa- pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_proscan.html)完成[28-29];跨膜区预测通过TMpred(http://www.ch.embnet.org/software/TMPRED_form.html)完成;3D结构的构建通过在线网站SWISS MODEL WORKSPACE(http://swissmodel. expasy.org)和软件Deep View4.0.1完成。系统进化树通过MEGA 4.0软件的neighbor-joining法构建,非参数重复取样估计方法评估系统发育树节点的可靠性(1 000次重复)。
1.4 Clock生物钟基因的表达水平检测
器官特异性检测:在进入光期1 h(ZT1),提取2日龄雌雄蛾头、胸、腹、足、翅、脑、触角、复眼的总RNA。以下试验的样本均采自2日龄雌雄蛾复眼。昼夜节律性检测:(1)在14L﹕10D光环境下饲养的雌雄蛾,从ZT1开始每隔2 h取样;(2)在14L﹕10D光环境下饲养的雌雄蛾转移至24 h黑暗中,从CT1开始每隔2 h取样;(3)在14L﹕10D光环境下饲养的幼虫,化蛹后转移至24 h持续黑暗环境,羽化至2日龄蛾时,从CT1开始每隔2 h取样。光照后检测:根据棉铃虫视网膜电位的研究[30],在进入暗期时(ZT14)设置500 lx照度的UV(峰值365 nm)、蓝光(峰值450 nm)和绿光(峰值505 nm),照射6 h后(ZT20)取样,在14L﹕10D 光环境下的雌雄蛾作为对照(CK)。交配后检测:在进入暗期时,将20对雌雄处女蛾放入交配笼(20 cm×25 cm×30 cm)中配对,每隔15 min观察交配是否发生,通过这种方式获得交配结束0 h和3 h的雌雄蛾,处女蛾作为对照(ZT15和ZT18)。饥饿后检测:雌雄蛾羽化后不再饲喂蜂蜜水,饥饿饲养至2日龄,正常饲喂的棉铃虫作为对照,当进入光期1 h取样。以上每个RNA样本取3个生物学重复。
在qRT-PCR之前,通过测序保证PCR产物是目的基因片段,生物钟基因表达量检测在ABI 7300仪器上进行(ABI Power SYBR Green PCR Master Mix购自ABI公司)。选取棉铃虫和为内参基因[22,27,31-32],qRT-PCR引物见表1。qRT-PCR反应程序:95℃预变性10 min;95℃15 s,55℃40 s,72℃35 s,40个循环;此外再加上qRT-PCR仪器自带的熔解步骤。保证熔解曲线为平滑的单峰且峰值单一,无杂峰。检测所取样本中目的基因和内参基因的Ct值,每个样本设置3次点样重复,基因相对表达量的计算采用2-ΔΔCt方法[33]进行。
1.5 数据分析
数据分析采用Tukey比较和独立样本检验方法进行,<0.05视为显著性差异。所有统计分析均借助SPASS 16.0软件包完成。
2 结果
2.1 棉铃虫Clock生物钟基因的克隆及生物信息学分析
成功克隆得到棉铃虫基因的完整开放阅读框,包含1 860个碱基,编码619个氨基酸组成的多肽,命名为,GenBank登录号KM233158。的cDNA序列及其推导得到的氨基酸序列见图1。
HeCLK的理论推测分子量(Mw)为69.32 kD,等电点(pI)为5.71。跨膜拓扑结构预测显示HeCLK在91—112位和491—512位有2个由外向内趋性的氨基酸跨膜拓扑结构,在416—437位有1个由内向外趋性的氨基酸跨膜拓扑结构。功能位点的分析表明,HeCLK含有3个N-糖基化位点(N-glycosylation site)、2个cAMP-和cGMP-蛋白激酶磷酸化位点(cAMP- and cGMP-dependent protein kinase phosphorylation site)、13个蛋白激酶C磷酸化位点(protein kinase C phosphorylation site)、15个酪蛋白激酶Ⅱ磷酸化位点(casein kinase II phosphorylation site)、9个N-豆蔻酰化位点(N-myristoylation site)。HeCLK蛋白序列的3D结构模型的构建基于鼠模型4f3i.1.B(2.27Å)完成(图2),序列一致性(sequence identity)57.66%,E-value:0.42,QMEAN Z-Score:-2.25。
用BLAST在线进行同源性比较分析,HeCLK氨基酸序列与已报道的昆虫CLK蛋白同源性较高,其中与甜菜夜蛾()同源性高达97%,与黑脉金斑蝶()同源性高达74%,与柞蚕()同源性高达71%,与切叶蚁(和)同源性达到68%,与赤拟谷盗()同源性达到65%,与长须罗蛉()同源性达到56%,与点蜂缘蝽()同源性达到53%,与马铃薯甲虫()同源性达到52%。如进化树(图3)所示,棉铃虫与甜菜夜蛾亲缘关系最近,鳞翅目、鞘翅目和膜翅目昆虫分别聚为一类,通过CLK氨基酸序列构建的进化关系与传统分类布局基本一致。
2.2 棉铃虫Clock生物钟基因在复眼中低表达
以持家基因和为内参基因,借助qRT-PCR技术检测在棉铃虫成虫头、胸、腹、足、翅、脑、触角和复眼中的相对表达量。如图4所示,在棉铃虫雌雄蛾的不同器官中均有表达,不具有器官特异性,且雌雄蛾间基因表达水平变化趋势相似(头:=0.179,=4,=0.866;胸:=0.642,=4,=0.556;腹:=1.254,=4,=0.278;足:=1.007,=4,=0.371;翅:=0.761,=4,=0.489;脑:=0.724,=4,=0.509;触角:=1.658,=4,=0.173;复眼:=0.930,=4,=0.405)。表达量在不同器官中具有波动性,在雌雄蛾腹、翅和触角中高表达,在复眼中表达水平最低(雌虫:7,16=11.946,<0.001;雄虫:7,16=20.382,<0.001)。以下试验均选取棉铃虫复眼为试验材料,检测在外周组织复眼中的表达模式。
2.3 棉铃虫Clock生物钟基因在复眼中的昼夜表达节律
在14L﹕10D的光环境下(图5-A),表达量在光期上升,暗期下降,且雌雄蛾中表达趋势一致(雌蛾:11,24=8.628,<0.001;雄蛾:11,24=12.062,<0.001)。饲养于14L﹕10D光环境下的棉铃虫转移至黑暗条件,检测的表达是否在外周组织复眼中存在固有节律。如图5-B所示,的表达趋势与处理14L﹕10D一致,并未发生变化(雌蛾:11,24=6.690,<0.001;雄蛾:11,24=13.469,<0.001)。在持续黑暗(棉铃虫自化蛹饲养于黑暗环境)下(图5-C),的这种表达模式被打破,表达量随着时间的推移不存在显著性差异(雌蛾:11,24=0.552,=0.848;雄蛾:11,24=0.388,=0.948)。

碱基和氨基酸的位置均标识在左侧。起始密码子加粗表示,终止密码子加星号表示,PAS保守区用阴影表示,HLH保守区标下划线,N-糖基化位点加框标出,cAMP-和cGMP-蛋白激酶磷酸化位点加虚框标出The positions of nucleotide and amino acid were indicated in the left margin. The start codon was in bold, and the stop codon was indicated with an asterisk. Conserved domain of PAS was outlined in gray, conserved domain of HLH was underlined, N-glycosylation sites were indicated with boxes, and cAMP- and cGMP-dependent protein kinase phosphorylation sites were indicated with dashed boxes

蓝绿色代表α螺旋,红色代表β折叠,洋红色代表Ω环,蓝色代表PAS-B保守区域,绿色代表PAS-A保守区,黄色代表HLH保守区
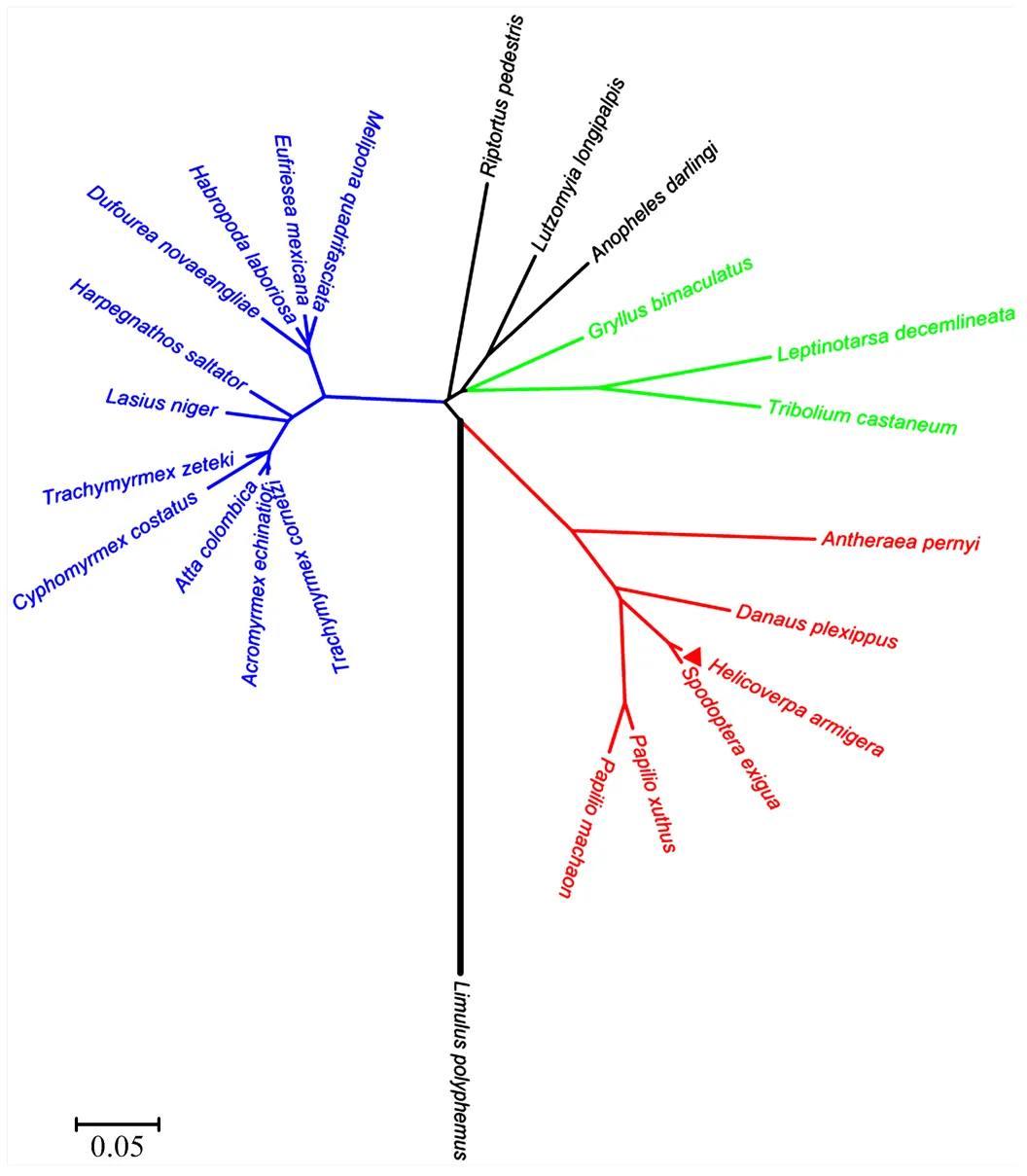
物种登录号The accession number of listed species:Antheraea pernyi柞蚕(AAR14936),Danaus plexippus黑脉金斑蝶(EHJ69324),Spodoptera exigua甜菜夜蛾(AEJ38222),Lutzomyia longipalpis长须罗蛉(AKN63486),Gryllus bimaculatus双斑蟋(BAM76759),Leptinotarsa decemlineata马铃薯甲虫(AKG92749),Tribolium castaneum赤拟谷盗(NP_001106937),Limulus polyphemus美洲鲎(ANO53967),Papilio xuthus柑橘凤蝶(KPI91944),Papilio machaon金凤蝶(KPJ06357),Cyphomyrmex costatus蚁类(KYN07352),Harpegnathos saltator印度跳蚁(EFN76178),Eufriesea mexicana蜂类(OAD62154),Habropoda laboriosa回条蜂(KOC70150),Anopheles darlingi达氏按蚊(ETN62614),Riptortus pedestris点蜂缘蝽(BAN20981),Trachymyrmex cornetzi蚁类(KYN13948),Trachymyrmex zeteki蚁类(KYQ51193),Atta colombica哥伦比亚美洲切叶蚁(KYM91193),Acromyrmex echinatior顶切叶蚁(EGI62057),Lasius niger黑蚁(KMR01088),Melipona quadrifasciata蜂类(KOX77563) and Dufourea novaeangliae蜂类(KZC11045)

图中数据为平均值±标准误(n=3)。下同 Value was the mean±SE of three collections. The same as below

阴影区域代表暗期 Shadowed area indicated dark period
2.4 棉铃虫Clock生物钟基因在光照下表达量上调,交配后表达量下调
暗期光照6 h后,表达水平显著上升,且不同波段光处理之间无显著性差异,雌雄蛾之间表达水平相似(雌蛾:3,8=4.407,=0.041;雄蛾:3,8=4.114,=0.049)(图6)。在棉铃虫交配后,表达水平有下降趋势(0 h雄蛾:=3.891,df=4,=0.018;3 h雄蛾:=2.993,df=4,=0.040)(图7)。
2.5 饥饿对棉铃虫Clock生物钟基因表达的影响
如图8所示,饥饿处理对的表达无显著性影响(雌蛾:=0.023,df=4,=0.983;雄蛾:=1.192,df=4,=0.299)。

在ZT20时,提取14L﹕10D光环境下棉铃虫复眼RNA作为CK样本。U:UV,B:蓝光,G:绿光

“*”表示基因表达差异显著(独立样本t检验,P<0.05) “*” indicated significant differences in gene expression at P<0.05 level according to independent t-tests

在ZT1时,取正常饲喂棉铃虫的复眼RNA作为CK样本 RNA samples were collected at ZT1 from the moths fed as CK
3 讨论
光感受器在同步生物体自身节律与外界光环境的过程中起到关键作用,因此光感受器中生物钟基因的研究十分重要[34-38]。本试验利用RT-PCR和RACE技术从棉铃虫复眼中成功克隆得到生物钟基因(),其与已报道的昆虫生物钟基因同源性较高,且保守区域相似,PAS和HLH保守区被报道是CLK-CYC二聚体结合的关键区域[39]。表达量在不同器官中波动较大,其在复眼中表达水平最低,触角中表达水平最高,但其表达不具有器官特异性,暗示在外周组织中可能承担着一定的生物学功能。与前人研究相似,棉铃虫和生物钟基因[22]、甘蓝夜蛾()和生物钟基因[40]、二化螟()滞育生物钟蛋白基因[41]、家蚕()、、和生物钟基因[3-4]在所检测的成虫和幼虫组织器官中均不存在表达特异性,生物钟基因的表达不具有器官特异性在其他动物中的报道也比较多[42-44]。鳞翅目昆虫对光的反应分化,蛾类具有夜行性视觉,Yan等[27]研究表明,棉铃虫复眼视觉基因的表达受生物钟基因的调控。本试验比较了生物钟基因在复眼与其他器官中的表达水平,虽然在复眼中的表达水平低,但其很可能参与复眼中生物节律的调控。
生物钟基因昼夜表达结果表明,在14L﹕10D光周期下,复眼中的表达量在光期上升,暗期下降。与前人对西方蜜蜂()和鹿角珊瑚()的研究结果相似,生物钟基因的表达量在光期上升,暗期下降[6,45]。将饲养于14L﹕10D的棉铃虫转移至黑暗条件,的表达模式没有变化,而在持续黑暗下此模式被打破,结果表明在外周组织复眼中的表达具有节律性,推测其执行着调节生物节律的功能;持续的黑暗可以扰乱生物钟基因的表达模式,进而打破生物节律。复眼作为昆虫最重要的感光器官,其生物钟的作用机制非常重要,结合之前的研究,初步明确了棉铃虫复眼中第一环路生物钟基因的表达模式:生物钟基因表达量在进入光期5 h达到高峰,随后表达量下降,生物钟基因表达量在光期下降,暗期升高[28]。综上所述,所检测的棉铃虫复眼生物钟基因的表达均存在固有节律性,笔者推测棉铃虫复眼中生物钟基因执行着调节生物节律的功能。对双斑蟋()视叶[24]、斜纹夜蛾()触角[46]和烟草天蛾()触角[47]中生物钟基因的研究表明,外周组织中存在内源性的调节生物钟基因表达的机制。
生物钟基因的表达水平受到外界光环境的影响[3,9,23]。黑腹果蝇()TIM和PER蛋白在持续光照下表达下调,TIM蛋白在450 nm波段下下调最多,600 nm以上波段几乎没有影响[7,48-49]。根据棉铃虫视网膜电位和视觉基因的敏感波段[27,30],笔者检测了棉铃虫在3种敏感波段光照射后,的表达水平。虽然在复眼中的表达存在固有节律,但暗期光照可以显著增强其表达,与进入光期表达量上调结果相一致,不同波段光之间表达上调程度相似。棉铃虫和生物钟基因在黑光灯照射2 h后表达量有下降趋势[21]。在蛋白水平上,持续光照下,TIM和PER蛋白表达量下调[2,7,49],CRY1蛋白降解TIM蛋白,导致PER-TIM二聚体或者CRY2蛋白转录负调控作用下降。
棉铃虫交配后,和生物钟基因的表达有下降趋势[21]。棉铃虫平均交配持续时间1 h,的表达水平在交配后有下降趋势。蛾类交配行为(求偶、性信息素合成和释放)存在一定的节律性,受到外源(光周期和温度)和内源性因子(神经中枢和激素)的调控[50-53]。有研究表明棉铃虫和烟青虫()的交配行为也具有节律性,弱光可以促进二者快速完成种内交配[54-55]。夜行性蛾类交配一般发生在黄昏或者黎明,光信号在其中起着重要作用[56-59]。本试验还进行了饥饿处理,但的表达不受饥饿影响。综上所述,的表达更易受到外界光环境的影响。
4 结论
利用RT-PCR和RACE技术从棉铃虫复眼中成功克隆得到了的cDNA序列,推导得到的HeCLK氨基酸序列具有典型的CLK蛋白的保守序列(PAS和HLH),其与甜菜夜蛾同源性最高。在外周组织复眼中的表达受到自身节律、外界光环境和生理状态的影响。光照和交配可以分别上调和下调的表达;的表达量在光期高于暗期,黑暗下表达模式没有发生改变,说明的表达存在固有节律性,可能在外周组织(复眼)中执行着生物钟基因的功能。
[1] Tanoue S, Nishioka T. A receptor-type guanylyl cyclase expression is regulated under circadian clock in peripheral tissues of the silk moth. Light-induced shifting of the expression rhythm and correlation with eclosion., 2001,276(50): 46765-46769.
[2] Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, Emery P, Reppert S M. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation., 2008, 6(1): e4.
[3] 张达燕, 梁辉, 司马杨虎, 徐世清. 温度与光照节律对家蚕成虫生物钟基因和表达的影响. 蚕业科学, 2013, 39(3): 453-459.
Zhang D Y, Liang H, Sima Y H, Xu S Q. Effects of temperature and light rhythm on expression of clock genesandadult., 2013, 39(3): 453-459. (in Chinese)
[4] 王文栋, 束梅影, 张达艳, 徐世清. 家蚕昼夜节律生物钟基因的生物信息学分析. 四川动物, 2016, 35(2): 275-282.
Wang W D, Shu M Y, Zhang D Y, Xu S Q. Bioinformatics analysis of circadian rhythm biological clock genes in., 2016, 35(2): 275-282. (in Chinese)
[5] Zhu L, Liu W, Tan Q Q, Lei C L, Wang X P. Differential expression of circadian clock genes in two strains of beetles reveals candidates related to photoperiodic induction of summer diapause., 2017, 603: 9-14.
[6] Rubin E B, Shemesh Y, Cohen M, Elgavish S, Robertson H M, Bloch G. Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee () and shed new light on the molecular evolution of the circadian clock., 2006, 16(11): 1352-1365.
[7] Gegear R J, Casselman A, Waddell S, Reppert S M. Cryptochrome mediates light-dependent magnetosensitivity in., 2008, 454(7207): 1014-1018.
[8] Barberà M, Collantes-Alegre J M, Martínez-Torres D. Characterisation, analysis of expression and localization of circadian clock genes from the perspective of photoperiodism in the aphid., 2017, 83: 54-67.
[9] Kontogiannatos D, Gkouvitsas T, Kourti A. The expression patterns of the clock genesandare affected by photoperiod in the Mediterranean corn stalk borer,., 2017, 94(1): e21366.
[10] Chang D C, McWatters H G, Williams J A, Gotter A L, Levine J D, Reppert S M. Constructing a feedback loop with circadian clock molecules from the silkmoth,., 2003, 278(40): 38149-38158.
[11] Shirasu N, Shimohigashi Y, Tominaga Y, ShimohigashiM. Molecular cogs of the insect circadian clock., 2003, 20(8): 947-955.
[12] Sandrelli F, Costa R, Kyriacou C P, Rosato E. Comparative analysis of circadian clock genes in insects., 2008, 17(5): 447-463.
[13] Tomioka K, Matsumoto A. A comparative view of insect circadian clock systems., 2010, 67(9): 1397-1406.
[14] 任爽, 魏慧敏, 郝友进, 陈斌. 昆虫钟基因研究进展. 昆虫学报, 2016, 59(3): 353-364.
Ren S, Wei H M, Hao Y J, Chen B. Research progress in circadian clock genes in insects., 2016, 59(3): 353-364. (in Chinese)
[15] Cashmore A R. Cryptochromes: enabling plants and animals to determine circadian time., 2003, 114(5): 537-543.
[16] Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the twocryptochrome structure domains in circadian photoreception., 2004, 304(5676): 1503-1506.
[17] Lin C, Todo T. The cryptochromes., 2005, 6(5): 220.
[18] Zhu H, Yuan Q, Froy O, Casselman A, Reppert S M. The two CRYs of the butterfly., 2005, 15(23): R953-R954.
[19] Boothroyd C E, Wijnen H, Naef F, Saez L, Young M W. Integration of light and temperature in the regulation of circadian gene expression in., 2007, 3(4): e54.
[20] Fan J Y, Muskus M J, Price J L. Entrainment of thecircadian clock: more heat than light., 2007, 413: pe65.
[21] Ni H, Yan S, Liu X X, Zhang Q W.mRNA expression under mating and black light treatment on., 2011, 2(3): 20-23, 30.
[22] Yan S, Ni H, Li H T, Zhang J, Liu X X, Zhang Q W. Molecular cloning, characterization, and mRNA expression of twogenes in(Lepidoptera: Noctuidae)., 2013, 106(1): 450-462.
[23] 刘孝明, 张松斗, 马木提·赛丽蔓, 邹驰, 李贞, 张青文, 刘小侠. 光周期和温度对生物钟基因在棉铃虫幼虫节律表达的影响. 应用昆虫学报, 2016, 53(5): 942-952.
Liu X M, Zhang S D, Mamuti S, Zou C, Li Z, Zhang Q W, Liu X X. The effect of photoperiod and temperature on the diurnal expression of the circadian clock genein larvae of cotton bollworm,(Hübner)., 2016, 53(5): 942-952. (in Chinese)
[24] Uryu O, Karpova S G, Tomioka K. The clock geneplays an important role in the circadian clock of the cricket., 2013, 59(7): 697-704.
[25] Wu K J, Gong P Y. A new and practical artificial diet for the cotton bollworm., 1997, 4(3): 277-282.
[26] Yan S, Li H T, Zhang J, Zhu J L, Zhang Q W, Liu X X. Sperm storage and sperm competition in the(Lepidoptera: Noctuidae)., 2013, 106(2): 708-715.
[27] Yan S, Zhu J L, Zhu W L, Zhang X F, Li Z, Liu X X, Zhang Q W. The expression of three opsin genes from the compound eye of(Lepidoptera: Noctuidae) is regulated by a circadian clock, light conditions and nutritional status., 2014, 9(10): e111683.
[28] Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997., 1997, 25(1): 217-221.
[29] Combet C, Blanchet C, Geourjon C, Deléage G. NPS@: network protein sequence analysis., 2000, 25(3): 147-150.
[30] 魏国树, 张青文, 周明牂, 吴卫国. 棉铃虫[(Hübner)]蛾复眼视网膜电位研究. 生物物理学报, 1999, 15(4): 682-688.
Wei G S, Zhang Q W, Zhou M Z, Wu W G. Studies on the electroretinogram of the compound eyes of(Hübner) moth., 1999, 15(4): 682-688. (in Chinese)
[31] Fuller R C, Claricoates K M. Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity., 2011, 20(16): 3321-3335.
[32] 闫硕, 朱家林, 朱威龙, 潘李隆, 张青文, 刘小侠. 棉铃虫-微管蛋白基因的克隆、序列分析及表达模式检测. 中国农业科学, 2013, 46(9): 1808-1817.
Yan S, Zhu J L, Zhu W L, Pan L L, Zhang Q W, Liu X X. Molecular cloning, sequence analysis and expression pattern detection of a-tubulin gene from(Hübner)., 2013, 46(9): 1808-1817. (in Chinese)
[33] Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCtmethod., 2001,25(4): 402-408.
[34] Sancar A. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception., 2000, 69: 31-67.
[35] Saunders D S. Insect photoperiodism: seeing the light., 2012, 37(3): 207-218.
[36] Ruan G X, Gamble K L, Risner M L, Young L A, McMahon D G. Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators., 2012, 7(6): e38985.
[37] Bobu C, Sandu C, Laurent V, Felder-Schmittbuhl M P, Hicks D. Prolonged light exposure induced widespread phase shifting in the circadian clock and visual pigment gene expression of theretina., 2013, 19(1): 1060-1073.
[38] Buonfiglio D C, Malan A, Sandu C, Jaeger C, Cipolla-Neto J, Hicks D, Felder-Schmittbuhl M P. Rat retina shows robust circadian expression of clock and clock output genes in explant culture., 2014, 20(6): 742-752.
[39] Huang N, Chelliah Y, Shan Y, Taylor C A, Yoo S H, Partch C, Green C B, Zhang H, Takahashi J S. Crystal structure of the heterodimeric CLOCK: BMAL1 transcriptional activator complex., 2012, 337(6091): 189-194.
[40] Merlin C, François M C, Queguiner I, Maïbèche- Coisné M, Jacquin-Joly E. Evidence for a putative antennal clock in: molecular cloning and characterization of two clock genes-and-in antennae., 2006,15(2): 137-145.
[41] 鲁艳辉, 赵燕燕, 张发成, 郑许松, 朱平阳, 吕仲贤. 二化螟滞育生物钟蛋白TIME-EA4基因的克隆及时空和温度诱导表达分析. 昆虫学报, 2016, 59(4): 392-401.
Lu Y H, Zhao Y Y, Zhang F C, Zheng X S, Zhu P Y, Lü Z X. Cloning and spatiotemporal and temperature-induced expression profiling of diapause bioclock protein TIME-EA4 gene in the rice stem borer,(Lepidoptera: Pyralidae)., 2016, 59(4): 392-401. (in Chinese)
[42] Chong N W, Chaurasia S S, Haque R, Klein D C, Iuvone P M. Temporal-spatial characterization of chicken clock genes: circadian expression in retina, pineal gland, peripheral tissues., 2003, 85(4): 851-860.
[43] Liu S, Cai Y N, Sothern R B, Guan Y Q, Chan P. Chronobiological analysis of circadian patterns in transcription of seven key clock genes in six peripheral tissues in mice., 2007, 24(5): 793-820.
[44] Singh D, Rani S, Kumar V. Daily expression of six clock genes in central and peripheral tissues of a night-migratory songbird: evidence for tissue-specific circadian timing., 2013, 30(10): 1208-1217.
[45] Brady A K, Snyder K A, Vize P D. Circadian cycles of gene expression in the coral,., 2011, 6(9): e25072.
[46] Merlin C, Lucas P, Rochat D, François M C, Maïbèche-Coisne M, Jacquin-Joly E. An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth., 2007, 22(6): 502-514.
[47] Schuckel J, Siwicki K K, Stengl M. Putative circadian pacemaker cells in the antennae of the hawkmoth., 2007, 330(2): 271-278.
[48] Suri V, Qian Z, Hall J C, Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in., 1998, 21(1): 225-234.
[49] Ceriani M F, Darlington T K, Staknis D, Más P, Petti A A, Weitz C J, Kay S A. Light-dependent sequestration of TIMELESS by CRYPTOCHROME., 1999, 285(5427): 553-556.
[50] Hollander A, Yin C M. Neurological influences on pheromone release and calling behaviour in the gypsy moth,(L.)., 1982, 7(2): 163-166.
[51] Raina A K, Klun J A. Brain factor control of sex pheromone production in the female corn earworm moth., 1984, 225(4661): 531-533.
[52] Delisle J, McNeil J N. Calling behaviour and pheromone titre of the true armyworm(Haw.) (Lepidoptera: Noctuidae) under different temperature and photoperiodic conditions., 1987, 33(5): 315-324.
[53] Raina A K. Neuroendocrine control of sex pheromone biosynthesis in Lepidoptera., 1993, 38: 329-349.
[54] 闫硕, 李慧婷, 朱威龙, 朱家林, 张青文, 刘小侠. 光强度对棉铃虫交配行为的影响. 昆虫学报, 2014, 57(9): 1045-1050.
Yan S, Li H T, Zhu W L, Zhu J L, Zhang Q W, Liu X X. Effects of light intensity on the sexual behavior of the cotton bollworm,(Lepidoptera: Noctuidae)., 2014, 57(9): 1045-1050. (in Chinese)
[55] Li H T, Yan S, Li Z, Zhang Q W, Liu X X. Dim light during scotophase enhances sexual behavior of the oriental tobacco budworm(Lepidoptera: Noctuidae)., 2015, 98(2): 690-696.
[56] Schal C, Cardé R T. Effects of temperature and light on calling in the tiger moth(Freeman) (Lepidoptera: Arctiidae)., 1986, 11(1): 75-87.
[57] Kamimura M, Tatsuki S. Effects of photoperiodic changes on calling behavior and pheromone production in the oriental tobacco budworm moth,(Lepidoptera: Noctuidae)., 1994, 40(8): 731-734.
[58] Burks C S, Brandl D G, Higbee B S. Effect of natural and artificial photoperiods and fluctuating temperature on age of first mating and mating frequency in the navel orangeworm,., 2011,11(1): 48.
[59] Kawazu K, Adati T, Tatsuki S. The effect of photoregime on the calling behavior of the rice leaf folder moth,(Lepidoptera: Crambidae)., 2011, 45(2): 197-202.
(责任编辑 岳梅)
Daily Expression ofGene in Compound Eye of
YAN Shuo1,2, LIU YanJun1, ZHANG XinFang3, QIN Meng2, LIU Hui2, ZHU JiaLin4, LI Zhen1, ZHANG QingWen1, LIU XiaoXia1
(1College of Plant Protection, China Agricultural University, Beijing 100193;2National Agricultural Technology Extension and Service Center, Beijing 100125;3Changli Institute of Pomology, Hebei Academy of Agriculture and Forestry Sciences, Changli 066600, Hebei;4Beijing Entry-Exit Inspection and Quarantine Bureau, Beijing 100026)
【】The objective of this study is to clone and analyze a circadian clock gene,() in the compound eyes of, examine the diurnal changes and determinants ofmRNA levels in compound eyes of cotton bollworm, and to determine whetherperformed circadian functions in compound eyes, which will provide a theoretical reference for understanding the circadian clock machinery in compound eyes. 【】The total RNA was isolated from the compound eyes of 2-day-oldmoths, and thewas cloned by reverse transcription polymerase chain reaction (RT-PCR) and rapid amplification of cDNA ends (RACE). The amino acid sequence of CLK from cotton bollworm was analyzed using bioinformatics softwares. The relative mRNA levels ofwere determined among various adult organs (head, thorax, abdomen, leg, wing, brain, antennae and compound eye) ofby quantitative real-time PCR (qRT-PCR). The diurnal change ofmRNA levels in compound eyes was measured under different photoperiods. Moths were illuminated by UV, blue and green lights, respectively, for 6 h from the beginning of the scotophase, and the expression levels ofin compound eyes were determined after light exposure. Female and male moths were paired for mating, and the expression levels ofin compound eyes were determined from the moths that had completed mating 0 h and 3 h. The expression levels ofin compound eyes were determined from the moths that were not fed. 【】An open reading frame of 1 860 bp was cloned, encoding 619 amino acids, designed as “” (GenBank accession number KM233158). The molecular mass of the deduced protein was predicted to be 69.32 kD, and the calculated isoelectric point (pI) was 5.71. Deduced amino acids sequence contained three transmembrane topologies, and several conserved domains of insect CLK (PAS and HLH). HeCLK showed high homology with(97% identity) and(74% identity), and low homology with(53% identity) and(52% identity). qRT-PCR revealed thatshowed the lowest mRNA levels in compound eyes, and the highest mRNA levels in antennas among tested adult organs. The mRNA levels ofin compound eyes increased during the day, and decreased during the night under 14L﹕10D. The cycling of the circadian clock gene mRNA levels persisted for 1 d under dark condition, but did not persist further under constant darkness.was up-regulated in compound eyes after light exposure, but there was no significant difference in mRNA levels ofamong different wavelengths of light. The expression levels ofwere tended to be down-regulated in the compound eyes after copulation, and there was a significant difference in mRNA levels ofbetween mated males and virgin males. The expression levels ofin compound eyes were not influenced by starvation. 【】from the compound eyes of a nocturnal moth,, was cloned. Deduced amino acids sequence contained the conserved domains of CLK proteins, and shared high homology with insect CLK.showed the lowest mRNA levels in compound eyes among tested adult organs. The expression levels ofin peripheral tissues (compound eyes) were regulated by the circadian rhythms, light condition and physiological status of moths, confirming thatplayed an important role in circadian rhythms in compound eyes. Whether the circadian clock machinery is similar between compound eyes and the central nervous system need to be further studied.
; circadian clock; compound eye; photoreceptor; peripheral tissue; rhythm
10.3864/j.issn.0578-1752.2017.19.010
2017-05-12;接受日期:2017-07-15
国家自然科学基金(31572018)
闫硕,E-mail:yanshuo2011@foxmail.com。通信作者刘小侠,E-mail:liuxiaoxia611@cau.edu.cn
