PW11Fe/CS/CNTs/C复合膜电极的制备及在H2O2电化学传感器中的应用
2017-11-07王鑫章金明蒋晓薇韩雷云王莉莉黄燕霞游诚航华英杰王崇太
王鑫,章金明,蒋晓薇,韩雷云,王莉莉,黄燕霞,游诚航,华英杰,王崇太
(海南师范大学化学与化工学院, 海南 海口 571158)
PW11Fe/CS/CNTs/C复合膜电极的制备及在H2O2电化学传感器中的应用
王鑫,章金明,蒋晓薇,韩雷云,王莉莉,黄燕霞,游诚航,华英杰,王崇太
(海南师范大学化学与化工学院, 海南 海口 571158)
以壳聚糖(CS)为吸附剂和成膜剂,Keggin 型铁取代杂多阴离子PW11O39Fe(Ⅲ)(H2O)4-(PW11Fe)为电催化剂,碳纳米管(CNTs) 为导电助剂,集成吸附法和溶胶-凝胶法将PW11Fe固载在石墨电极表面制备了PW11Fe/CS/CNTs /C复合膜电极,并用电化学方法研究了该电极的电化学行为及其影响因素,同时研究了电极对H2O2还原的电催化活性及作为H2O2电化学传感器的可能性。实验结果表明,PW11Fe/CS/CNTs/C复合膜电极具有PW11Fe类似的电化学行为及对H2O2还原的电催化活性,用于H2O2的安培检测,电流响应灵敏度为0.30 mA·cm-2·(μmol/L)-1,检出限为7.3 μmol/L (S/N=3)。
Keggin型铁取代杂多阴离子;循环伏安;壳聚糖;电催化;碳纳米管

将PW11Fe修饰到电极表面的方法已有许多报道,如电化学沉积法[7-8]、吸附法[9-10]、电聚合法[6,11]、层-层组装法[12-13]和溶胶-凝胶法[14-15]等,这些方法根据研究目的的不同,各有千秋。本文集成吸附法和溶胶-凝胶法,利用壳聚糖(CS)作为吸附剂和成膜剂的特点[16-17], 通过CS聚阳离子和PW11Fe多阴离子的静电相互作用,将PW11Fe固定在石墨电极表面。为了提高导电性,在电极制备过程中加入碳纳米管(CNTs)。如此制备的PW11Fe/CS/CNTs/C复合膜电极,具有制备方法简单的优点。
1 实验部分
1.1 试剂和仪器
钨酸钠(SCRC国药集团化学试剂有限公司), 壳聚糖(上海伯奥生物科技有限公司),NaOH(天津市河北区海晶精细化工厂), 碳纳米管 (SWCNTs,-OH型,深圳市纳米港有限公司),丙酮(广州化学试剂厂), NaAc (广州文龙化工有限公司), HAc (天津市福晨化学试剂厂), HNO3(广州化学试剂厂), Na2HPO4(天津市化学试剂一厂), NaHSO4(广州化学试剂厂), Fe(NO3)3(国药集团化学试剂有限公司),φ=30% H2O2(西陇化工股份有限公司), 混合磷酸盐(pH=6.86和4.00,上海雷磁创益仪器仪表有限公司)。上述药品均为分析纯, Keggin型缺位杂多酸盐Na7PW11O39和铁取代杂多酸盐Na4PW11O39Fe(Ⅲ)按参考文献[18-19]的方法合成。实验用水为超纯水(电导率<5.0 μs·cm-1)。
电化学测量在CHI电化学工作站(60 D,上海辰华仪器有限公司)上进行,使用单室电解池,制备的PW11Fe/CS/CNTs复合膜电极为工作电极,Pt丝为辅助电极,Ag/AgCl (3 mol/L KCl)为参比电极。每次扫描前,溶液先充N2赶氧10 min。除非指明,实验温度为298 K。
1.2 PW11Fe/CS/CNTs/GCE复合膜电极的制备
室温下在0.15 mL 0.1 mol/L NaAc-HAc(pH=3.5)的缓冲溶液中加入0.005 g CS 和0.004 g CNTs,超声5 min,得到CS/CNTs溶胶。然后将CS/CNTs溶胶均匀滴涂到事先用1200#金相砂纸打磨好的石墨电极(Φ=3 mm,S≈0.071 cm2)表面,静置12 h,这时电极表面形成一层薄膜,将该电极在0.1 mol/L 的PW11Fe溶液中浸泡12 h后取出自然晾干,便得到PW11Fe/CS/CNTs/C复合膜电极。
2 结果与讨论
2.1 PW11Fe/CS/CNTS/C复合膜电极的电化学行为
图1(a)是PW11Fe/CS/CNTS/C复合膜电极在0.1 mol/L NaHSO4-Na2SO4(pH=2.2)溶液中的循环伏安图。从图中可以看到3对还原氧化波,分别归属于Fe(Ⅲ)/Fe(Ⅱ)电对和2个W(Ⅵ)/W(Ⅴ)电对的还原氧化反应。第二个W(Ⅵ)/W(Ⅴ)电对的还原峰由于和质子的竞争还原峰重叠而难于区分,导致峰形有些变形。此外,PW11Fe被CS膜固载在石墨电极表面后,阴极峰电位和阳极峰电位之差(ΔEp)与均相溶液相比明显增大,例如,对于PW11Fe/CS/CNTS/C复合膜电极的Fe波,ΔEp=355 mV,而PW11Fe在均相溶液中的ΔEp=(60~90) mV[1-3],表明固载后电子传递的可逆性变差,这是因为膜的存在增加了电阻的缘故。
为了考察PW11Fe/CS/CNTs/C复合膜电极的稳定性,将电极置于0.1 mol/L 的NaHSO4-Na2SO4(pH=2.2)溶液中连续扫描50周,发现阴极峰电流和阳极峰电流的大小以及波形几乎不变(图1(b)),表明电极具有较好的稳定性。
改变扫描速率,峰电流随扫描速率的增加而增加。取阴极峰电流和阳极峰电流对扫描速率作图,得到两条直线(图2),表明电极反应受表面过程控制。

图1 (a) PW11Fe/CS/CNTs/C复合膜电极在0.1 mol/L NaHSO4-Na2SO4 (pH=2.2)溶液中的循环伏安曲线扫描速率:10 mV·s-1,扫描范围:0.4~-0.85 V(b) 对Fe中心连续扫描50圈的循环伏安曲线Fig.1 (a) Cyclic voltammograms of the PW11Fe/CS/CNTs/C composite film electrode in 0.1 mol/L NaHSO4-Na2SO4 (pH=2.2) solution; scan rate: 10 mV·s-1, scan potential range: 0.4~-0.85 V (b) Cyclic voltammogram of Fe center for continuous scanning 50 cycles
2.2 CNTs剂量对PW11Fe/CS/CNTS/C循环伏安行为的影响
在电极的制备过程中加入CNTs的目的是为了提高膜的导电性。从图3可以看到,Fe(Ⅲ)/Fe(Ⅱ)电对的峰电流随着CNTs剂量的增加而增大。但当CNTs的剂量超过0.004 g时,峰电流反而下降,这是因为CNTs达到一定量时,在空间上阻止了CS对PW11Fe的吸附,使膜中电活性成分PW11Fe的含量减少,从而导致响应电流减小。因此,在本实验条件下,CNTs的最佳剂量是0.004 g。
2.3 溶液pH对PW11Fe/CS/CNTS/C循环伏安行为的影响
PW11Fe对质子比较敏感,因此合成时须严格控制反应体系的pH值。在均相水溶液中,PW11Fe稳定存在的pH值为2.0~8.0[3, 20]。但当溶液pH值改变时,Fe(Ⅲ)/Fe(Ⅱ)和W(Ⅵ)/W(Ⅴ)电对的还原氧化峰电位负移,且ΔEp增大,电子传递的可逆性变差,换言之,高浓度的H+由于与PW11Fe形成“紧密离子对”,在动力学上有利于促进电子的传递,提高电极过程的可逆性[20]。将PW11Fe固载到电极表面后,这种电化学行为依然可以观察到。如图4所示,当溶液的pH从2.2增大到7.0时,PW11Fe/CS/CNTs/C复合膜电极的还原氧化峰电位负移,导致在0.4~-0.8 V的电位范围内,只观察到Fe(Ⅲ)/Fe(Ⅱ)电对的还原氧化峰,且ΔEp明显增大。
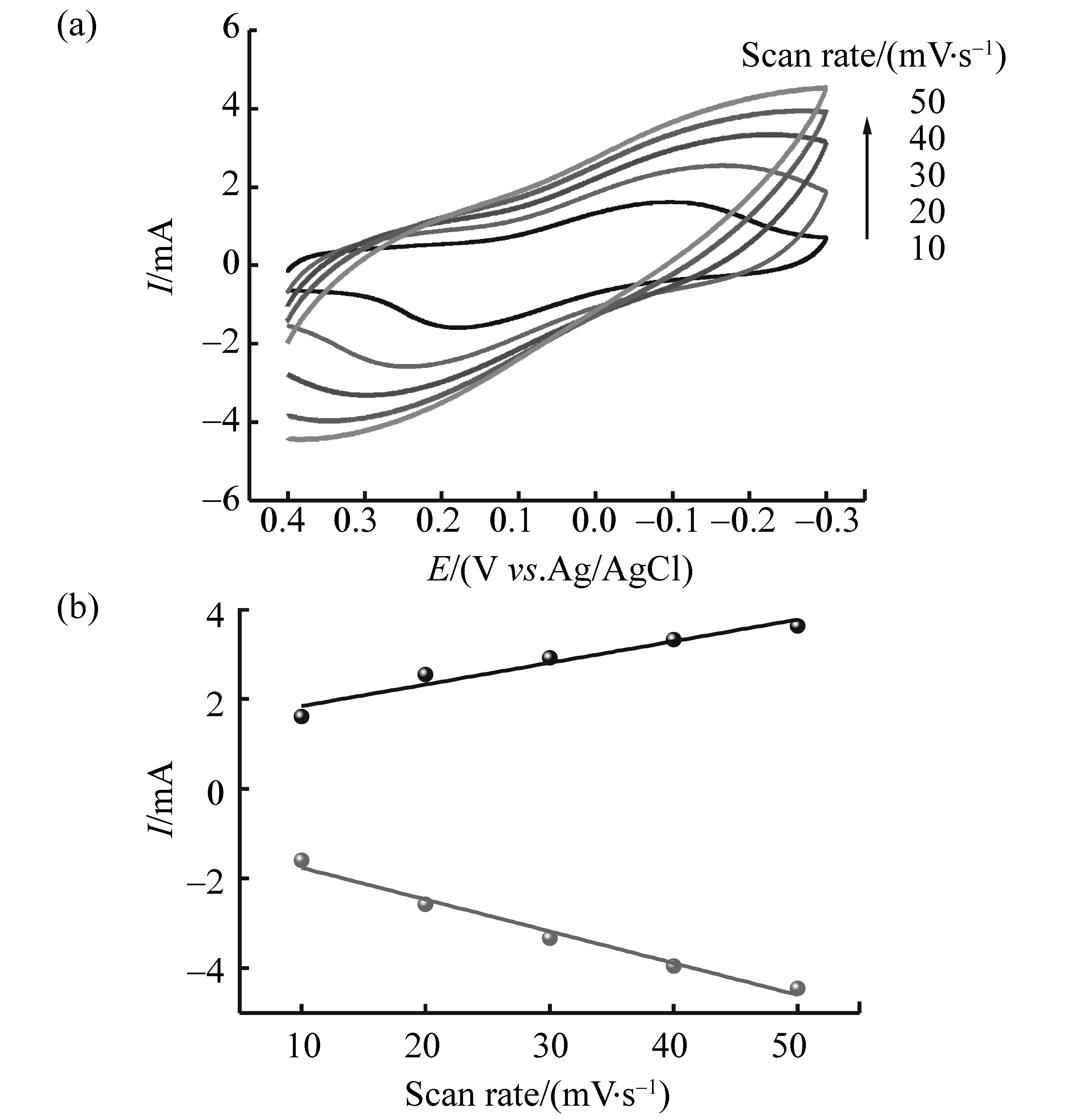
图2 (a)不同扫描速度下PW11Fe/CS/CNTs/C复合膜电极在0.1 mol/L NaHSO4-Na2SO4 (pH=2.2)溶液中的循环伏安曲线;(b)阴极峰电流和阳极峰电流与扫描速率的关系Fig.2 (a) Cyclic voltammograms of the PW11Fe/CS/CNTs/C composite film electrode at different scan rate in 0.1 mol/L NaHSO4-Na2SO4(pH=2.2) solution;(b) Relationship of peak current with scan rate

图3 不同CNTs剂量下PW11Fe/CS/CNTs/C复合膜电极在0.1 mol/L NaHSO4-Na2SO4 (pH=2.2)溶液中的循环伏安曲线Fig.3 Cyclic voltammograms of the PW11Fe/CS/CNTs/C composite film electrode at different dosage of CNTs in 0.1 mol/L NaHSO4-Na2SO4(pH=2.2) solution Scan rate: 10 mV·s-1
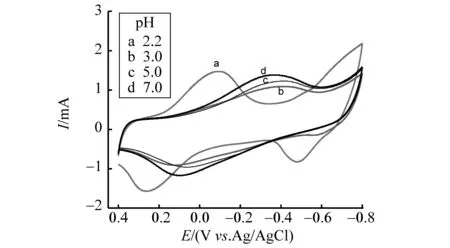
图4 PW11Fe/CS/CNTs/C复合膜电极在不同pH值的 NaHSO4-Na2SO4溶液中的循环伏安曲线Fig.4 Cyclic voltammograms of the PW11Fe/CS/CNTs/C composite film electrode in NaHSO4-Na2SO4 solution with different pH; Scan rate: 10 mV·s-1
2.4 PW11Fe/CS/CNTS/C复合膜电极对H2O2的电催化性能
PW11Fe/CS/CNTs/C复合膜电极能否用于检测H2O2首先取决于它对H2O2还原的电催化活性。如图5(a)所示,在PW11Fe/CS/CNTs/C复合膜电极体系中加入H2O2,响应电流明显提高,且随H2O2浓度的增加而增大,表明电极对H2O2的还原产生了电催化作用,因为H2O2在裸碳电极上的还原速率很慢,几乎观察不到H2O2的响应电流[3-4]。PW11Fe/CS/CNTs/C电极对H2O2还原的电催化作用源于其电活性组分PW11Fe中的Fe(Ⅲ)/Fe(Ⅱ)电对,实验表明,Fe(Ⅲ)很容易从碳电极上获得电子,并迅速传递给H2O2分子使之还原。换句话说,Fe(Ⅲ)的存在改变了H2O2分子在碳电极上发生还原反应的途径,降低了反应的活化能,从而导致H2O2还原速率的提高。从另一方面来看,H2O2分子很容易结合到PW11Fe/CS/CNTs/C电极的活性位点—Fe中心,并在还原电位下迅速清除来自电极的电子,产生去极化作用,从而降低了电子传递的阻力,使反应加速。电化学阻抗谱证实了这一点。从图5(b)中可以看到,在PW11Fe/CS/CNTs/C复合膜电极体系中加入H2O2,交流阻抗明显下降,且加入的H2O2越多,交流阻抗的下降越明显。
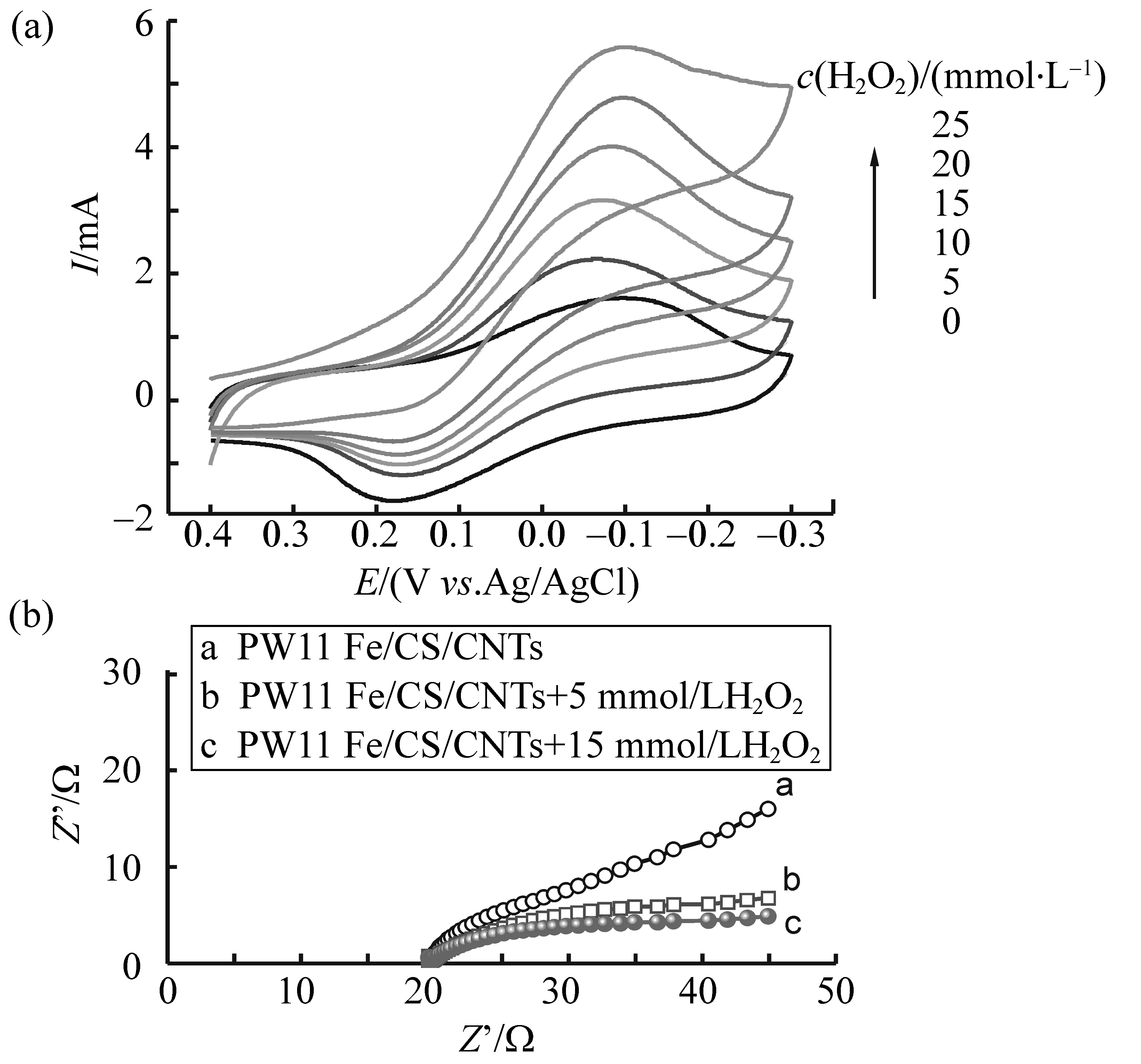
图5 (a)不同浓度H2O2下PW11Fe/CS/CNTs/C复合膜电极在0.1 mol/L NaHSO4-Na2SO4 (pH=2.2)溶液中的循环伏安曲线;(b) 不同浓度H2O2下PW11Fe/CS/CNTs/C复合膜电极的电化学阻抗谱Fig.5 (a)Cyclic voltammograms of the PW11Fe/CS/CNTs/C composite film electrode at different H2O2 concentration in 0.1 mol/L NaHSO4-Na2SO4 (pH=2.2) solution; Scan rate: 10 mV·s-1 (b) EIS of the PW11Fe/CS/CNTs/C composite film electrode at different H2O2 concentration in 0.1 mol/L NaHSO4-Na2SO4 (pH=2.2) solution; frequency =100 000~0.1 Hz, ΔE=5 mV, E=-0.08 V
鉴于PW11Fe/CS/CNTs/C复合膜电极对H2O2还原具有较高的电催化活性,我们用它进行了H2O2的安培检测实验,结果如图6(a)所示。从图中可以看到,当在插入PW11Fe/CS/CNTs/C复合膜电极的0.1 mol/L NaHSO4-Na2SO4(pH=2.2) 溶液中连续滴加5 μmol/L H2O2时,可以观察到迅速且稳定的电流响应,响应电流在5~35 μmol/L的范围内与H2O2浓度成线性关系(图6(b)),线性方程为
J(mA/cm2)=1.971 83 +0.304 12[c(μmol/L) ],
R=0.997 66
从直线的斜率可知,电极对H2O2响应的灵敏度为0.30 mA·cm-2·(μmol/L)-1。根据方程式(1)[21]估算电极对H2O2的检出限为7.3 μmol/L (S/N=3)。
cL=KSb/m
(1)
式中,K为信噪比,Sb为空白的标准偏差,m为直线的斜率。

图6 (a) 在PW11Fe/CS/CNTs/C复合膜电极体系中连续滴加5 μmol/L H2O2时的计时电流响应曲线(b)响应电流与H2O2浓度的关系支持电解质:0.1 mol/L NaHSO4-Na2SO4 (pH 2.2) 溶液Fig.6 (a) Chronoamperometric response of the PW11Fe/CS/CNTs/C composite film electrode towards H2O2 on successive addition of 5 μmol/L H2O2 Supporting electrolyte: 0.1 mol/L NaHSO4-Na2SO4 (pH=2.2) solution, Applied potential: -0.084 V (b) The relationship of response current with H2O2 concentration
3 结 论
以CS为吸附剂和成膜剂,CNTs为导电助剂,将PW11Fe固载到石墨电极表面制备的PW11Fe/CS/CNTs/C复合膜电极,对H2O2的还原具有明显的电催化活性,应用于H2O2的安培检测,电流响应灵敏,检出限低,因此有可能在H2O2电化学传感器方面获得应用。
[1] TOTH J E, ANSON F C. Electrochemical properties of iron(Ⅲ)-substituted heteropolytungstate anions [J]. Journal of Electroanalytical Chemistry, 1988, 256(2):361-370.
[2] TOTH J E, ANSON F C. Electrocatalytic reduction of nitrite and nitric oxide to ammonia with iron-substituted polyoxotungstates [J]. J Am Chem Soc, 1989, 20(25):2444-2451.
[3] WANG C, HUA Y, LI G, et al. Indirect cathodic electrocatalytic degradation of dimethylphthalate with PW11O39Fe(Ⅲ)(H2O)4-and H2O2in neutral aqueous medium [J]. Electrochimica Acta, 2008, 53(16):5100-5105.
[4] WANG C, HUA Y, TONG Y. A novel electro-fenton-like system using PWOFe(Ⅲ)(HO) as an electrocatalyst for wastewater treatment [J]. Electrochimica Acta, 2010, 55(22):6755-6760.
[5] 华英杰, 王崇太, 童叶翔, 等. Keggin型杂多阴离子PW11O39Fe(Ⅲ)(H2O)4-电催化降解硝基苯[J]. 化学学报, 2009, 67(23):2650-2654.
HUA Yingjie, WANG Chongtai, TONG Yexiang,et al. Electrocatatytic degradation of nitrgbbenzene with Keggin-type PW11O39Fe(Ⅲ)(H2O)4-[J]. Acta Chimica Sinica, 2009, 67(23):2650-2654.
[6] GASPAR S, MURESAN L, PATRUT A, et al. PFeW11-doped polymer film modified electrodes and their electrocatalytic activity for H2O2, reduction [J]. Analytica Chimica Acta, 1999, 385(1):111-117.
[7] NAKAYAMA M, KOMATSU H, OZUKA S, et al. Cathodic deposition of molybdenum and vanadium mixed oxyhydroxide films from V-substituted polymolybdophosphate [J]. Electrochimica Acta, 2006, 51(2):274-280.
[8] TANG Z, LIU S, WANG A E, et al. Preparation, structures, and electrochemistry of a new polyoxometalate-based organic/inorganic film on carbon surfaces [J]. Langmuir, 2000, 16(13):5806-5813.
[9] PRODROMIDIS M I, VELTSISTAS P G, EFSTATHIOU C E, et al. Amperometric detection of periodate using a graphite electrode modified with a novel α-Keggin-type silicotungstic acid salt and determination of ethylene glycol in antifreeze fluids [J]. Electroanalysis, 2001, 13(11):960-966.
[10] RONG C, ANSON F C. Unusually strong adsorption of highly charged heteropolytungstate anions on mercury electrode surfaces [J]. Analytical Chemistry, 2002, 66(19):3124-3130.

[12] JIANG K, ZHANG H, CURTIS S A, et al. Preparation and characterization of polyoxometalate/protein ultrathin films grown on electrode surfaces using layer-by-layer assembly [J]. Langmuir, 2008, 24(7):3584-3589.
[13] YANG G, GONG J, YANG R, et al. Modification of electrode surface through electrospinning followed by self-assembly multilayer film of polyoxometalate and its photochromic [J]. Electrochemistry Communications, 2006, 8(5):790-796.
[14] SONG W, CHEN X, JIANG Y, et al. Fabrication of a chemically modified electrode containing 12-molybdophosphoric acid by the sol-gel technique and its application as an amperometric detector for iodate [J]. Analytica Chimica Acta, 1999, 394(1):73-80.
[15] SONG W, LIU Y, LU N, et al. Application of the sol-gel technique to polyoxometalates: towards a new chemically modified electrode [J]. Electrochimica Acta, 2000, 45(10):1639-1644.
[16] HUA Y, WANG C, HUI D, et al. Fabrication, characterization and electrocatalytic properties of a solid modified electrode based on PW11O39Fe(Ⅲ)(H2O)4-, and chitosan [J]. Electrochimica Acta, 2011, 58(1):99-104.
[17] FENG Y, HAN Z, PENG J, et al. Fabrication and characterization of multilayer films based on Keggin-type polyoxometalate and chitosan [J]. Materials Letters, 2006, 60(13/14):1588-1593.
[18] BREVARD C, SCHIMPF R, TOURNÉ G, et al. Tungsten-183 NMR: A complete and unequivocalassignment of the tungsten-tungsten connectivities in heteropolytungstates via two-dimensional183W NMR techniques [J]. J Am Chem Soc,1983, 105(24):7059-7063.
[19] ZONNEVIJLLE F, TOURNÉ C M, TOURNÉ G F. Preparation and characterization of iron(Ⅲ)- and rhodium(Ⅲ)-containing heteropolytungstates. Identification of novel oxo-bridged iron(Ⅲ) dimers [J]. Inorg Chem, 1982, 21 (7):2751-2757.
[20] 王崇太. 过渡金属Cr(Ⅲ)和Fe(Ⅲ)取代磷钨杂多配合物的制备及电催化氧化性能研究 [D]. 广州:中山大学, 2008.
WANG Chongtai. Preparation and study of the electrocatalytic oxidation property of the transition metal Cr(Ⅲ)-and Fe(Ⅲ)-substituted phosphorus tungsten heteropoly complexes [D]. Guangzhou: Sun Yat-sen University, 2008.
[21] GAO R M, LIU H G. Discussion on the concept of detection limit: IUPC and other detection limits of the comprehensive discussion and experimental demonstration [J]. Chinese J Anal Chem, 1993, 21(10):1232-1236.
PreparationofPW11Fe/CS/CNTs/CcompositefilmelectrodeandapplicationinH2O2electrochemicalsensor
WANGXin,ZHANGJinming,JIANGXiaowei,HANLeiyun,WANGLili,HUANGYanxia,YOUChenghang,HUAYingjie,WANGChongtai
(School of Chemistry and Chemical Engineering, Hainan Normal University, Haikou 571158, China)
A composite film electrode PW11Fe/CS/CNTs/C was prepared by the integration of adsorption method and sol-gel method using chitosan as both an adsorbent and film-forming agent, Keggin type Fe(Ⅲ)-substituted heteropolyanion PW11O39Fe(Ⅲ)(H2O)4-(PW11Fe) as an electrocatalyst, carbon nano tubes (CNTs) as a conductivity improver in this paper. The electrochemical behavior and its influence factors of the as-prepared electrode were investigated by using the electrochemical method. The electrocatalytic activity towards H2O2reduction and the possibility also used to be an electrochemical sensor detecting H2O2were evaluated. The results showed that the PW11Fe/CS/CNTs/C composite film electrode possessed a similar electrochemical behavior with PW11Fe and a high electrocatalytic activity towards H2O2reduction. For detecting H2O2reduction. Applied to detect H2O2, the electrode exhibits a high sensitivity of 0.30 mA·cm-2·(μmol/L)-1and a detection limit of 7.3 μmol/L (S/N=3).
Keggin-type iron substituted heteropolytungstate; cycle voltammetry; chitosan;electrocatalysis;carbon nano tubes
O646
A
0529-6579(2017)05-0079-06
10.13471/j.cnki.acta.snus.2017.05.011
2017-04-05
国家自然科学基金( 21606061);海南省国际科技合作项目(KJHZ2014-08);海南省自然科学基金项目(20162022);海口市重点科技项目(2016-032);海南省科协青年科技英才创新计划项目(201502);全国大学生创新创业训练项目(201511658004)
王鑫 (1991年生), 男;研究方向电化学储能与光能转换材料;E-mail : 544317402@qq.com
华英杰(1966年生),女;研究方向:电化学储能与光能转换材料;E-mail:521000hua282@sina.com;王崇太(1962年生),男;研究方向:电化学储能与光能转换材料;E-mail: oehy2014@163.com
