基于柔性四羧酸配体构筑的两个镉配位聚合物的晶体结构及荧光性质
2017-11-01许春莺王吉超张裕平王键吉
刘 露 许春莺 李 英 王吉超 张裕平 王键吉
刘 露*,1许春莺2李 英1王吉超1张裕平*,1王键吉3
(1河南科技学院化学化工学院博士后研发基地,新乡 453003)
(2洛阳师范学院化学化工学院,洛阳 471934)
(3河南师范大学化学化工学院,新乡 453003)
通过溶剂热和水热合成的方法制备了2个Cd配位聚合物[Cd2(L)(DMF)1.5(H2O)2]n(1)和{[Cd(L)0.5(4,4′-bpy)(H2O)]·2H2O}n(2)(H4L=5,5′-(己烷-1,6)-双-(氧基)-二-间苯二甲酸)。 结构分析表明配合物 1 是一个(4,4)-连接的 sql拓扑网络,拓扑符号为{44·62}2。 配合物2是一个(4,4,4)-连接的三重穿插的bbf网络,拓扑符号为(66)(64·82)。配合物1和2都呈现出较好的热稳定性和荧光性质。
Cd;晶体结构;拓扑;荧光
Metal-organic frameworks (MOFs)have been quickly developed into an active research field and induced considerable attention from both academia and industry,because of their fascinating architectures and distinctive physical properties[1-12].The studies in this field have been focused on modifying the building blocks and regulating the assembled motifs for requisite products by choosing disparate organic ligands[13-14].Hence,the rationalization design of organic ligands are usually vital for the formation of fresh skeletons.As far as we know,many multidentate aromatic carboxylic acid ligands[15-17]have been extensively engaged to fabricate coordination complexes on account of their robustness[18].Based on the above mentioned,we designed a flexible ligand 5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalic acid (H4L)(Scheme 1)as a functional ligand.The H4L ligand can be deprotonated to the corresponding carboxylate species,which will admit it′s all kinds of coordination modes to the inorganic connectors[19].In H4L,the existence of theCH2nspacers and two methoxy groups between the two oxyisophthalic acid fragments will render the carboxyl groups bind with metal ions in different directions,which can offer more possibilities for the construction of versatile frameworks with untouchable functional properties[20-22].
Scheme 2 Schematic view of the versatile coordination modes of the L4-ligand
Given this,the reactions of Cdsalts and H4L were performed in the presence/absence of auxiliary ligand 4,4′-bipyridine (4,4′-bipy).Two different structural complexes,[Cd2(L)(DMF)1.5(H2O)2]n(1)and{[Cd(L)0.5(4,4′-bpy)(H2O)]·2H2O}n(2)have successfully been obtained.Their crystal structures and topological analyses will be represented and discussed.The thermal stabilities and fluorescent properties of 1~2 have also been investigated in detail.
1 Experimental
1.1 Materials and methods
All of the reagents and solvents were commercially practicable.H4L was synthesized in accordance with a revised procedure from reported literature[23].The Fourier transform infrared(FT-IR)spectra were registered on a Bruker-ALPHA spectrophotometer employing KBr pellets within 400~4 000 cm-1scale.Thermogravimetric analyses were implemented adopting a Netzsch STA 449C thermal analyzer with a 10℃·min-1heating speed in the air stream.Elemental analyses of C,H,and N were performed introducing a FLASH EA 1112 analyzer.Powder X-ray diffraction patterns were accomplished on a PANalytical X′Pert PRO diffractometer making use of Cu Kα1radiation(λ=0.154 06 nm)at 40 kV and 40 mA.The XRD patterns were collected in the range of 5°~50°.Diffuse reflectivity spectra of the solid samples were gathered by virtue of a Cary 500 spectrophotometer,which is equipped with a 110 nm diameter integrating sphere.The whole testing is from 200 to 800 nm utilizing barium sulfate(BaSO4)as a standard with 100%reflectance.The luminescence spectra for the powdered solid samples were measured at room temperature on a Hitachi 850 fluorescence spectrophotometer.The excitation slit and emission slit were both 2.0 nm.
1.2 Synthesis of[Cd2(L)(DMF)1.5(H2O)2]n(1)
A mixture of Cd(NO3)2·4H2O(0.030 8 g,0.1 mmol)and L(0.022 3 g,0.05 mmol)in DMF/H2O(VDMF/VH2O=2)was placed in a Teflon-lined stainless steel container(25 mL),and then the vessel was sealed and heated at 85℃for 48 h.After the mixture was cooled to ambient temperature at a rate of 5℃·h-1,colorless block-shaped crystals of 1 were obtained with a yield of 62% (based on Cd).Anal.Calcd.for C28H34Cd2N2O14(%):C,39.69;H,4.04;N,3.31.Found(%):C,39.72;H,4.03;N,3.34.IR(KBr,cm-1):3 415(m),3 073(w),2 943(m),2 866(w),1 658(s),1 542(s),1 454(m),1 384(s),1 318(m),1 260(m),1 132(w),1 042(s),933(w),887(w),812(w),778(s),733(s),673(w).
1.3 Synthesisof{[Cd(L)0.5(4,4′-bpy)(H2O)]·2H2O}n(2)
A mixture of Cd(NO3)2·4H2O(0.030 8 g,0.1 mmol),L(0.022 3 g,0.05 mmol),4,4′-bipy(0.007 8 g,0.05 mmol),and NaOH(0.2 mmol)in H2O(8 mL)was kept in a 25 mL Teflon-lined stainless steel vessel at 130℃for 72 h.After the mixture was cooled to room temperature at a rate of 5 ℃·h-1,colorless rodshaped crystals suitable for X-ray diffraction were obtained with a yield of 75%(based on Cd).Anal.Calcd.for C21H23CdN2O8(%):C,46.38;H,4.26;N,5.15.Found(%):C,46.34;H,4.28;N,5.17.IR(KBr,cm-1):3 349(m),3 130(w),2 871(m),1 669(m),1 606(s),1 549(s),1 416(s),1 386(s),1 324(w),1 275(m),1 221(m),1 125(s),1 045(s),886(w),812(s),778(s),733(w),693(w),630(s),518(w).
1.4 Crystal structural determination
The collections of crystallographic data of 1~2 were fulfilled at 293(2)K adopting a Rigaku Saturn 724 CCD diffractomer,which was equipped with Mo Kα radiation(λ=0.071 073 nm).Absorption corrections were enforced via utilizing multi-scan program.The data were corrected on the basis of Lorentz and polarization effects.The structures were solved by direct methods and refined by full matrix least square on F2using the SHELX-97 crystallographic software package[24].All of the non-hydrogen atoms were refined anisotropically.All the H atoms were positioned geometrically and refined using a riding model.Crystallographic data and structure refinement details for 1~2 are listed in Table 1.
CCDC:1497548,1;1497549,2.
2 Results and discussion
2.1 Structure description of
[Cd2(L)(DMF)1.5(H2O)2]n(1)
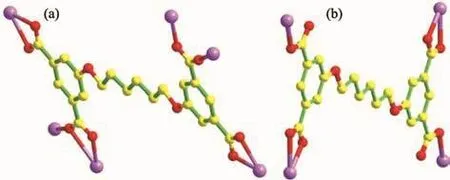
X-ray single-crystal diffraction analysis reveals that 1 crystallizes in triclinic space group P1,generating a 2D layer network.In the structure of complex 1,the two crystallographically independent Cdions,one L4-ligand,one and a half associated DMF molecules together with two coordinated water moleculesreside in the asymmetric unit.As shown in Fig.1a,the octahedral coordination around Cd1 is provided by four carboxylate oxygens(O3,O13A,O11Band O12B)from three different L4-moieties generating the square base,and the axial coordination is endowed by the DMF oxygen atom(O1)and O2 atom from coordinated water.The 50%ellipsoid figure of 1 is displayed in Fig.1b.The Cd1…O distance ranges from 0.221 8(4)to 0.238 7(4)nm.Cd2 also possesses a distorted octahedral coordination in which the square base is offered by the chelated coordination of the carboxylate oxygen atoms(O5 and O6)fromthe L4-ligand(Cd2-O5 0.225 7(4),Cd2-O6 0.247 8(4)nm)and the monodentate bridging coordination of the carboxylate oxygen atoms(O4C and O14D)originating from two separate L4-ligands(Cd2-O4C 0.221 8(4);Cd2-O14D 0.227 4(4)nm).Axial coordination comes from one DMF oxygen atom(O8)(Cd2-O8 0.232 8(5)nm)and one coordinated water oxygen atom(O7)(Cd2-O7 0.230 4(5)nm).Each L4-anion severs as a μ6-bridge to connect six Cdions through its four carboxylate groups,and the coordination mode of (κ2)-(κ1-κ1)-(κ2)-(κ2-κ1-μ2)-μ6for L4-is found in complex 1(Scheme 2a).The Cd1 ion and symmetry-related Cd2C(Symmetry codes:C:2-x,-y,2-z)ion are bridged by one μ2-η1∶η1bridging carboxylate group and one μ2-η2∶η1chelating/bridging carboxylate group to furnish a binuclear[Cd2(CO2)2]unit.Meanwhile,the Cd2 ion and symmetry-related Cd1C ion are also bridged by one μ2-η1∶η1bridging carboxylate group and one μ2-η2∶η1chelating/bridging carboxylate group to give a binuclear[Cd2(CO2)2]unit.Within the two bimetallic subunits,the intermetallic distance of Cd…Cd is both 0.393 6 nm.Further expansion of the structure through the L4-anions creates a ladder-like 2D layer propagating in ab plane(Fig.1c).The cooperative of two kinds of theπ…π stacking interactions(between the two aromatic phenyl ringswith a centroid-centroid distance of about 0.366 6 and 0.372 9 nm,respectively)join the adjacent 2D layers into a 3D supramolecular architecture(Fig.1d).

Table 1 Crystal data and structure refinement details for complexes 1~2

Fig.1 (a)Coordination environment of Cdion with hydrogen atoms omitted for clarity;(b)50%ellipsoid figure of 1;(c)Ladder-like 2D layer structure of 1 viewed along the c-axis;(d)3D supramolecular architecture of 1;(e)Schematic description of a(4,4)-connected sql topology net with point symbol of{44·62}2 for 1
Topologically,the above-mentioned bimetallic subunit can be considered as a 4-connected node since it links four L4-ligands,and the L4-ligand can be seen as a 4-connector by connecting four bimetallic subunits.Thus,the resulting structure of 1 is described as a(4,4)-connected net with its point(Schläfli)symbol of{44·62}2,which isreferred toas sql topology(Fig.1e).2.2 Structure description of{[Cd(L)0.5(4,4′-bpy)
(H2O)]·2H2O}n(2)
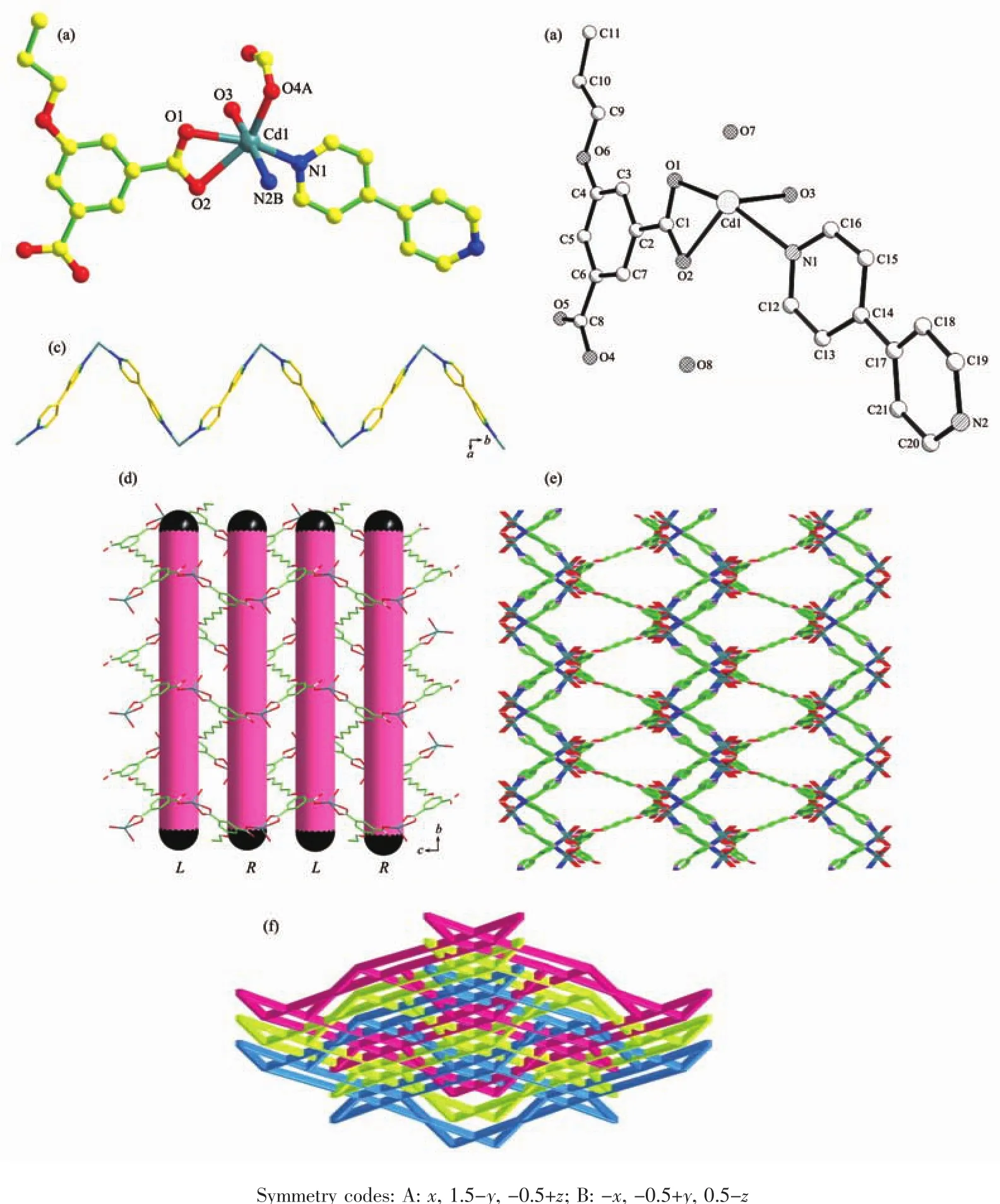
Fig.2 (a)Coordination environment of Cdion with hydrogen atoms omitted for clarity;(b)50%ellipsoid figure of 2;(c)View of the 1D Cd-4,4′-bpy zigzag chain along the c-axis;(d)View of the 2D Cd-L4-net built by alternately left-and right-handed helical chains parallel to the bc plane;(e)Schematic view of 3D structure of 2;(f)Schematic representation of the 3-fold interpenetrated topology net for 2
Complex 2 is a 3D framework with the monoclinic space group of P21/c.As depicted in Fig.2a,the asymmetric unit of 2 consists of one Cdion,half a L ligand,one 4,4′-bpy ligand,one coordinated water molecule and two lattice water molecules.The 50%ellipsoid figure of 2 is displayed in Fig.2b.Each Cdion issix-coordinated by three carboxylate oxygen atoms(O1,O2 and O4A)from two L4-anions,one oxygen atom(O3)from one coordinated water molecule and two nitrogen atoms(N1 and N2B)fromtwo 4,4′-bpy ligands,in turn,forming a distorted octahedral geometry.The O1,O2,O4A and N1 atoms comprise the equatorial plane,and the O3 and N2B atoms occupy axial positions.The Cd-O bond distances vary from 0.222 1(3)to 0.246 9(3)nm,while the Cd-N1 and Cd-N2B bond lengths are 0.230 8(3)and 0.234 3(4)nm,respectively.The bond angles around Cd1 range from 54.85(1)°to 169.65(1)°.
In 2,each 4,4′-bpy ligand bridges two Cdions to product a 1D zigzag chain with a Cd…Cd distance of 1.161 4 nm propagating along the c-axis(Fig.2c).The L4-anions connects four Cdcations,where two carboxylate groups take μ1-η1∶η1coordination modes,the other two carboxylate groups adoptμ1-η1∶η0fashions(Scheme 2b).The Cdions are bridged by the L4-anions to generate a 2D sheet with alternately arranged left-and right-handed helical chains along the a-axis(Fig.2d).The screw axes of these helices are all parallel to the b-axis,and the pitch is 1.442 9(3)nm.The Cd-L4-2D layers are pillared by 1D Cd-4,4′-bpy chain through sharing the common Cdions to result in the formation of a 3D framework(Fig.2e).Three identical nets are further interpenetrating with each other,leading to the formation of the final 3-fold interpenetrating network (Fig.2f).To get deep insight into the structure of 2,the topological analysis was carried out.The Cdion can be assigned to a 4-connected node,linking with two L4-anions and two 4,4′-bpy ligands.The L4-ligands connect four Cdions.Thus,the L4-ligands are considered as 4-connected linkers.Finally,the 3D framework presents a binodal (4,4)-connected bbf topology with the Schläfli symbol of(66)(64·82)(Fig.2f).
2.3 Effects of the coordination modes of the L4-anion and 4,4′-bpy ligand on the frameworks of complexes 1~2
On the basis of the structure descriptions of 1~2,we find that the L4-anion can adopt miscellaneous coordination modes,linking to six(complex 1)or four(complex 2)metal ions.The structural diversities of 1~2 can be attributed to the various coordination modes of carboxylate groups in the H4L molecule.For 1,the carboxylate group of L4-anion bond with the metal ions in a(κ2)-(κ1-κ1)-(κ2)-(κ2-κ1-μ2)-μ6fashion.This kind of coordination fashion in 1 brings about the formation of a ladder-like 2D layer Cd-L4-net.For 2,two carboxylate groups of L4-anion take μ1-η1∶η1coordination modes,while the other two carboxylate groups adopt μ1-η1∶η0fashions.This kind of coordination fashion in 2 afford a 2D Cd-L4-net with alternately arranged leftand right-handed helical chains.The N-donor ligands have also a far-reaching influence on the final structures of the complexes.In 2,The 4,4′-bpy bridge Cdions to generate a 1D infinite chain.The Cd-L4-2D layers are connected by Cd-4,4′-bpy 1D chain inducing a 3-fold interpenetrating 3D architecture.
2.4 XRD patterns and thermal analyses
The PXRD patterns of complexes 1~2 are shown in Fig.3.The PXRD patterns of the two complexes determined by experiment are in line with the simulated ones of single crystals,which indicates that each of the two complexes is pure phase.To appraise the stability of the coordination architectures,thermogravimetric analyses(TGA)of 1~2 were carried out(Fig.4).The TGA curve of complex 1 exhibits that it loses weight from 114 to 273℃,corresponding to the decomposition of two coordinated water molecules and one and a half associated DMF molecules(Obsd.17.35%;Calcd.17.74%).The decomposition of L4-is observed in the range of 375~527 ℃.A residue of CdO (Obsd.31.26%,Calcd.31.29%)is discovered.The TGA data of complex 2 display a two-step weight loss.The first weight loss of 3.75%is observed in the temperature range of 84~156 ℃,which corresponds to the loss of two lattice water molecules and one coordinated water molecule (Calcd.3.31%).The second step weight loss from 259~497 ℃ corresponds to the decomposition of L4-and 4,4′-bpy,accompanying with the CdO residue of 24.52%(Calcd.23.61%).
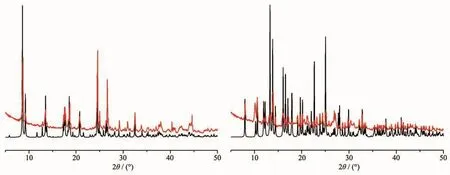
Fig.3 Experimental(top)and simulated(bottom)PXRD patterns of complexes 1~2
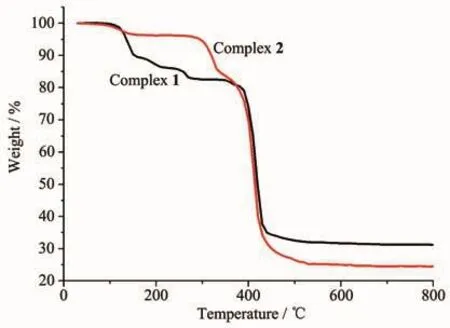
Fig.4 TGA curves of complexes 1~2
2.5 Diffuse-reflectance UV-Vis spectra and photoluminescence properties
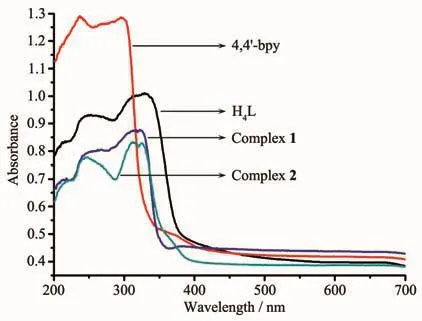
Fig.5 UV-Vis absorption spectra at room temperature for the free organic ligands and complexes 1~2
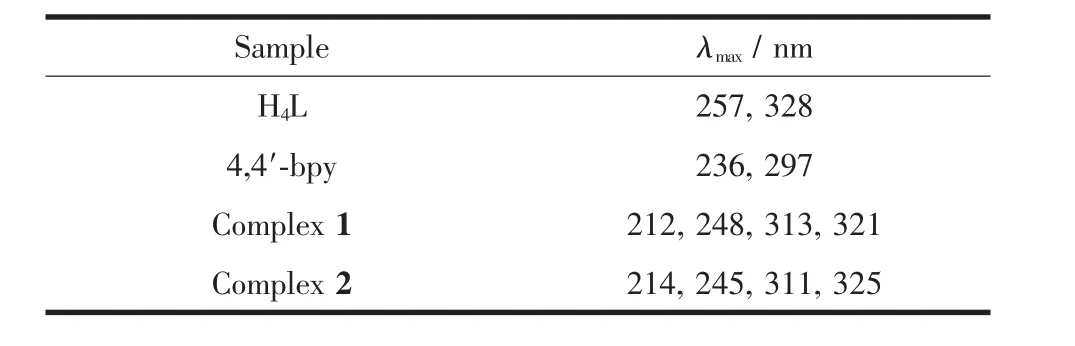
Table 2 Main absorption bands for the free organic ligands and complexes 1~2
As depicted in Fig.5 and Table 2,diffusereflectance UV-Vis spectrum of the ligand H4L demonstrates two intense absorption bands (λmax=257,328 nm)in the range of 200~400 nm,which are assigned to the typicalπ→π*transitions[25-27].Besides,the UV-Vis absorption spectra of 4,4′-bpy manifest 236 and 297 nm absorption bands in the scale of 200~400 nm.In accordance with the relevant literatures[28],the absorption bands of 4,4′-bpy belong to the π→π*transitionsof thearomatic rings.Complexes1~2 display the analogous absorption bands,which are also extremely similar to the peak of H4L.Besides,the lowest energy absorption band of 4,4′-bpy are larger than that of H4L in the energy level,which indicates that 4,4′-bpy might have less influence than the lowest energy absorption band of H4L through the coordination with the metal ion.For 1 and 2,their lowest energy absorption bands show smaller hypochromatic shift(7 and 3 nm,respectively),comparing with that of H4L.The situation may be attributed to the coordination of the H4L to the Cdion increasing the energy gap of the intraligand(IL,π→π*)transition.

Fig.6 Solid-state photoluminescent spectra of complexes 1~2 and free ligands
Moreover,the solid-state luminescences of complexes 1~2 and the free ligand H4L are also surveyed at ambient temperature.As reported before[29-30],the emission of 4,4′-bipy is 428 nm(λex=350 nm).The photoluminescent spectrum of H4L exhibits a weak emission at 429 nm (λex=330 nm,Fig.6),which is very close to that of 4,4′-bipy.For complex 1,excited at 321 nm,it gives rise to an emission band at 389 nm(Fig.6).As compared to the H4L ligand,a hypochromic shift(40 nm)is observed.The emission spectrum of complex 2(λem=412 nm,λex=319 nm)is blue-shifted(17 and 16 nm)corresponding to those of H4L and 4,4′-bipy,respectively.Illustrated by the Fig.6,the emission spectra of 1~2 are parallel to that of the H4L ligand,which also be mainly attributed to the intraligand emission of H4L[31].
3 Conclusions
[1]Zheng A X,Si J,Tang X Y,et al.Inorg.Chem.,2012,51:10262-10273
[2]Jiang H,Feng D,Liu T,et al.J.Am.Chem.Soc.,2012,134:14690-14693
[3]Suh M.P,Park H.J,Prasad T.K,et al.Chem.Rev.,2012,112:782-835
[4]Li J,Sculley R J,Zhou H C.Chem.Rev.,2012,112:869-932[5]Liu Y,Xuan W,Cui Y.Adv.Mater.,2010,22:4112-4135
[6]Nugent P S,Rhodus V L,Pham T,et al.J.Am.Chem.Soc.,2013,135:10950-10953
[7]Bauer C A,Timofeeva T V,Settersten T B,et al.J.Am.Chem.Soc.,2007,129:7136-7144
[8]Zhang C L,Zhang M D,Qin L,et al.Cryst.Growth Des.,2014,14:491-499
[9]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126-1162
[10]Cook T R,Zheng Y R,Stang P J.Chem.Rev.,2013,113:734-777
[11]Peng Y,Gong T,Cui Y.Chem.Commun.,2013,49:8253-8255
[12]Wang Y L,Fu JH,Wei JJ,et al.Cryst.Growth Des.,2012,12:4663-4668
[13]Kanoo P,Gurunatha K L,Maji T K.Cryst.Growth Des.,2009,9:4147-4156
[14]Hagrman P J,Hagrman D,Zubieta J.Angew.Chem.,Int.Ed.,1999,38:2638-2684
[15]Zhang X P,Zhou J M,Shi W,et al.CrystEngComm,2013,15:9738-9744
[16]Liu W L,Yu J H,Jiang J X,et al.CrystEngComm,2011,13:2764-2773
[17]Ordonez C,Fonari M,Lindline J,et al.Cryst.Growth Des.,2014,14:5452-5465
[18]Eddaoudi M,Moler D B,Li H,et al.Acc.Chem.Res.,2001,34:319-330
[19]Karmakar A,Goldberg I.CrystEngComm,2011,13:339-349
[20]Hawxwell SM,Espallargas G M,Bradshaw D,et al.Chem.Commun.,2007:1532-1534
[21]Perry JJ,Kravtsov V C,McManus G J,et al.J.Am.Chem.Soc.,2007,129:10076-10077
[22]Qu L L,Zhu Y L,Li Y Z,et al.Cryst.Growth Des.,2011,11:2444-2452
[23]Berl V,Schmutz M,Krische M J,et al.Chem.Eur.J.,2002,8:1227-1244
[24]Sheldrick GM.Acta Crystallogr.,Sect.A:Found.Crystallogr.,2008,A64:112-122
[25]He Y H,Feng Y L,Lan Y Z,et al.Cryst.Growth Des.,2008,8:3586-3594
[26]Zhang L Y,Zhang J P,Lin Y Y,et al.Cryst.Growth Des.,2006,6:1684-1689
[27]Zhang R B,Li Z J,Qin Y Y,et al.Inorg.Chem.,2008,47:4861-4876
[28]Ohkoshi S,Tokoro H,Hozumi T,et al.J.Am.Chem.Soc.,2006,128:270-277
[29]Censo D D,Fantacci S,Angelis F D,et al.Inorg.Chem.,2008,47:980-989
[30]Stadler M,Puntoriero F,Campagna S,et al.Chem.Eur.J.,2005,11:3997-4009
[31]Fang SM,Chen M,Yang X G,et al.CrystEngComm,2012,14:5299-5304
Two coordination polymers,[Cd2(L)(DMF)1.5(H2O)2]n(1)and{[Cd(L)0.5(4,4′-bpy)(H2O)]·2H2O}n(2),have been designed and synthesized by solvothermal or hydrothermal reactions of 5,5′-(hexane-1,6-diyl)-bis(oxy)diisophthalic acid ligand (H4L)with Cdmetal ions in the presence/absence of N-donor ligands.The structures of 1~2 have been determined by single-crystal X-ray diffraction analyses and further characterized via infrared spectra(IR),powder X-ray diffraction(PXRD),elemental analyses and thermogravimetric(TG)analyses.Complex 1 features a(4,4)-connected sql topology net with point symbol of{44·62}2.Complex 2 presents a trinodal(4,4,4)-connected 3D 3-fold interpenetrating bbf network with a Schläfli symbol of(66)(64·82).Additionally,complexes 1 and 2 present better thermal stabilities,and show different photoluminescence behaviors in the solid state.CCDC:1497548,1;1497549,2.
Cd;crystal structure;topology;fluorescence
O614.24+2
A
1001-4861(2017)10-1817-08
10.11862/CJIC.2017.233
LIU Lu*,1XU Chun-Ying2LI Ying1WANG Ji-Chao1ZHANG Yu-Ping*,1WANG Jian-Ji3
(1Postdoctoral Research Base,School of Chemistry and Chemical Engineering,Henan Institute of Science and Technology,Xinxiang,Henan 453003,China)
(2College of Chemistry and Chemical Engineering,Luoyang Normal University,Luoyang,Henan 471934,China)
(3Henan Normal University,Xinxiang,Henan 453007,China)
2016-12-19。收修改稿日期:2017-09-07。
河南科技学院高层次人才科研启动项目、河南省博士后科研项目、河南省高等学校重点科研项目(No.16A150007)和河南科技学院标志性创新工程项目(No.2015BZ02)资助。
*通信联系人。 E-mail:liululiulu2012@126.com,beijing2008zyp@163.com
