尖晶石型氧化物催化剂与金属-空气电池
2017-09-16雷凯翔孙洪明程方益
陈 祥,雷凯翔,孙洪明,程方益,陈 军
尖晶石型氧化物催化剂与金属-空气电池
陈 祥,雷凯翔,孙洪明,程方益,陈 军
(南开大学化学学院,先进能源材料化学教育部重点实验室,天津化学化工协同创新中心,天津 300071)
金属-空气电池具有高能量密度,是极具吸引力的电化学能量储存与转化器件,阴极反应动力学缓慢是制约其性能的关键因素之一,需要使用高效催化剂。本文简要介绍金属-空气电池的结构和工作原理,并综述近年来尖晶石型金属氧化物阴极催化剂的研究进展。
尖晶石;电催化;氧还原;氧析出;金属空气电池
电池在开发新型可再生能源、实现低碳经济的过程中起着重要作用[1-3]。作为高效储能器件,锂离子电池在便携式电子市场已得到广泛应用,但是能量密度不高(理论极限值约400 W·h/kg),限制了其在电动汽车、智能电网和可再生能源大规模储能体系中的应用[4]。金属-空气电池具有开放的电池结构,由于能量密度高、成本低和环境友好等优点受到广泛关注[5-7],其中锂-空气电池理论能量密度远远超过商业化锂离子电池{Cheng, 2012 #6;Lee, 2011 #8},应用前景广阔[8-9]。
金属-空气电池的研究已取得较大进展,然而与其它电池技术一样,仍面临一系列科学和技术问题,包括阳极利用率低、阴极动力学过程缓慢、过电位高、可逆性差等,其实际能量密度与能量效率较低[10-14]。开发廉价高效的阴极催化剂是金属-空气电池领域的研发热点和重点之一。尖晶石型过渡金属氧化物是一类非贵金属阴极催化剂,因具有储量丰富、价格低廉以及结构和价态多变等特点而广受关注。通过结构、组分、物相、价态、形貌和缺陷的调控,有望使其获得与贵金属催化剂相媲美的催化活性[15-23]。本文简要介绍了金属-空气电池的结构原理,重点论述近年来尖晶石材料作为阴极催化剂所取得的研究进展。
1 空气电池的种类、结构与原理
1.1 金属-空气电池的种类
目前金属-空气电池研究主要集中在锂-空气电池[24-32]、钠-空气电池[33-35]、锌-空气电池[36-38]、镁-空气电池[39-41]以及铝-空气电池[42-45]。铁-空气电池也被报道[46-47],但由于放电电压和质量比能量都较低、开发使用成本相对于其它体系要高,所以这类空气电池目前研究相对较少。图1比较了几种典型金属-空气电池与碱性锌锰电池、锂离子电池的理论电压、质量能量密度和体积能量密度,表1为对应不同种类电池的具体理论参数。其中金属-空气电池的质量能量密度和体积能量密度的数据来源于文献[6,9,36]。Zn-MnO2和Li离子电池(负极:石墨,正极:LiFePO4)的相关数据来源于文献[126]。可以看出,金属-空气电池特别是锂-空气电池,能量密度显著高于常见的锌锰一次电池与可充锂离子电池,其发展空间巨大。
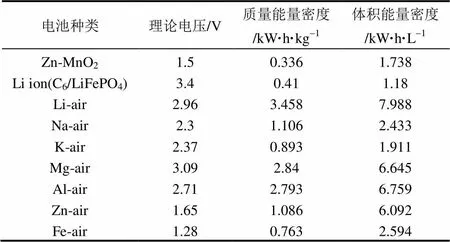
表1 不同种类电池的具体理论参数
1.2 金属-空气电池的结构与原理
金属-空气电池是由金属阳极、空气电极、隔膜和电解液组成,如图2所示。空气电极由活性催化层、集流体和气体扩散层三部分构成,氧还原反应(ORR)和氧析出反应(OER)发生在空气电极结构中活性催化层的三相界面上,催化活性层通常由导电剂、黏接剂(聚四氟乙烯、聚乙烯等)和催化剂组成。集流体一般选择具有高导电性的金属网,提供空气进入电池内部的孔道和用于电流收集的导电通路。气体扩散层可以使氧气均匀地分散到电池内部界面上,兼具防水透气、防止电解液泄露的功能。
对于可充金属-空气电池,在放电过程中,金属负极被氧化为离子形态并释放出电子,同时氧气扩散到空气电极并接受电子被还原成含氧物种。由于氧气在液相中的溶解度较低,因此游离的金属离子会与含氧物种在空气电极结合形成金属氧化物。充电时,这个过程是放电过程的逆向反应,金属会在阳极析出,氧气在阴极析出。在整个充放电过程中,氧在电解液中的传输对电池的性能具有决定作用。不同种类的金属-空气电池涉及不同的电化学反应和产物,其工作原理取决于所选择的金属、电解液和催化材料。本节中,空气电池的工作原理将从水系和非水体系电解液角度进行阐述。
金属阳极在酸性电解液中会发生剧烈反应,产生严重的腐蚀或析氢现象。此外,大部分阴极电催化材料在强酸环境下不稳定,这使得酸性电解质不适合实际应用。因此,金属-空气电池通常采用碱性电解液[7]。
在水系金属-空气电池中,氧气一般被还原为氢氧负离子。因此在水系电解液中金属-空气电池的工作原理如下[48]:
正极(空气电极):
O2+2H2O+4e-4OH-(1)
负极(金属电极):
MM++e-(2)
式中的M为金属,为金属氧化过程中价态的变化。在电池中发生的总反应为:
4M+O2+2H2O4M(OH)(3)
其中,阴极氧还原过程涉及一系列多步电子转移和复杂的含氧中间产物[49-51]。
O2+2H2O+4e-4OH-(0=0.40 V) (4)
O2+H2O+2e-HO2-+OH-(0=0.07 V) (5)
HO2-+H2O+2e-3OH-(0=0.87 V) (6)
2HO2-2OH-+O2(7)
目前一般认为在水系碱性电解液中氧还原有两种途径:一类为四电子反应机理,由氧气直接还原为氢氧根离子,中间不生成可检测的过氧化物;另一类为二电子反应机制,产物中除了氢氧根离子外,还包含过氧化氢中间产物的生成,这不仅降低了氧还原反应效率,产生的过氧化物对电池隔膜有一定破坏作用从而影响电池性能。如果这一系列反应中间产物的还原进行得足够快,产生的过氧化氢会迅速被还原为氢氧根离子,这种情况也可以认为是准四电子进程[52-54]。
水系电解液体系具有廉价、来源广泛和高离子电导率等优点。但是,由于受到析氢和析氧反应的影响,水系电解液的电压窗口较小,导致金属-空气电池实际电压远低于理论电压。相较于水系电解液,非水电解液能有效减少水系电解液对金属锂的腐蚀,增加电池的安全性。在非水电解液中金属-空气电池(以锂-空气电池为例)的工作原理如下:
正极(空气电极):
2Li++O2+2e-Li2O2(0=2.96 V,Li/Li+)(8)
4Li++O2+4e-Li2O(0=2.91 V,Li/Li+)(9)
负极(金属电极):
LiLi++e-(10)
与水系电解液氧还原反应进程相似,在非水系电解液中氧还原进程也是逐步发生的。对于非水系的进程也有不同的反应机理被提出。LAOIRE等[55-56]提出以下可能的阴极反应:
O2+Li++e-LiO2(11)
2LiO2Li2O2+O2(12)
LiO2+Li++e-Li2O2(13)
Li2O2+2Li++2e-2Li2O (14)
上述反应进程受溶剂和电解质影响很大,当电解液中含有较大的阳离子时,阴极进行O2/O2-单电子可逆反应。电解液中阳离子为Li+时,O2将发生不可逆或是准可逆过程,被还原成O2-、O22-和O2-等产物,电解液中阳离子对反应进程的影响可以用Pearson软硬酸碱理论和Li+溶剂复合物的相对稳定性来解释。Li+、O2-和O22-分别表现出路易斯强酸、中等弱碱和路易斯强碱的性质,形成的Li+(溶剂)复合物会降低Li+的酸性,这与溶剂的给电子能力有关。溶剂的给电子能力越强,形成的Li+(溶剂)-O2-复合物就越稳定,有利于LiO2的形成,对于给电子能力弱的溶剂,O2-会快速分解成O22-或进行完全的电化学过程被还原为O2-。此外,催化剂表面的吸附氧键能大小对阴极反应路径也有影响。YANG 等[57]认为氧分子首先接受一个电子形成超氧自由基,该自由基与Li+结合生成LiO2,吸附在催化剂表面。当催化剂表面的吸附氧键能较弱时,LiO2能够快速得到电子还原为Li2O2,而当吸附氧键能较强时,电子转移受到阻碍,LiO2会更多地还原为Li2O。对于非水系电解液,由于金属-空气电池反应产物金属过氧化物和超氧化物不溶于有机电解液,因此长时间放电后会在空气电极孔道中进行沉积,沉积的产物会降低电池的导电性,增加过电位。同时沉积物会导致O2通路孔道堵塞并覆盖催化剂活性位点,从而影响电池性能和循环寿命。近年来,除了锂-空气电池外,钠-空气电池、镁-空气电池等可逆二次电池在非水系中的充放电机理也有大量报道。目前,对钠空气电池充放电机理的认识仍然存在较大争议,有两种机理被普遍认可:过氧化钠路径和超氧化钠路径[58-59]。在非水系镁-空气电池中,金属镁的反应活性非常低,因此电解质通常为中性或碱性电解质,其工作原理与其它水系金属-空气电池类似[60]。深入了解电池充放电反应机理对发展高性能金属-空气电池具有重要的指导意义。
2 尖晶石型氧化物催化剂
正极(空气电极)是影响金属-空气电池性能的关键因素,氧还原反应和氧析出反应的过电势严重降低了金属-空气电池的输出功率和循环效率,使用空气电极催化剂是改善反应动力学、提升性能的必要手段。尖晶石型氧化物由于在碱性电解液中具有良好的催化活性和稳定性,兼具资源丰富、价格低廉的优点,成为氧还原反应和氧析出反应最具应用前景的非贵金属催化剂之一[61-62],获得越来越多的关注。
2.1 尖晶石组分和结构
尖晶石型金属氧化物是由两种或多种金属元素组成的氧化物,通式为AB2X4,其中X多为-2价的元素,A一般为+2价或+4价,B相对应地为+3价或+2价。本节将以常见的尖晶石型催化剂NiCo2O4为例做详细介绍。其中Ni2+阳离子处于由O2-阴离子构成的四面体中心,Co3+阳离子处于O2-构成的八面体空隙,O2-组成多面体的顶点[63-64]。
尖晶石存在不同阳离子的分布现象,根据阳离子分布情况可以将尖晶石分为三类[65-67],以NiCo2O4为例:Ni2+都填充在四面体空隙,而Co3+都填充在八面体空隙时,该结构的尖晶石称作正尖晶石;当Ni2+占据八面体空隙,而Co3+同时占据四面体空隙和八面体空隙,该结构尖晶石称作反尖晶石;当Ni2+、Co3+两种离子对四面体空隙和八面体空隙没有选择性占据时,它们既填充在四面体空隙内,又填充在八面体空隙内,就形成无序的混合尖晶石构型。为了准确区分这些尖晶石,通式可以写成A1-B(AB2-)X4,式中括号前的离子位于四面体位置,而圆括号中的离子位于八面体位置=0时,为正尖晶石,=1时,为反尖晶石,0<<1时为混合尖晶石。图3显示了集中典型尖晶石晶体结构图,尖晶石中阳离子分布由几种因素共同决定,如阳离子半径,阳离子之间的库仑相互作用,晶体场效应和八面体择位能(OSPF)等。根据晶体场理论,对OSPF的定义为八面体场和四面体场之间的晶体场稳定能差,OSPF的绝对值更高的阳离子更倾向于占据八面体间隙[68]。
(a)
(b)
2.2 尖晶石催化剂的电催化反应路径
氧分子的吸附和还原是整个氧还原反应的速率控制步骤。根据分子轨道理论,氧分子的吸附实际上是氧分子和催化剂活性中心空轨道的相互重叠,吸附方式分为三种:端基式、桥式或侧基式。此外,氧还原反应也是一种结构敏感型反应,其途径和机理与催化材料密切相关[69-70],氧分子在催化剂的活性部位吸附方式的不同,取决于催化剂的晶体结构、表面结构和结合能[71-73]。对于金属氧化物,其表面的阳离子除了与氧气分子配位外,还会与H2O中的氧发生配位。在反应过程中,活性中心M自身发生还原反应得到电子使表面的含氧配体质子化,从而实现OH-类物质的形成。M-OH-进一步与在氧化物表面通过端基式吸附的氧分子反应[9]。因此氧还原反应在氧化物表面的反应路径可能是[51]:
2M+-O2-+2H2O+2e-2M(m-1)+-OH-+2OH-(15)
O2+e-O2,ads-(16)
2M(m-1)+-OH-+O2,ads-+e-2M+-O2-+2OH-(17)
或者:
M+-O2-+H2O+e-M(m-1)+-OH-+OH-(18)
O2+e-O2,ads-(19)
M(m-1)+-OH-+O2,ads-M+-O-O2-+OH-(20)
M+-O-O2-+H2O+e-M(m-1)+-O-HO-+OH-(21)
M(m-1)+-O-OH-+e-M+-O2-+OH-(22)
M+-O-O2-+H2O+e-M(m-1)+-OH-+HO-(23)
其中反应(17)和(23)是氧还原过程的速控步[74]。
在碱性溶液中氧析出反应的路径开始于溶液中OH-离子在催化剂表面的吸附和排出,溶液中的OH-会与表面吸附的OH物种反应,形成H2O和吸附的O*并产生电子。表面吸附的O*进一步与溶液中的OH-反应形成OOH,继续与额外的OH-阴离子反应,形成吸附的O2和H2O,最终吸附的O2脱附。可能的反应路径如下[75-76]:
M+OH-M-OH+e-(24)
M-OH+OH-M-O*+e-+H2O (25)
M-O*+OH-M-OOH+e-(26)
M-OOH+OH-M-O2+e-+H2O (27)
M-O2M+O2(28)
其中反应(26)为影响氧析出反应速度的控制步骤。深入了解氧还原/氧析出反应机理对高活性催化剂的设计与合成具有重要的指导意义。
2.3 尖晶石催化剂研究现状
目前,尖晶石型催化剂的研究主要集中在通过优化制备方法来得到特定的形貌、组分和结构以探究其构效关系,进而探索相应的策略提高材料的氧还原/氧析出性能。综合起来可以归纳为三类:①设计特殊的微纳结构,提高材料的比表面积,增加活性位点的暴露程度;②调控本征组分、物相和缺陷,调整界面电子排布,优化反应过程中与氧分子的相互作用;③与碳基底复合,提高材料导电性。
2.3.1 调控微纳结构
与块体材料相比,纳米材料具有更高的比表面积和更多的催化活性位点。因此,研究人员通过采用不同的合成方法得到不同维度、形貌和尺寸的尖晶石型纳米材料催化剂,从而实现对催化剂活性的提高。CHEN等[77]通过水热法合成的一维NiCo2O4纳米线阵列表现出优异的氧析出性能[图4(a),4(d)],电流密度为10 mA/cm2时,过电位约0.32 V。LIN团队[16]通过共沉淀和热分解的方法得到核-环结构的NiCo2O4二维纳米片[图4(b)],TEM能谱表征结果显示Co在纳米片的核区富集,边缘环区镍钴比例为1∶2。该材料展现出优异的氧析出性能,电流密度为100 mA/cm2时过电位为0.315 V(. SCE)。此外,LIN团队[78]报道了一种分级结构的三维中空微米立方体NiCo2O4[图4(c)],得益于其独特的三维分级中空结构提供的大量催化活性位点,其具有1.46 V的氧析出起始电位(起始电位定义为 电流密度=1.0 mA/cm2时的电位),电流密度为 10 mA/cm2时,电位为1.52 V。
多孔纳米结构具有高比表面积和短扩散传质路径,这有利于提高催化活性[79-80]。CHEN等[81]利用微波辅助水热法合成了具有内部互连结构的介孔Co3O4纳米片。得益于其相互连接的介孔结构,材料展现出较商业化材料更低的过电位0.452 V(. Ag/AgCl)和更小的塔菲尔斜率48 mV/dec。JIAO课题组[82]通过选择性刻蚀Mg掺杂的介孔Co3O4得到了具有超高比表面积的介孔Co3O4纳米片,表现出优异的氧析出性能。
尖晶石的尺寸对其催化性能也有较大影响。SHI等[83采用热注入升温的方法,通过改变反应温度制备了不同尺寸(2.0 nm、3.9 nm、5.4 nm)的CoMn2O4量子点,适宜的尺寸调节材料的能带和表面吸附氧的量,从而影响体系中载流子的浓度,其中3.9 nm的CoMn2O4/CNT表现出优异的电催化性能(OER:10 mA/cm2电流密度时,电位为1.61 V;ORR:半波电位为0.89 V,塔菲尔斜率为126 mV/dec)。
此外,催化剂的组分相同但暴露的晶面不同,其催化活性也会存在一定差异。这主要是因为不同的晶面其原子和电子的排列不同,从而导致氧气分子的吸附方式不同,进而引起电化学性能的差异。SWITZER课题组[84]通过直接在单晶金的(100)晶面回流电解液的方法得到了特定晶面的Co3O4薄膜,开辟了尖晶石型材料不同暴露晶面与催化活性关联性研究的先河。SU等[85]合成了具有不同暴露晶面的单晶Co3O4纳米晶(纳米立方体,准八面体、纳米片、六边形薄片),不同的暴露晶面展现出的氧析出催化活性顺序为:(111)>(110)>(112)>(100)。同时,根据密度泛函理论计算得到的Co3O4不同晶面的表面能大小的顺序与其催化活性顺序一致,高表面能晶面具有大量位于台阶和扭结位置具有高反应活性的低配位原子,有利于离子在表面和内部的快速传导,因此具有高的催化反应活性,验证了其实验结果。与此同时,CHEN等[86]还发现Co3O4的(111)晶面具有更高的氧析出活性而(100)晶面材料则具有较好的稳定性。此外,也有不少研究从理论计算的角度对晶面和催化活性之间的关系进行研究解释。MONTOYA等[87]采用密度泛函理论的GGA+U框架,以Co3O4表面自由能评价(110)、(111)和(100)不同晶面的热力学稳定性,从而预测其催化活性。结果显示在任意给定的温度和氧分压下,(100)晶面最稳定,这说明(100)晶面的催化活性不高。LI等[88]通过密度泛函模拟的方法对比了β-MnO2(211)和(2-2-1)氧还原催化活性。构建的二氧化锰(211)和(2-2-1)表面有相同数量的配位和活性位点。结果显示(2-2-1)面具有更高的HOMO能级和导电性。这表明(2-2-1)的HOMO能级和氧气分子的LUMO能级之间的能带宽度更小,更有利于氧气和β-MnO2之间的电子传递。同时,OH在(2-2-1)上的吸附较弱有利于OH的脱附并为后续反应的进行提供活性位点。因此β-MnO2(2-2-1)具有更好的氧还原催化活性。理论计算研究可为高效催化剂材料的设计合成提供指导。
2.3.2 调控本征组分、晶相和缺陷
对于含有多种变价过渡金属元素的尖晶石催化剂,其组成和结构难以实现同时调控。以钴锰氧尖晶石为例,因三价锰离子的姜-泰勒效应会使锰氧正八面体拉伸为扭曲八面体,钴锰比例对晶体对称性有很大影响,这为同时控制这类材料的组成和晶相,进而研究其与催化活性之间的构效关系带来了挑战。LI等[89]报道了一种“氧化-沉淀晶化”方法,在温和条件下快速合成MMn3-O4(M为二价金属如Co、Mg、Zn),实现了对组分和晶相的调控并揭示其组成、结构与电催化氧还原性能的构效关系。结果表明,立方相高锰含量钴锰氧尖晶石氧还原性能最佳,这归因于其高导电性、Mn混合价态以及适宜的表面氧吸附能。
WEI课题组[90]通过调控CoFeO尖晶石中的铁含量,使其结构由正尖晶石转变为反尖晶石从而显著提高了钴铁尖晶石的催化性能。反尖晶石结构的转变,使不同的金属原子同时存在于MO6八面体中,这种“相异作用”调节了氧的吸附能并使 O—O键变长,加速了O—O键的活化和断裂。其中,(Co)[FeCo]O4反尖晶石在碱性电解液中的氧还原催化性能甚至可以与商业碳载铂相媲美。
催化剂的催化活性在很大程度上取决于氧气分子在催化剂表面的吸附能,缺陷(阳离子缺陷和氧缺陷)的存在能调节材料的电子结构,改善本征导电性,促进氧在催化剂表面的吸附,从而加速氧还原/氧析出进程。低温或室温得到的尖晶石更容易形成缺陷。CHENG等[91]利用“还原-转晶”法以无定形的MnO2为前驱体在室温条件下首次合成出一系列具有高催化活性的立方相和四方相的CoMn3-O4。合成的Co-Mn-O纳米晶因为保留了前驱体的形貌而具有较高的比表面积和大量的缺陷。实验结果表明,立方相的Co-Mn-O尖晶石其氧还原活性要优于四方相,而氧析出活性却低于四方相。理论计算结果显示,性能的晶相依赖特性是由于两种相的表面氧吸附能存在一定的差异,立方晶相上缺陷对氧气分子的吸附(吸附能Co缺陷-2.42 eV、Mn缺陷-2.23 eV)要比四方相(吸附能Co缺陷-0.26 eV、Mn缺陷-0.6 eV)更容易,同时单位表面活性位点密度存在差异。
XIE课题组[92]以NiCo氢氧化物为前驱体采用局部化学转换的方法制备了富含氧缺陷的NiCo2O4超薄纳米片。氧缺陷的存在降低了H2O在催化剂表面的吸附能,增强了活性位点的催化活性,而其超薄的纳米片结构则会提供更多的活性位点,从而使NiCo2O4超薄纳米片表现出优异的氧析出性能,在电位为0.8 V(. Ag/AgCl)时电流密度达到 285 mA/cm2,过电位减小至0.32 V。
(a)
(b)
(c)
(d)
图8 (a)水分子在尖晶石结构表面的吸附和含氧空位的NiCo2O4的部分电荷密度示意图;(b)富含氧空位的NiCo2O4超薄纳米片Co 2p和O 1s的高分辨XPS谱图;(c)不同NiCo2O4样品的极化曲线,插图为起始电位附近区域的放大及(d)对应样品的塔菲尔曲线[92]
Fig.8 Schematic illustration (a) of the adsorption of H2O molecules on the spinel structure and the partial charge density of NiCo2O4with oxygen vacancies; High-resolution Co 2p spectra and O 1s spectra (b) of the samples; Polarization curves (c) of various NiCo2O4samples, and inset shows enlargement of the region near the onset potential. Corresponding Tafel plots (d) of the samples[92]
2.3.3 与导电碳基底复合
与常见的金属氧化物相似,尖晶石催化剂导电性较差,不利于氧还原/氧析出过程中的电子转移。与不同类型的导电碳材料[93-96]复合来提高催化剂的导电性和结构稳定性是目前常用的方法之一。一维碳原子具有定向电子传递途径,有利于电子的快速传导。HAN等[97]通过共沉淀再硫化的方法制备了NiCo2S4/CNT、NiCo2S4/N-CNT和块体的NiCo2S4复合材料,其中NiCo2S4/N-CNT的性能最佳,展现出接近商业碳载铂的催化性能。这是由于尺寸效应使催化剂具有大的表面积,N的掺杂对表面电子排布的调节以及复合材料存在的协同效应。
石墨烯材料由于其表面积大、高机械稳定性和化学稳定性高及导电性突出受到广泛关注。具有协同催化作用的杂原子掺杂的碳可以进一步提高整体性能和表面化学性质。DAI课题组[98]发现与单纯的Co3O4以及氮掺杂的石墨烯和二者的物理混合物相比,原位生长的Co3O4纳米颗粒/N-rGO复合材料具有更好的氧还原/氧析出性能。这是由于原位生长得到的复合材料具有更强的键合以及协同催化效应。
QIAO课题组[99]以非均相反应法得到了具有分级结构的氮掺杂还原石墨烯/NiCo2O4复合材料,其分级多孔结构构筑了三维导电网络,不仅提供大量的催化活性位点,也提高了催化剂的导电性。氧析出测试结果显示,电流密度为5 mA/cm2时过电位为0.373 V,塔菲尔斜率为156 mV/dec。
(a)
(b)
(c)
(d)
图10 Co3O4/N-rmGO复合物的TEM图片和电化学性能测试图(a,b)[93];PNG-NiCo三维复合材料的SEM图片(c),PNG-NiCo,NG-NiCo和PNG三种材料在0.1 mol/L氢氧化钾溶液中的极化曲线(d),插图为电流密度对过电位图片[99]
Fig.10 TEM images (a) and the corresponding electrocatalyst behavior (b) of Co3O4/N-rmGO hybrid respectively[93]; SEM image (c) of the as-fabricated PNG-NiCo 3D hybrid catalyst. LSV plots (d) of PNG-NiCo, NG-NiCo and PNG in 0.1 mol/L KOH; the inset shows the corresponding data replotted as the current density. overpotential[99]
3 尖晶石催化剂在空气电池的应用
目前关于尖晶石催化剂在空气电池器件中的应用研究主要集中在锂-空气电池和锌-空气电池上。KIM等[100]采用模板热解法得到三维有序大孔CoFe2O4用作锂-空气电池正极催化剂,电流密度为200 mA/g时,其首圈容量能达到11658.5 mA·h/g。WANG等[101]通过水热法合成了介孔CuCo2O4并用于锂-空气电池正极催化剂,在50 mA/g电流密度下,首圈容量为8000 mA·h/g。
(a)
(b)
(c)
(d)
图11 尖晶石型CoFe2O4和CuCo2O4用做锂-空气电池正极催化剂:(a~b)为二者采用的锂-空气电池结构图片,(c)为CoFe2O4在200 mA/g条件下首圈充放电曲线图,(d)为CuCo2O4分别在50 mA/g、100 mA/g和200 mA/g电流密度下首圈充放电曲线图[100-101]
Fig.11 Spinel type of CoFe2O4, CuCo2O4as cathode catalysts for Li-O2battery: photograph of the assembled Li-O2battery (a~b); first discharge/charge voltage profiles of CoFe2O4at 200 mA/g (c) and CuCo2O4at 50 mA/g, 100 mA/g, and 200 mA/g (d)[100-101]
CHEN课题组[102]通过模板热解法合成了三维有序介孔的Co3O4并应用于锌-空气电池,所得到的催化剂充放电电位可媲美商业碳载铂催化剂,同时具有更好的循环稳定性,在电流密度为10 mA/cm2时,稳定工作400小时,充放电电位差无显著增加。FU等[103]结合电沉积和化学气相沉积的方法构筑了由一维N掺杂碳纳米管和二维Co3O4纳米片形成的形态仿真头发的三维结构阵列用作柔性锌-空气电池空气电极,与商业碳载铂与碳载铱构筑的空气电池相比,该催化剂具有更小的过电位,在电流密度为25 mA/cm2时,工作600小时后,充放电电位差无显著增加。同时在不同扭曲状态下过电位无明显变化,具有柔性。

表2 尖晶石型催化剂在金属-空气电池中应用总结[104-121]

续表2
金属-空气电池体系结构丰富,我们可以根据自己的需求进行选择。图13列举了目前常见的几种空气电池器件,包括扣式、软包、堆栈式以及柔性线状空气电池[122-125]。众所周知,扣式和软包空气电池由于电极集成和组装工艺的限制,导致其密闭性较差,容易出现漏液等问题,从而严重影响了电池的电化学性能。此外,通过优化空气扩散层、改善电池结构,研究人员开发出了密闭性较好的电池模具[图13(c)和(d)]。氧气通过特定的通道进入电池内部进行电化学反应,排除了其它因素的干扰,为我们探究催化剂对电池性能的影响提供了保障。此外,随着智能电子器件和微型可穿戴电子器件的发展,作为电子设备的供能装置,对电池的形态可塑性提出了新要求。因此柔性空气电池[如图13(e)]的研究成为近年热点之一。
4 结 语
金属-空气电池是最具发展前景的能源存储与转换技术之一,但仍面临实际能量密度低和循环性能差等问题,廉价、高效、稳定空气电极催化剂的开发制备以及电池器件的组装工艺优化是提升电池性能的关键。尖晶石型催化剂在金属-空气电池中展现出巨大的应用潜力,但需要进一步提高催化活性、稳定性和导电性。发生在空气电极上的氧还原/氧析出是一个涉及多相的表界面反应,氧气的吸附和活化、催化活性位点数量和催化过程中的传质是影响催化活性的主要因素。研究表明,可以通过以下途径来对尖晶石氧化物催化材料进行改性:①调控材料组分、晶相和缺陷(包括阳离子缺陷和氧缺陷),改变表面电子结构,优化反应过程中与氧分子的结合能;②减小尺寸,构筑多孔和三维骨架结构,提高比表面积和增加活性位点;③与不同导电碳基底复合,提高导电性和结构稳定性。
对于尖晶石型催化剂,还需进一步深入认识催化机制与构效关系,以指导材料设计与制备。采用密度泛函理论、分子动力学模拟有助于揭示活性位点、表面氧气吸附构型和吸附能,预测高活性组分与结构。通过原位表征技术(如原位X射线衍射、透射电子显微镜、表面增强拉曼光谱、X射线吸收光谱等),可以从不同尺度分析精细结构信息,探究空气电池富氧气氛下尖晶石氧化物与含氧物种的相互作用规律。
针对金属-空气电池器件组装,可通过对空气电极结构合理设计和优化来提升电池性能。例如,在与空气接触的一侧采用氧气选择性透过膜,降低水对活泼金属负极的腐蚀(有机电解液体系),抑制二氧化碳与电解质副反应(碱性水系电解液体系),减弱副反应生成物(如碳酸盐)堵塞空气电极的不利影响。通过优化空气扩散层、改善电池结构或设计固态/凝胶态电解质,解决电解液挥发和漏液等问题。通过三维分级孔道结构集流的设计构筑,有利于反应物/生成物储运与电荷传导。此外,在实际应用中供能装置需适应多种工作环境,并且随着智能化可穿戴电子器件的发展,合理设计具有特殊属性(如超轻薄、良好弯折与延展性、宽工作温度范围等)的金属-空气电池也是值得关注的开发方向。
[1] ARMAND M, TARASCON J M. Building better batteries[J]. Nature, 2008, 451: 652-657.
[2] BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al. Li-O2and Li-S batteries with high energy storage[J]. Nature Materials, 2011, 11: 19-29.
[3] BRUCE P G, SCROSATI B, TARASCON J M. Nanomaterials for rechargeable lithium batteries[J]. Angewandte Chemie-International Edition, 2008, 47: 2930-2946.
[4] WAGNER F T, LAKSHMANAN B, MATHIAS M F. Electrochemistry and the future of the automobile[J]. J. Phys. Chem. Lett., 2010, 1: 2204-2219.
[5] CHRISTENSEN J, ALBERTUS P, SANCHEZ-CARRERA R S, et al. A critical review of Li/air batteries[J]. Journal of the Electrochemical Society, 2012, 159: R1-R30.
[6] LI Y, DAI H. Recent advances in zinc-air batteries[J]. Chemical Society Reviews, 2014, 43: 5257-5275.
[7] RAHMAN M A, WANG X, WEN C. High energy density metal-air batteries: A review[J]. Journal of the Electrochemical Society, 2013, 160: A1759-A1771.
[8] SONG M K, PARK S, ALAMGIR F M, et al. Nanostructured electrodes for lithium-ion and lithium-air batteries: The latest developments, challenges, and perspectives[J]. Materials Science & Engineering R-Reports, 2011, 72: 203-252.
[9] CHENG F, CHEN J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts[J]. Chemical Society Reviews, 2012, 41: 2172-2192.
[10] 魏子栋, 李莉, 李兰兰, 等. 氧电极催化材料的研究现状[J]. 电源技术, 2004(2): 116-120.
WEI Z D, LI L, LI L L, et al. State-of-art of electrocatalysts for oxygen electrode[J]. Chinese Journal of Power Sources, 2004(2): 116-120.
[11] 张涛, 张晓平, 温兆银. 固态锂空气电池研究进展[J]. 储能科学与技术, 2016, 5(5): 702-712.
ZHANG T, ZHANG X P, WEN Z Y. Progress in rechargeable solid-state lithium-air battery[J]. Energy Storage Science and Technology, 2016, 5(5): 702-712.
[12] 马景灵, 许开辉, 文九巴, 等. 铝空气电池的研究进展[J]. 电源技术, 2012, 36(1): 139-141.
MA J L, XU K H, WEN J B, et al. Progress of research on aluminum air batteries[J]. Chinese Journal of Power Sources, 2012, 36(1): 139-141.
[13] 刘春娜. 锌空气电池技术进展[J]. 电源技术, 2012, 36(6): 782-783.
LIU C N. Progress in zinc air battery technology[J]. Chinese Journal of Power Sources, 2012, 36(6): 782-783.
[14] 朱明骏, 袁振善, 桑林, 等. 金属/空气电池的研究进展[J]. 电源技术, 2012, 36(12): 1953-1955.
ZHU M J, YUAN Z S, SANG L, et al. Research progress of metal/air battery[J]. Chinese Journal of Power Sources, 2012, 36(12): 1953-1955.
[15] CHEN J Y C, MILLER J T, GERKEN J B, et al. Inverse spinel NiFeAlO4 as a highly active oxygen evolution electrocatalyst: promotion of activity by a redox-inert metal ion[J]. Energy & Environmental Science, 2014, 7: 1382-1386.
[16] CUI B, LIN H, LI J B, et al. Core-ring structured NiCo2O4nanoplatelets: Synthesis, characterization, and electrocatalytic applications[J]. Advanced Functional Materials, 2008, 18: 1440-1447.
[17] LIU Y, CAO L J, CAO C W, et al. Facile synthesis of spinel CuCo2O4nanocrystals as high-performance cathode catalysts for rechargeable Li-air batteries[J]. Chemical Communications, 2014, 50: 14635-14638.
[18] MAIYALAGAN T, JARVIS K A, THERESE S, et al. Spinel-type lithium cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen reduction reactions[J]. Nature Communications, 2014, 5: 3949-3956.
[19] YAN X, JIA Y, CHEN J, et al. Defective-activated-carbon-supported Mn-Co nanoparticles as a highly efficient electrocatalyst for oxygen reduction[J]. Advanced Materials, 2016, 28: 8771-8778.
[20] YANG H, HU F, ZHANG Y, et al. Controlled synthesis of porous spinel cobalt manganese oxides as efficient oxygen reduction reaction electrocatalysts[J]. Nano Research, 2016, 9: 207-213.
[21] ZHAO A, MASA J, XIA W, et al. Spinel Mn-Co oxide in N-doped carbon nanotubes as a bifunctional electrocatalyst synthesized by oxidative cutting[J]. Journal of the American Chemical Society, 2014, 136: 7551-7554.
[22] DU J, CHEN C, CHENG F, et al. Rapid synthesis and efficient electrocatalytic oxygen reduction/evolution reaction of CoMn2O4nanodots supported on graphene[J]. Inorganic Chemistry, 2015, 54: 5467-5474.
[23] ZHANG K, HAN X, HU Z, et al. Nanostructured Mn-based oxides for electrochemical energy storage and conversion[J]. Chemical Society Reviews, 2015, 44: 699-728.
[24] HAN X, CHENG F, CHEN C, et al. Uniform MnO2nanostructures supported on hierarchically porous carbon as efficient electrocatalysts for rechargeable Li-O2batteries[J]. Nano Research, 2015, 8: 156-164.
[25] HAN X, HU Y, YANG J, et al. Porous perovskite CaMnO3as an electrocatalyst for rechargeable Li-O2batteries[J]. Chemical Communications, 2014, 50: 1497-1499.
[26] HU X, CHENG F, HAN X, et al. Oxygen bubble-templated hierarchical porous epsilon-MnO2as a superior catalyst for rechargeable Li-O2batteries[J]. Small, 2015, 11: 809-813.
[27] HU X, CHENG F, ZHANG N, et al. Nanocomposite of Fe2O3@C@MnO2as an efficient cathode catalyst for rechargeable lithium-oxygen batteries[J]. Small, 2015, 11: 5545-5550.
[28] HU X, HAN X, HU Y, et al. Epsilon-MnO2nanostructures directly grown on Ni foam: A cathode catalyst for rechargeable Li-O2batteries[J]. Nanoscale, 2014, 6: 3522-3525.
[29] HU X, WANG J, LI T, et al. MCNTs@MnO2nanocomposite cathode integrated with soluble O2-carrier Co-salen in electrolyte for high-performance Li-air batteries[J]. Nano Letters, 2017, 17: 2073-2078.
[30] HU Y, ZHANG T, CHENG F, et al. Recycling application of Li-MnO2batteries as rechargeable lithium-air batteries[J]. Angewandte Chemie-International Edition, 2015, 54: 4338-4343.
[31] JIN Q, PEI L, HU Y, et al. Solvo/hydrothermal preparation of MnO@rGO nanocomposites for electrocatalytic oxygen reduction[J]. Acta Chimica Sinica, 2014, 72: 920-926.
[32] 程方益, 陈军. 可充锂空气电池多孔纳米催化剂[J]. 化学学报, 2013, 71(4): 473-477
CHENG F Y, CHEN J. Nanoporous catalysts for rechargeable Li-air batteries[J. Acta Chimica Sinica, 2013, 71(4): 473-477.
[33] 张三佩, 温兆银. 钠-空气电池研究评述[J]. 储能科学与技术, 2016, 5(3): 249-257.
ZHANG S P, WEN Z Y. Review on sodium-air battery[J]. Energy Storage Science and Technology, 2016, 5(3): 249-257.
[34] YADEGARI H, LI Y, BANIS M N, et al. On rechargeability and reaction kinetics of sodium-air batteries[J]. Energy & Environmental Science, 2014, 7: 3747-3757.
[35] YANG S, SIEGEL D J. Intrinsic conductivity in sodium-air battery discharge phases: sodium superoxide vs sodium peroxide[J]. Chemistry of Materials, 2015, 27: 3852-3860.
[36] FU J, CANO Z P, PARK M G, et al. Electrically rechargeable zinc-air batteries: Progress, challenges, and perspectives[J]. Advanced Materials, 2017, 29: doi: 10.1002/adma.201604685.
[37] LIU Q, WANG Y, DAI L, et al. Scalable fabrication of nanoporous carbon fiber films as bifunctional catalytic electrodes for flexible Zn-air batteries[J]. Advanced Materials, 2016, 28: 3000-3006.
[38] MENG F, ZHONG H, BAO D, et al. In situ coupling of strung Co4N and intertwined N-C fibers toward free-standing bifunctional cathode for robust, efficient, and flexible Zn-Air batteries[J]. Journal of the American Chemical Society, 2016, 138: 10226-10231.
[39] LI W, LI C, ZHOU C, et al. Metallic magnesium nano/mesoscale structures: Their shape-controlled preparation and Mg/air battery applications[J]. Angewandte Chemie-International Edition, 2006, 45: 6009-6012.
[40] SHIGA T, HASE Y, YAGI Y, et al. Catalytic cycle employing a TEMPO-anion complex to obtain a secondary Mg-O2battery[J]. Journal of Physical Chemistry Letters, 2014, 5: 1648-1652.
[41] ZHANG T, TAO Z, CHEN J. Magnesium-air batteries: From principle to application[J]. Materials Horizons, 2014, 1: 196-206.
[42] EGAN D R, DE LEON C P, WOOD R J K, et al. Developments in electrode materials and electrolytes for aluminium-air batteries[J]. Journal of Power Sources, 2013, 236: 293-310.
[43] 王诚, 邱平达, 蔡克迪, 等. 铝空气电池关键技术研究进展[J]. 化工进展, 2016, 35(5): 1396-1403.
WANG C, QIU P D, CAI K D, et al. Research progress of the key technologies for aluminum air battery[J]. Chemical Industry and Engineering Progress, 2016, 35(5): 1396-1403.
[44] YUAN J, WANG J, SHE Y, et al. BiOCl micro-assembles consisting, of ultrafine nanoplates: A high performance electro-catalyst for air electrode of Al-air batteries[J]. Journal of Power Sources, 2014, 263: 37-45.
[45] ZHANG Z, ZUO C, LIU Z, et al. All-solid-state Al-air batteries with polymer alkaline gel electrolyte[J]. Journal of Power Sources, 2014, 251: 470-475.
[46] BUI THI H, DOAN HA T, NGUYEN TUYET N, et al. Nanoparticle Fe2O3-loaded carbon nanofibers as iron-air battery anodes[J]. Journal of the Electrochemical Society, 2013, 160: A1442-A1445.
[47] HANG B T, WATANABE T, EASHIRA M, et al. The electrochemical properties of Fe2O3-loaded carbon electrodes for iron-air battery anodes[J]. Journal of Power Sources, 2005, 150: 261-271.
[48] BLURTON K F, SAMMELLS A F. Metal/air batteries: Their status and potential—A review[J]. Journal of Power Sources, 1979, 4: 263-279.
[49] SPENDELOW J S, WIECKOWSKI A. Electrocatalysis of oxygen reduction and small alcohol oxidation in alkaline media[J]. Physical Chemistry Chemical Physics, 2007, 9: 2654-2675.
[50] CHRISTENSEN P A, HAMNETT A, LINARES-MOYA D. Oxygen reduction and fuel oxidation in alkaline solution[J]. Physical Chemistry Chemical Physics, 2011, 13: 5206-5214.
[51] VIELSTICH W, YOKOKAWA H, GASTEIGER H A. Handbook of fuel cells: Fundamentals technology and applications[M]. New York: John Wiley & Sons, 2009.
[52] LIMA F H B, CALEGARO M L, TICIANELLI E A. Electrocatalytic activity of manganese oxides prepared by thermal decomposition for oxygen reduction[J]. Electrochimica Acta, 2007, 52: 3732-3738.
[53] CHENG F, SHEN J, JI W, et al. Selective synthesis of manganese oxide nanostructures for electrocatalytic oxygen reduction[J]. Acs Applied Materials & Interfaces, 2009, 1: 460-466.
[54] CHENG F, SU Y, LIANG J, et al. MnO2-based nanostructures as catalysts for electrochemical oxygen reduction in alkaline media[J]. Chemistry of Materials, 2010, 22: 898-905.
[55] LAOIRE C O, MUKERJEE S, ABRAHAM K M. Elucidating the mechanism of oxygen reduction for lithium-air battery applications[J]. Journal of Physical Chemistry C, 2009, 113: 20127-20134.
[56] LAOIRE C O, MUKERJEE S, ABRAHAM K M. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium-air battery[J]. Journal of Physical Chemistry C, 2010, 114: 9178-9186.
[57] LU Y C, GASTEIGER H A, SHAO-HORN Y. Catalytic activity trends of oxygen reduction reaction for nonaqueous Li-air batteries[J]. Journal of the American Chemical Society, 2011, 133: 19048-19051.
[58] 张三佩, 温兆银, 俊靳, 等. 二次钠空气电池的研究进展[J]. 电化学, 2015, 21: 425-431.
ZHANG S P, WEN Z Y, JIN J, et al. The research progress of secondary sodium/air batteries[J]. Journal of Electrochemistry, 2015, 21: 425-432.
[59] HARTMANN P, BENDER C L, VRAČAR M, et al. A rechargeable room-temperature sodium superoxide (NaO2) battery[J]. Nature Materials, 2013, 12: 228-232.
[60] 高婧, 吴晓梅, 邹建新, 等. 镁空气电池空气阴极研究进展[J]. 电源技术, 2016, 40: 1148-1151.
GAO J, WU X M, ZOU J X, et al. Research progress of air cathodes for magnesium-air batteries[J]. Chinese Journal of Power Sources, 2016, 40: 1148-1151.
[61] DU J, PAN Y, ZHANG T, et al. Facile solvothermal synthesis of CaMn2O4nanorods for electrochemical oxygen reduction[J]. Journal of Materials Chemistry, 2012, 22: 15812-15818.
[62] 王洪波, 程方益, 陶占良, 等. 空心ZnMn2O4纳米球和纳米立方体的室温合成及氧还原催化性能[J]. 高等学校化学学报, 2011, 32: 595-600.
WANG H B, CHENG F Y, TAO Z L, et al. Room-temperature synthesis and oxygen-reduction catalytic performance of hollow ZnMn2O4nanospheres and nanocubes[J]. Chemical Journal of Chinese Universities-Chinese, 2011, 32: 595-600.
[63] HILL R J, CRAIG J R, GIBBS G. Systematics of the spinel structure type[J]. Physics and chemistry of minerals, 1979, 4: 317-339.
[64] SICKAFUS K E, WILLS J M, GRIMES N W. Structure of spinel[J]. Journal of the American Ceramic Society, 1999, 82: 3279-3292.
[65] GOODENOUGH J B, LOEB A L. Theory of ionic ordering, crystal distortion, and magnetic exchange due to covalent forces in spinels[J]. Physical Review, 1955, 98: 391-408.
[66] GRIMES R W, ANDERSON A B, HEUER A H. Predictions of cation distributions in AB2O4spinels from normalized ion energies[J]. Journal of the American Chemical Society, 1989, 111: 1-7.
[67] SEKO A, YUGE K, OBA F, et al. Prediction of ground-state structures and order-disorder phase transitions in II-III spinel oxides: A combined cluster-expansion method and first-principles study[J]. Physical Review B, 2006, 73: 184117-184121.
[68] BURNS R G, Mineralogical applications of crystal field theory[M]. UK: Cambridge University Press, 1993.
[69] SHAO M H, SASAKI K, ADZIC R R. Pd-Fe nanoparticles as electrocatalysts for oxygen reduction[J]. Journal of the American Chemical Society, 2006, 128: 3526-3527.
[70] ARIC A S, BRUCE P, SCROSATI B, et al. Nanostructured materials for advanced energy conversion and storage devices[J]. Nature Materials, 2005, 4: 366-377.
[71] STAMENKOVIC V R, MUN B S, ARENZ M, et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces[J]. Nature Materials, 2007, 6: 241-247.
[72] CHEN S, FERREIRA P J, SHENG W, et al. Enhanced activity for oxygen reduction reaction on “Pt3Co” nanoparticles: Direct evidence of percolated and sandwich-segregation structures[J]. Journal of the American Chemical Society, 2008, 130: 13818-13819.
[73] WANG J X, INADA H, WU L, et al. Oxygen reduction on well-defined core-shell nanocatalysts: Particle size, facet, and Pt shell thickness effects[J]. Journal of the American Chemical Society, 2009, 131: 17298-17302.
[74] GE X, SUMBOJA A, WUU D, et al. Oxygen reduction in alkaline media: From mechanisms to recent advances of catalysts[J]. ACS Catalysis, 2015, 5: 4643-4667.
[75] WANG H Y, HUNG S F, CHEN H Y, et al. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4[J]. Journal of the American Chemical Society, 2016, 138: 36-39.
[76] YEO B S, BELL A T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen[J]. Journal of the American Chemical Society, 2011, 133: 5587-5593.
[77] CHEN R, WANG H Y, MIAO J, et al. A flexible high-performance oxygen evolution electrode with three-dimensional NiCo2O4core-shell nanowires[J]. Nano Energy, 2015, 11: 333-340.
[78] GAO X, ZHANG H, LI Q, et al. Hierarchical NiCo2O4hollow microcuboids as bifunctional electrocatalysts for overall water-splitting[J]. Angewandte Chemie-International Edition, 2016, 55: 6290-6294.
[79] CHENG F, CHEN J. Nanoporous catalysts for rechargeable Li-air batteries[J]. Acta Chimica Sinica, 2013, 71: 473-477.
[80] HAN X, ZHANG T, DU J, et al. Porous calcium-manganese oxide microspheres for electrocatalytic oxygen reduction with high activity[J]. Chemical Science, 2013, 4: 368-376.
[81] CHEN S, ZHAO Y, SUN B, et al. Microwave-assisted synthesis of mesoporous Co3O4nanoflakes for applications in lithium ion batteries and oxygen evolution reactions[J]. Acs Applied Materials & Interfaces, 2015, 7: 3306-3313.
[82] ROSEN J, HUTCHINGS G S, JIAO F. Ordered mesoporous cobalt oxide as highly efficient oxygen evolution catalyst[J]. Journal of the American Chemical Society, 2013, 135: 4516-4521.
[83] SHI J, LEI K, SUN W, et al. Synthesis of size-controlled CoMn2O4quantum dots supported on carbon nanotubes for electrocatalytic oxygen reduction/evolution[J]. Nano Research, 2017, doi: 10.1007/s12274-017-1597-0.
[84] KOZA J A, HE Z, MILLER A S, et al. Electrodeposition of crystalline Co3O4—A catalyst for the oxygen evolution reaction[J]. Chemistry of Materials, 2012, 24: 3567-3573.
[85] SU D, DOU S, WANG G. Single crystalline Co3O4nanocrystals exposed with different crystal planes for Li-O2batteries[J]. Scientific Reports, 2014, 4: 5767-5775.
[86] CHEN Z, KRONAWITTER C X, KOEL B E. Facet-dependent activity and stability of Co3O4nanocrystals towards the oxygen evolution reaction[J]. Physical Chemistry Chemical Physics, 2015, 17: 29387-29393.
[87] ALEJANDRO MONTOYA, HAYNES B S. Periodic density functional study of Co3O4surfaces[J]. Chemical Physics Letters, 2011, 502: 63-68.
[88] LI L,WEI Z,CHEN S, et al. A comparative DFT study of the catalytic activity of MnO2(211) and (2-2-1) surfaces for an oxygen reduction reaction[J]. Chemical Physics Letters, 2012, 539-540: 89-93.
[89] LI C, HAN X, CHENG F, et al. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis[J]. Nature Communication, 2015, 6: 7345-7352.
[90] WU G, WANG J, DING W, et al. A strategy to promote the electrocatalytic activity of spinels for oxygen reduction by structure reversal[J]. Angewandte Chemie International Edition, 2016, 55: 1340-1344.
[91] CHENG F, SHEN J, PENG B, et al. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts[J]. Nature Chemistry, 2011, 3: 79-84.
[92] BAO J, ZHANG X, FAN B, et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation[J]. Angewandte Chemie-International Edition, 2015, 54: 7399-7404.
[93] CHENG H, SU Y Z, KUANG P Y, et al. Hierarchical NiCo2O4nanosheet-decorated carbon nanotubes towards highly efficient electrocatalyst for water oxidation[J]. Journal of Materials Chemistry A, 2015, 3: 19314-19321.
[94] LEE D U, KIM B J, CHEN Z. One-pot synthesis of a mesoporous NiCo2O4nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst[J]. Journal of Materials Chemistry A, 2013, 1: 4754-4762.
[95] ZHANG H, LI H, WANG H, et al. NiCo2O4/N-doped graphene as an advanced electrocatalyst for oxygen reduction reaction[J]. Journal of Power Sources, 2015, 280: 640-648.
[96] ZHANG H, QIAO H, WANG H, et al. Nickel cobalt oxide/carbon nanotubes hybrid as a high-performance electrocatalyst for metal/air battery[J]. Nanoscale, 2014, 6: 10235-10242.
[97] HAN X, WU X, ZHONG C, et al. NiCo2S4nanocrystals anchored on nitrogen-doped carbon nanotubes as a highly efficient bifunctional electrocatalyst for rechargeable zinc-air batteries[J]. Nano Energy, 2017, 31: 541-550.
[98] LIANG Y, LI Y, WANG H, et al. Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction[J]. Nature Materials, 2011, 10: 780-786.
[99] CHEN S, QIAO S Z. Hierarchically porous nitrogen-doped graphene NiCo2O4hybrid paper as an advanced electrocatalytic water-splitting material[J]. Acs Nano, 2013, 7: 10190-10196.
[100] KIM J G, NOH Y, KIM Y, et al. Fabrication of three-dimensional ordered macroporous spinel CoFe2O4as efficient bifunctional catalysts for the positive electrode of lithium-oxygen batteries[J]. Nanoscale, 2017, 9: 5119-5128.
[101] WANG P X, SHAO L, ZHANG N Q, et al. Mesoporous CuCo2O4nanoparticles as an efficient cathode catalyst for Li-O2batteries[J]. Journal of Power Sources, 2016, 325: 506-512.
[102] PARK M G, LEE D U, SEO M H, et al. 3D ordered mesoporous bifunctional oxygen catalyst for electrically rechargeable zinc-air batteries[J]. Small, 2016, 12: 2707-2714.
[103] FU J, HASSAN F M, LI J, et al. Flexible rechargeable zinc-air batteries through morphological emulation of human hair array[J]. Advanced Materials, 2016, 28: 6421-6428.
[104] ABIRAMI M, HWANG S M, YANG J, et al. A metal-organic framework derived porous cobalt manganese oxide bifunctional electrocatalyst for hybrid Na-air/seawater batteries[J]. ACS Applied Materials & Interfaces, 2016, 8: 32778-32787.
[105] HUANG Z, CHI B, JIAN L, et al. CoFe2O4@multi-walled carbon nanotubes integrated composite with nanosized architecture as a cathode material for high performance rechargeable lithium-oxygen battery[J]. Journal of Alloys and Compounds, 2017, 695: 3435-3444.
[106] JADHAV H S, KALUBARME R S, JADHAV A H, et al. Iron-nickel spinel oxide as an electrocatalyst for non-aqueous rechargeable lithium-oxygen batteries[J]. Journal of Alloys and Compounds, 2016, 666: 476-481.
[107] JADHAV H S, KALUBARME R S, ROH J W, et al. Facile and cost effective synthesized mesoporous spinel NiCo2O4as catalyst for non-aqueous lithium-oxygen batteries[J]. Journal of the Electrochemical Society, 2014, 161: A2188-A2196.
[108] KALUBARME R S, JADHAV H S, DUC TUNG N, et al. Simple synthesis of highly catalytic carbon-free MnCo2O4@Ni as an oxygen electrode for rechargeable Li-O2batteries with long-term stability[J]. Scientific Reports, 2015, 5: 13266-13277.
[109] LI J, ZOU M, WEN W, et al. Spinel MFe2O4(M=Co, Ni) nanoparticles coated on multi-walled carbon nanotubes as electrocatalysts for Li-O2batteries[J]. Journal of Materials Chemistry A, 2014, 2: 10257-10262.
[110] LI P, SUN W, YU Q, et al. An effective three-dimensional ordered mesoporous CuCo2O4as electrocatalyst for Li-O2batteries[J]. Solid State Ionics, 2016, 289: 17-22.
[111] LUO Y, LU F, JIN C, et al. NiCo2O4@La0.8Sr0.2MnO3core-shell structured nanorods as efficient electrocatalyst for Li-O2battery with enhanced performances[J]. Journal of Power Sources, 2016, 319: 19-26.
[112] WANG L, ZHU T, LYU Z, et al. Facile synthesis of flower-like hierarchical NiCo2O4microspheres as high-performance cathode materials for Li-O2batteries[J]. RSC Advances, 2016, 6: 98867-98873.
[113] ZHANG J, WANG L, XU L, et al. Porous cobalt-manganese oxide nanocubes derived from metal organic frameworks as a cathode catalyst for rechargeable Li-O2batteries[J]. Nanoscale, 2015, 7: 720-726.
[114] ZOU L, CHENG J, JIANG Y, et al. Spinel MnCo2O4nanospheres as an effective cathode electrocatalyst for rechargeable lithium-oxygen batteries[J]. RSC Advances, 2016, 6: 31248-31255.
[115] HAN X, CHENG F, CHEN C, et al. A Co3O4@MnO2/Ni nanocomposite as a carbon- and binder-free cathode for rechargeable Li-O2batteries[J]. Inorganic Chemistry Frontiers, 2016, 3: 866-871.
[116] JUNG K N, HWANG S M, PARK M S, et al. One-dimensional manganese-cobalt oxide nanofibres as bi-functional cathode catalysts for rechargeable metal-air batteries[J]. Scientific Reports, 2015, 5: 7665-7674.
[117] PRABU M, KETPANG K, SHANMUGAM S. Hierarchical nanostructured NiCo2O4as an efficient bifunctional non-precious metal catalyst for rechargeable zinc-air batteries[J]. Nanoscale, 2014, 6: 3173-3181.
[118] SHEN Q, YANG J, CHEN K L, et al. Co3O4nanorods-graphene composites as catalysts for rechargeable zinc-air battery[J]. Journal of Solid State Electrochemistry, 2016, 20: 3331-3336.
[119] YU M, WANG Z, HOU C, et al. Nitrogen-Doped Co3O4mesoporous nanowire arrays as an additive-free air-cathode for flexible solid-state zinc-air batteries[J]. Advanced Materials, 2017, 29: doi: 10.1002/adma.201602868.
[120] PRABU M, RAMAKRISHNAN P, SHANMUGAM S. CoMn2O4nanoparticles anchored on nitrogen-doped graphene nanosheets as bifunctional electrocatalyst for rechargeable zinc-air battery[J]. Electrochemistry Communications, 2014, 41: 59-63.
[121] LEE D U, SCOTT J, PARK H W, et al. Morphologically controlled Co3O4nanodisks as practical bi-functional catalyst for rechargeable zinc-air battery applications[J]. Electrochemistry Communications, 2014, 43: 109-112.
[122] HU X, ZHU Z, CHENG F, et al. Micro-nano structured Ni-MOFs as high-performance cathode catalyst for rechargeable Li-O2batteries[J]. Nanoscale, 2015, 7: 11833-11840.
[123] LEE J S, LEE T, SONG H K, et al. Ionic liquid modified graphene nanosheets anchoring manganese oxide nanoparticles as efficient electrocatalysts for Zn-air batteries[J]. Energy & Environmental Science, 2011, 4: 4148.
[124] ZENG M,LIU Y,ZHAO F, et al. Metallic cobalt nanoparticles encapsulated in nitrogen-enriched graphene shells: Its bifunctional electrocatalysis and application in zinc-air batteries[J]. Advanced Functional Materials, 2016, 26: 4397-4404.
[125] PARK J, PARK M, NAM G, et al. All-solid-state cable-type flexible zinc-air battery[J]. Advanced Materials, 2015, 27: 1396-1401.
[126] 彭佳悦, 祖晨曦, 李泓. 锂电池基础科学问题(I)——化学储能电池理论能量密度的估算[J]. 储能科学与技术, 2013, 2(1): 55-62.
PENG J Y, ZU C X, LI H. Fundamental scientific aspects of lithium batteries (I)—Thermodynamic calculations of theoretical energy densities of chemical energy storage systems[J]. Energy Storage Science and Technology, 2013, 2(1): 55-62.
Spinel-type transition metal oxide electrocatalysts for metal-air batteries
CHEN Xiang, LEI Kaixiang, SUN Hongming, CHENG Fangyi, CHEN Jun
(Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Collaborative Innovation Center of Chemical Science and Engineering, College of Chemistry, Nankai University, Tianjin 300071, China)
Metal air batteries are attractive devices for electrochemical energy storage and conversion because of high energy density. The slow kinetics of the cathodic electrochemical oxygen reduction/evolution is a key factor limiting the performance of metal air batteries, necessitating the use of active catalyst. In this review, we briefly introduce the structure and mechanism of prevailing metal-air batteries and summarize recent progress in applying spinel-type transition metal oxides as cathode catalysts to build metal-air battery.
spinel; electrocatalyst; oxygen reduction; oxygen evolution; metal air battery
10.12028/j.issn.2095-4239.2017.00959
TM 911.41
A
2095-4239(2017)05-904-20
2017-06-07;
2017-07-29。
国家重点研发计划项目(2017YFA0206700)和国家自然科学基金项目(21231005和21322101)。
陈祥(1989—),男,博士研究生,主要研究方向为电催化与金属空气电池,E-mail:cx9528@mail.nankai.edu.cn;
程方益,研究员,主要研究方向为功能材料与化学电源,E-mail:fycheng@nankai.edu.cn。
