废旧锂离子电池中钴、锂的回收研究进展
2017-09-06孟奇张英杰董鹏梁风
孟奇,张英杰,董鹏,梁风
(昆明理工大学冶金与能源工程学院,锂离子电池及材料制备技术国家地方联合工程实验室,云南省先进电池材料重点实验室(筹),云南 昆明 650093)
废旧锂离子电池中钴、锂的回收研究进展
孟奇,张英杰,董鹏,梁风
(昆明理工大学冶金与能源工程学院,锂离子电池及材料制备技术国家地方联合工程实验室,云南省先进电池材料重点实验室(筹),云南 昆明 650093)
随着锂离子电池产业发展,废旧锂离子电池所带来的环境及资源问题日益突出,废旧锂离子电池中有价金属的资源化、无害化处理逐渐成为国内外的研究热点。为实现废旧锂离子电池中钴、锂资源绿色高效回收,本文介绍了废旧锂离子电池中有价金属回收的研究现状,主要包括预处理、正极材料处理、浸出液回收等环节,着重评述了各环节中新方法及工艺,简要对比了各方法及工艺的优缺点。现阶段研究主要集中于湿法浸出回收工艺,酸-还原剂为典型浸出模型,而动力学控制、离子转移路径等机理方面欠缺。最后展望了今后废旧锂离子电池中钴、锂资源回收研究方向,下一步主要是朝着有机酸浸-沉淀获得优质产品方向发展,需着重强化浸出效率、提升沉淀指标、简化工艺条件,以利于产业化推广。
废旧锂离子电池;钴;锂;回收;再生
20世纪90年代,锂离子电池正式进入商品化发展阶段,其具有能量密度大、工作电压高、循环寿命长、无记忆效应及安全性好等诸多优点,现已逐步取代传统二次电池,广泛用于移动电子设备、航天航空、医疗等领域,加之我国正大力发展的新能源汽车、智能电网、可再生能源等产业,锂离子电池作为良好的动力电池和储能材料,其需求量及产量势必进一步增加[1],同时,锂离子电池材料价格随之迅速上涨,锂离子电池生产成本不断加大。
锂离子电池经过几百或上千次的循环充放电后,活性材料同样会由于结构改变而失活报废,锂离子电池寿命一般3~5年。废旧锂离子电池中电解液的释放会污染环境和危害生态系统,同时电极材料含有大量锂、钴等稀有金属,大量废旧锂离子电池的堆弃造成资源极大浪费[2],而锂离子电池原料矿产资源日益减少,特别是我国钴、锂资源相对匮乏,制约了锂离子电池产业的良性发展。为此,进行废旧锂离子电池中有价金属回收研究,不仅解决潜在的环境问题,也可实现锂、钴等资源再生,降低锂离子电池生产成本,缓解资源紧张的现状。本文综述了近年来废旧锂离子电池中钴、锂回收的新工艺及方法,讨论了其优缺性并展望了今后研究方向。
目前,废旧锂离子电池回收主要是回收锂、钴等有价金属。废旧锂离子电池是由外壳、正极、负极、电解质、隔膜5部分以壳层包裹形式构成,其中,正极活性物质主要为钴酸锂、磷酸铁锂、锰酸锂、锂三元化合物等,成为钴、锂等有价金属来源物质[3],因此,正极材料中钴、锂有价金属成为回收重点,常用回收工艺主要有预处理、正极材料处理、浸出液回收等过程。
1 预处理
预处理主要是选择性分离出有较高价值的正极材料,脱除稍低价值的组分或有机溶剂,减少后续钴、锂金属浸出过程不利影响,主要有放电、破碎、溶解、热解等方法。
1.1 放电
废旧锂离子电池仍含有少量余电,残存的电量在拆解、破碎及浸出过程中会因急剧释放而引发局部过热或爆炸等危险,为此,预先放电工序必不可少,预放电可分为物理放电和化学放电。其中,物理放电为强制短路放电,先通过液氮等冷冻液进行低温冷冻,而后穿孔强制放电,物理放电适用于小批量电池的预放电,需要一定的设备,不宜大规模应用。化学放电是在导电溶液中通过电解的方式来消耗余电,导电溶液多为氯化钠溶液。宋秀玲等[4]通过构建以硫酸盐为电解液放电体系,确定了最佳放电条件为:电解液MnSO40.8mol/L、pH=2.78、稳定剂抗坏血酸2g/L、放电8h,最终消电电压降至0.54V,实现了高效绿色放电。化学放电成本相对较低,可用于大规模电池放电,但化学放电易造成金属或合金外壳腐蚀,引发电解液渗透或有价金属的流失。
1.2 破碎及联用技术
破碎是利用冲击、挤压、摩擦等作用破坏废旧锂离子电池的金属外壳、解离并选择性分离内部电极材料的过程,破碎工艺一般为粗碎、中碎、细碎等多级破碎。GRANATA等[5]进行了双轴和锤式两级破碎工艺处理废旧锂离子电池的试验,发现铁、铜、铝等金属主要分布于 +0.2mm粒级,电极材料则分布在 –0.1mm粒级。张涛[6]研究了废旧锂离子电池两级破碎的产物特征,发现破碎产物中–0.25mm和+2mm粒级的产率分别达56.21%、27.58%,同样电极材料富集在 –0.25mm粒级。因而,破碎常与粒度筛分联用进行电极材料与隔膜、大粒外壳、铝箔等材料的初步分离。
由于锂离子电池组成较为复杂,破碎筛分也很难彻底分离电极材料,为进一步强化电极材料的初步分离指标,破碎还可与磁选[7]、超声[8]、浮选等技术联用。其中,细粒级中仍含有石墨等杂质,重选、磁选、超声等技术在此粒级下对正极材料与石墨区分不明显,很难实现分离,而石墨与正极材料表面润湿性差异较大,因而,浮选成为分离正极、负极的较优方案[9]。李红等[10]在废旧锂离子电池破碎产物浮选试验中,发现纯钴酸锂与石墨天然可浮性差异大,而废旧电池破碎后钴酸锂颗粒表面有杂质附着,造成其与石墨润湿性相近,浮选分离指标变差。为此,表面改性再浮选成为解决方案。张伟刚等[11]采用Fenton试剂进行了废旧锂离子电池破碎得到富钴粉体的改性浮选试验,发现改性浮选获得钴回收率达99%,富集比为1.41,Fenton试剂可去除富钴粉体附着的有机杂质,增大了其与石墨的润湿性差异。
1.3 溶解
溶解是利用正极材料与黏结剂、铝箔等杂质材料在有机溶剂或酸碱溶液中溶解性质的差异使之分离的过程。黏结剂多为聚偏氟乙烯(PVDF),可选取强极性有机溶剂溶解脱除PVDF杂质,从而分离正极材料与铝箔。梁立君[12]对破碎后电极材料进行4种极性有机溶剂的溶解对比实验,发现最佳溶剂为N-甲基吡咯烷酮(NMP),在其浓度5mL/g、温度100℃、时间1h条件下,可实现正极活性物质与铝箔彻底分离。LI等[13]进行了正极活性物质与铝箔的除杂分离试验,选取NMP作溶剂溶解黏结剂,温度100℃、时间60min,筛分去除铝箔碎片,且过滤后NMP可重复使用。
铝箔属于两性金属,而正极材料不会与碱反应,可通过碱溶法去除铝箔进一步富集正极材料[14]。FERREIRA等[15]进行了碱溶液选择性溶解铝箔杂质试验,发现NaOH浓度由1%增加至10%,铝浸出率迅速增大,而温度改变对浸出率影响不明显。谭群英等[16]采用碱溶液二级逆流方式进行正极材料中铝的溶解试验,最佳工艺为:一级为先碱后进料,二级碱用量15%,一、二级碱量比40%∶60%,反应时间均为2h,温度95℃,铝浸出率为98.0%。
黏结剂多是采用NMP溶解去除,溶解指标较好,但NMP具有价格高、易挥发、低毒性等不足,这在一定程度上限制了其发展应用。碱溶解去除铝箔简单易行,也可工业应用。
1.4 热解
热解可用于去除正极材料富集物料中有机物、碳质等杂质,或分解黏结剂使得正极材料与集流体分离过程,热解主要依据有机物、黏结剂、铝箔、正极材料等物质分解或融化温度点不同而实现分离富集。温度超过350℃,黏结剂PVDF便会发生分解,温度达到660℃,铝箔发生熔化[17]。CHEN等[18]进行了正极材料的热解除杂试验,采用马弗炉中800℃、煅烧2h,脱除PVDF及碳质,分离出正极活性物质。LEE等[19]进行电极材料的两步热解除杂试验,首先,对物料在温度100~150℃保温1h,而后在温度500~900℃条件下煅烧0.5~2.0h,可以同时脱除黏结剂和导电炭黑等杂质,但热解过程会产生氟化物等有害物质,需增加收集净化装置,防止有害气体的释放。为提高热解效率,削弱有害气体的释放,孙亮[20]采用真空环境下热解预处理废旧锂离子电池正极材料,采用条件:压强低于1kPa、恒温600℃、时间30min,最终黏结剂基本除去,正极材料与铝箔分离。真空热解可实现快速分解黏结剂、分离铝箔与正极材料的目的,同时避免了有害气体的释放,但其对设备要求高、操作复杂,应用推广难度较大。而空气中热解除杂操作简单、易推广应用,但其耗能较大,易发生铝箔二次包裹,不利于后续金属回收,同时应注意有害气体的收集净化,防止危害大气环境。
2 正极材料处理
正极材料处理主要是对预处理得到的正极材料富集物进行选择性提取回收钴和锂的过程,主要方法有湿法浸出、微生物浸出、电化学浸出等。
2.1 湿法浸出
湿法浸出目的是将正极材料中有价金属转移到浸出液中,而利于后续沉淀、提纯工艺。废旧锂离子电池正极材料的湿法浸出主要是酸浸方案,同时由于Co(Ⅲ)化合物不易溶解浸出,还原剂H2O2作强化剂得到研究及应用,酸+H2O2成为常见浸出体系[21-23],常见湿法浸出流程见图1,现有报道的酸浸体系见表1。
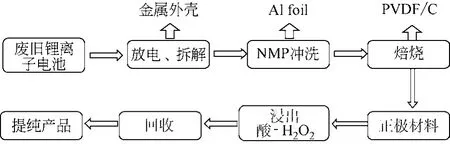
图1 常见湿法浸出流程
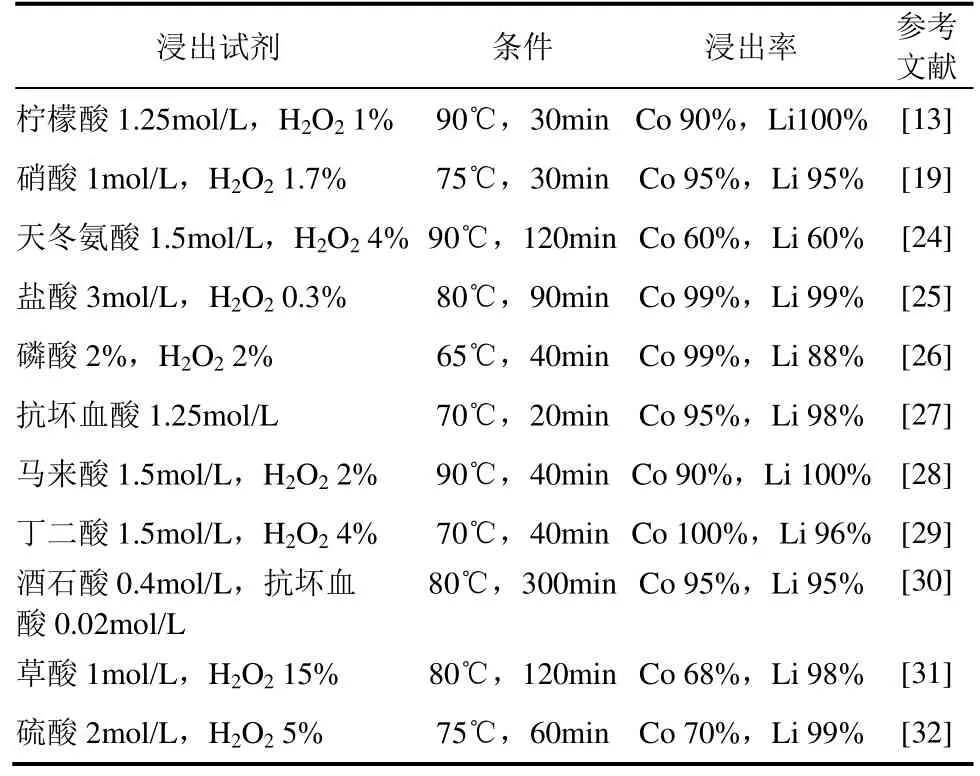
表1 正极材料的酸浸体系
常见的硫酸、盐酸、硝酸等无机酸-H2O2可以较好地浸出正极材料,但无机酸具有较强腐蚀性对设备要求较高,同时易产生Cl2、SO2等有害气体,有机酸替代无机酸成为选择,其相对绿色环保,现已研究的有机酸包括草酸、柠檬酸、马来酸、抗坏血酸、酒石酸、葡萄糖酸等[33-35]。LI等[36]采用丁二酸进行了废旧正极材料的浸出试验,发现在丁二酸1.5mol/L、H2O24%(体积分数)、固液比15g/L、温度70℃、时间40min条件下,Li、Co浸出率分别达96%、100%。ZENG等[28]采用草酸浸出废旧锂离子电池中LiCoO2正极物料,发现草酸浸出过程属于液固相非催化反应,其主要受化学反应控制,在草酸用量1mol/L、时间150min、温度95℃、固液比15g/L条件下,Co、Li回收率分别达97%、98%。由于酸+ H2O2浸出体系中H2O2在酸性环境易分解,H2O2替代性的还原剂也得以研究,主要有硫代硫酸钠、NaHSO3、麦秆粉等[29,37]。NAYAKA等[30]进行了混合有机酸浸出正极材料的试验,采用酒石酸0.4mol/L和抗坏血酸0.02mol/L混合作浸出剂,温度80℃、时间5h,Li、Co均全部浸出。
2.2 微生物浸出
微生物浸出法也用于从废旧锂离子电池材料中回收Ni、Co、Li等有价金属,其同样是利用某些特定的微生物及代谢产物的络合、还原、氧化、浸出等作用,实现有价金属回收溶解的目的。微生物浸出机理分为直接作用和间接作用[38-39],直接作用主要是微生物利用其胞内氧化酶系统产生氧化氛围,同时分泌酸性物质提供H+,使得金属离子溶解浸出。间接作用则是微生物优先氧化部分金属离子,而后利用氧化的离子提供氧化氛围,促进废旧材料中金属离子的溶解。
邓孝荣等[40]采用氧化亚铁硫杆菌进行了废旧锂离子电池的浸出试验,发现较优工艺为:硫酸亚铁初始量45g/L、接种量5%、振荡160r/min、振荡温度35℃、浸出时间10天、固液比为3%,最终钴浸出率达37.5%。辛亚云[41]进行了氧化硫硫杆菌、嗜铁钩端螺旋菌浸出废旧锂离子电池材料的试验,发现氧化硫硫杆菌体系对Li的浸出率较高,而两种菌系混合体系对Ni、Co、Mn的浸出率较高,原因在于Li浸出主要是依靠H2SO4溶解,Ni、Co、Mn的浸出则是依靠Fe2+的还原和酸溶的共同作用。常规微生物浸出周期长,为强化微生物浸出效果,外加强化剂促进微生物浸出方法也得到研究。ZENG等[42-43]通过向微生物浸出体系添加Cu2+、Ag+作催化剂,进行废旧锂电池浸出研究,发现添加Ag+0.02g/L或Cu2+0.75g/L,可使得Co浸出分别达到98.4%、99.9%,相比无添加催化剂情况下Co浸出率仅有43.1%。可见,适量Ag+或Cu2+可以提升Co浸出率。
微生物浸出具有环境友好、成本低、酸耗量小等优势,是值得发展的回收方案,但也存在周期长、菌种培育难度大、效率低等不足,废旧锂离子电池中有价金属含量均较高,微生物浸出周期更长。
2.3 电化学浸出
电化学浸出是基于电解原理选取废旧锂离子电池正极条作阴极,铅板作阳极,通过电解浸出实现Li、Co等有价金属的选择性溶解过程。阴极发生还原可以促进LiCoO2材料溶解,起到替代H2O2的作用,同时还原性氛围可保护铝箔避免溶解。常伟等[44]采用硫酸40g/L作电解液进行正极片的电解浸出试验,在电流密度15.6mA/cm2、稳定剂柠檬酸36g/L、浸出温度45℃、时间2h条件下,Co、Al浸出率分别为90.8%、7.9%,并发现硫酸具有维持阴极pH和溶解浸出正极材料的双重特性。张建等[45]对电解浸出进行了动力学方面研究,发现了阴极还原氛围未能保护铝箔表面氧化铝层,氧化铝溶解是浸出液中Al离子杂质主要来源,同时促进了正极材料的脱落分离。由于脱落的正极材料缺少阴极还原氛围辅助,浸出指标下降。
电解浸出法避免了碱浸除铝环节,采取阴极还原替代H2O2使用,降低药剂成本,利于简化工艺,但也存在一些不足,如电解能耗、正极材料溶解不彻底,电解渣二次浸出增加工艺复杂性。
3 浸出液回收
经预处理、浸出获得浸出液是Co、Li离子的混合体,同时含有少量Al、Fe、Mn、Cu杂质离子,为获得可用产品或制备电极材料,需要进行纯化分离操作,主要方法有萃取法、沉淀法、电化学等方法。
3.1 萃取法
萃取法是通过向浸出液中加入某种有机溶剂选择性地分离Co、Li、Ni有价金属,该方法可以获得较高纯度产物,条件温和,回收率好,成为研究重点和常用处理方案,常用萃取剂有P507、Cyanex272、Acorga M5640等[46-47]。
萃取剂P507,或称PC88A可以有选择性萃取Co(Ⅱ)分离Li(Ⅰ)、Ni(Ⅱ)。潘晓勇等[48]进行了浸出液中Co、Li萃取分离试验,采用P507从浸出液中单级萃取Co,最佳萃取条件为:萃取剂P507∶磺化煤油∶TBP=2.5∶7∶0.5、pH=3.5、水油比1∶2、皂化率70%、萃取时间10min。选取草酸进行反萃,反萃条件草酸3%、温度40℃,最后烘干获得草酸钴,纯度98.4%,钴回收率可达99%,萃余液选用饱和碳酸钠制得碳酸锂,纯度99.3%,锂回收率可达98%。GRANATA等[5]进行了废旧正极材料浸出液中Co萃取试验,发现Cyanex 272作萃取剂,在pH=5~6、萃取剂/Co=4条件下,可实现Co和Li较好的分离,若浸出液中有Mn(Ⅱ)存在,Cyanex 272对Co(Ⅱ)选择性变差,为此,需采用D2EHPA先将Mn (Ⅱ)提取,而后再进行Co和Li萃取分离。
为消除Al、Fe、Cu等杂质离子对选择性萃取Co影响,预先萃取除杂同样得到研究。PRANOLO等[49]选用混合萃取剂分离Al、Fe、Cu等杂质,采用7%Ionquest 801+2%Acorga M5640作萃取剂,在水油比1∶1、温度22℃条件下,Al、Fe、Cu同时萃取出,而后用15%Cyanex 272在pH=5.5~6.0萃取Co分离Li,最后获得较纯的钴、锂产品。
3.2 沉淀法
沉淀法是利用沉淀剂与浸出液中Li、Co离子发生选择性沉淀,而后经过滤分离出产品,沉淀法研究重点是防止杂质离子的共沉淀以及有价金属离子的顺序沉淀,以获得较高纯度的产品。由于碳酸盐、碱均可与Li、Co离子发生沉淀反应,而草酸盐仅与Co离子沉淀,因而,沉淀法多是先利用草酸盐沉淀Co离子生成CoC2O4,煅烧获得Co3O4产品,而后进行Li离子沉淀。
谭海翔等[50]进行了钴酸锂正极材料浸出液中离子的沉淀研究,先使用草酸酸化浸出液,再加入草酸铵,草酸钴沉淀率达99%,Li离子全部留在溶液中,最后经马弗炉600℃、空气氛围、恒温煅烧4h,获得高纯度的Co3O4产品。孟洋[51]采用沉淀法处理废旧锂离子电池正极材料浸出液,由于浸出液中含有Fe、Al、Cu这3种杂质,含量分别为2.76g/L、2.32g/L、0.66g/L,为此,选取Na2S沉淀Cu、调节pH=5.5沉淀Fe和Al,最终Fe、Al、Cu沉淀率分别达到100%、98.67%、98.56%,而后采用超声强化草酸铵沉淀获得产品草酸钴。
电池材料浸出液中Li离子多采用碳酸盐生成Li2CO3沉淀,HUANG等[52]采用磷酸盐进行了浸出液中Li离子沉淀试验,发现Na3PO4同样可以与Li离子反应生成Li3PO4沉淀,且相比Na2CO3沉淀效率更高,原因是Li2CO3溶解性稍大。
沉淀法处理量大,操作简单,无需特殊设备,易于推广工业化,但是由于杂质影响较大,产品纯度不易控制,相比萃取法指标略有下降。为此,选择适宜的、高选择性的沉淀剂,或优化杂质离子预沉淀、有价离子的沉淀顺序成为关键。
3.3 电化学
电化学法是利用浸出液中有价金属离子在阴极放电还原生成单质或沉积物[53],该方法可以获得更高纯度的产品,不易引入杂质,但多种金属存在时会发生共沉积,产品纯度降低。FREITAS等[54]进行了废旧锂离子电池浸出液中钴的电沉积回收研究,发现产品Co质量随着电沉积溶液pH升高而增大,在电势-1.0V、电荷密度10C/cm2、pH=5.4条件下,Co开始发生沉积,电荷最大效率96.9%,此过程中Co(Ⅲ)先转化成Co(OH)2再以Co单质形式沉积下来。GARCIA等[55]研究了废旧锂离子电池Co的电沉积过程,发现在pH=5.4时,电沉积钴还原量与电荷比为33g/mol,而在pH=2.7时,该比值为13g/mol,并伴随吸附氢的形成。可见,pH对浸出液中钴电沉积提纯影响较大。电沉积法具有纯度高的优点,但也存在电耗过大的不足,同时相关机理研究有待加强。
4 结语与展望
总结了废旧锂离子电池中有价金属回收的工艺及方法,现阶段研究重点以湿法浸出为中心,酸-还原剂为典型浸出模型,且以工艺参数研究居多,机理方面欠缺,如动力学控制、离子转移路径等。同时,手工拆解得到电极材料为主要前处理手段,大规模实践应用仍有距离。下一步主要是朝着有机酸浸-沉淀获得产品方向发展,着重强化浸出效率、提升沉淀产品质量及简化工艺条件,以利于产业化推广,同时注意控制二次污染及降低回收成本,以实现废旧锂离子电池绿色高效回收再用。
[1] 张笑笑,王鸯鸯,刘媛,等. 废旧锂离子电池回收处理技术与资源化再生技术进展[J]. 化工进展,2016,35(12):4026-4032.ZHANG X X,WANG Y Y,LIU Y,et al. Recent progress in disposal and recycling of spent lithium-ion batteries[J]. Chemical Industry and Engineering Progress,2016,35(12): 4026-4032.
[2] 李劲,邵威,毛洪仁. 废弃锂离子电池回收处理的污染物分析[J].化工进展,2016,35(5):1529-1538.LI J,SHAO W,MAO H R. Analysis of pollutants in the recycling of waste lithium batteries[J]. Chemical Industry and Engineering Progress,2016,35(5):1529-1538.
[3] KANG J,SOHN J,CHANG H,et al. Preparation of cobalt oxide from concentrated cathode material of spent lithium ion batteries by hydrometallurgical method[J]. Advanced Powder Technology,2010,21(2):175-179.
[4] 宋秀玲,戴书琪,徐永胜,等. 废旧锂离子电池放电的实验研究[J]. 应用化工,2015(4):594-597.SONG X L,DAI S Q,XU Y S,et al. Experimental study on the discharge of the waste lithium ion battery[J]. Applied Chemical Industry,2015(4):594-597.
[5] GRANATA G,PAGNANELLI F,MOSCARDINI E,et al.Simultaneous recycling of nickel metal hydride,lithium ion and primary lithium batteries:accomplishment of European guidelines by optimizing mechanical pre-treatment and solvent extraction operations[J]. Journal of Power Sources,2012,212:205-211.
[6] 张涛. 废弃锂离子电池破碎及富钴产物浮选的基础研究[D]. 徐州:中国矿业大学,2015.ZHANG T. Mechanical crushing of spent lithium-ion batteries and flotation of cobalt enriched crushed products[D]. Xuzhou:China University of Mining and Technology,2015.
[7] SHIN S M,KIM N H,SOHN J S,et al. Development of a metal recovery process from Li-ion battery wastes[J]. Hydrometallurgy,2005,79(3/4):172-181.
[8] LI J,SHI P,WANG Z,et al. A combined recovery process of metals in spent lithium-ion batteries[J]. Chemosphere,2009,77(8):1132-1136.
[9] 文瑞明,刘长辉,游沛清,等. 分离回收废旧锂离子电池电极材料的浮选实验研究[J]. 中南大学学报(自然科学版),2014,45(1):40-44.WEN R M,LIU C H,YOU P Q,et al. Flotation on recovery of electrode materials from spent lithium-ion batteries[J]. Journal of Central South University (Science and Technology),2014,45(1):40-44.
[10] 李红,何亚群,张涛,等. 废弃锂离子电池富钴破碎产物的可浮性[J]. 中国有色金属学报,2014,24(10):2530-2538.LI H,HE Y Q,ZHANG T,et al. Floatability of Co-enriched crushed products of spent lithium-ion batteries[J]. The Chinese Journal of Nonferrous Metals,2014,24(10):2530-2538.
[11] 张伟刚,何亚群,张涛,等. 废弃锂离子电池富钴粉体可浮性的改善[J]. 中国粉体技术,2016,22(1):23-27.ZHANG W G,HE Y Q,ZHANG T,et al. Floatability improvement ofco-enriched powders recovered from spent lithium-ion battery[J].China Powder Science and Technology,2016,22(1):23-27.
[12] 梁立君. 废旧锂离子电池正极材料LiFePO4的回收及合成的研究[D]. 大连:大连交通大学,2012.LIANG L J. Studies on recovery and synthetic of anode materials LiFePO4for waste lithium ions batteries[D]. Dalian:Dalian Jiaotong University,2012.
[13] LI L,DUNN J B,ZHANG X X,et al. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment[J]. Journal of Power Sources,2013,233:180-189.
[14] 刘正,戴长松,纪大龙,等. 废旧磷酸钒锂电池材料回收及再利用研究[J]. 电源技术,2016(1):35-38.LIU Z,DAI C S,JI D L,et al. Spent lithium vanadium phosphate battery material recycling and reusing[J]. Chinese Journal of Power Source,2016(1):35-38.
[15] FERREIRA D A,PRADOS L M Z,MAJUSTE D,et al.Hydrometallurgical separation of aluminum,cobalt,copper and lithium from spent Li-ion batteries[J]. Journal of Power Sources,2009,187(1):238-246.
[16] 谭群英,唐红辉,龙桂花,等. 二级逆流浸出废旧锂离子电池正极片中的铝[J]. 矿冶工程,2012(3):92-94.TAN Q Y,TANG H H,LONG G H,et al. Leaching aluminum from cathode electrode of spent lithium ion batteries by two-stage countercurrent process[J]. Mining and Metallurgical Engineering,2012(3):92-94.
[17] YANG Y,HUANG G,XU S,et al. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries[J].Hydrometallurgy,2015,165:390-396.
[18] CHEN X,ZHOU T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media[J].Waste Management & Research,2014,32 (11):1083-1093.
[19] LEE C K,RHEE K I. Reductive leaching of cathodic active materials from lithium ion battery wastes[J]. Hydrometallurgy,2003,68(1/2/3):5-10.
[20] 孙亮. 废旧锂离子电池回收利用新工艺的研究[D]. 长沙:中南大学,2012.SUN L. A novel reclamation process for spent lithium ions batteries[D]. Changsha:Central South University,2012.
[21] LIU K,ZHANG F S. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dichlorination of polyvinyl chloride in subcritical water[J]. Journal of Hazardous Materials,2016,316:19-25.
[22] BIAN D,SUN Y,LI S,et al. A novel process to recycle spent LiFePO4for synthesizing LiFePO4/C hierarchical microflowers[J].Electrochimica Acta,2016,190:134-140.
[23] NAYAKA G P,MANJANNA J,PAI K V,et al. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids[J].Hydrometallurgy,2015,151:73-77.
[24] XU J,THOMAS H R,FRANCIS R W,et al. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources,2004,177(2):512-527.
[25] NAYAKA G P,PAI K V,MANJANNA J,et al. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries[J].Waste Management,2016,51:234-238.
[26] PINNA E G,RUIZ M C,OJEDA M W,et al. Cathodes of spent Li-ion batteries:dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors[J]. Hydrometallurgy,2017,167:66-71.
[27] LI L,GE J,CHEN R,et al. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries[J].Waste Management,2010,30(12):2615-2621.
[28] ZENG X,LI J,SHEN B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid[J]. Journal of Hazardous Materials,2015,295:112-118.
[29] MESHRAM P,ABHILASH P B D,MANKHAND T R,et al.Comparison of different reductants in leaching of spent lithium ion batteries[J]. JOM,2016,68(10):2613-2623.
[30] NAYAKA G P,PAI K V,SANTHOSH G,et al. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co[J]. Hydrometallurgy,2016,161:54-57.
[31] SUN L,QIU K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management,2012,32(8):1575-1582.
[32] JHA M K,KUMARI A,JHA A K,et al. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone[J]. Waste Management,2013,33(9):1890-1897.
[33] GUO Y,LI F,ZHU H,et al. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid(HCl)[J]. Waste Management,2016,51:227-233.
[34] HOREH N B,MOUSAVI S M,SHOJAOSADATI S A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus Niger[J]. Journal of Power Sources,2016,320:257-266.
[35] LI L,LU J,REN Y,et al. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries[J]. Journal of Power Sources,2012,218:21-27.
[36] LI L,QU W,ZHANG X,et al. Succinic acid-based leaching system:a sustainable process for recovery of valuable metals from spent Li-ion batteries[J]. Journal of Power Sources,2015,282:544-551.
[37] 贺凤,满瑞林,刘琦,等. 燕麦秸秆酸浸回收废旧锂电池中Co的动力学[J]. 中国有色金属学报,2015,25(4):1103-1108.HE F,MAN R L,LIU Q,et al. Kinetics of acid leaching cobalt from waste lithium-ion batteries using oat straw[J]. The Chinese Journal of Nonferrous Metals,2015,25(4):1103-1108.
[38] BAJESTANI M I,MOUSAVI S M,SHOJAOSADATI S A.Bioleaching of heavy metals from spent household batteries using acidithiobacillus ferrooxidans:statistical evaluation and optimization[J]. Separation and Purification Technology,2014,132:309-316.
[39] NIU Z,ZOU Y,XIN B,et al. Process controls for improving bioleaching performance of both Li and Co from spent lithium ion batteries at high pulp density and its thermodynamics and kinetics exploration[J]. Chemosphere,2014,109:92–98.
[40] 邓孝荣,曾桂生,李卓,等. 氧化亚铁硫杆菌浸出废旧锂离子电池的工艺条件[J]. 环境化学,2012,31(9):1381-1386.ZHENG X R,ZENG G S,LI Z,et al. Optimization conditions of bioleaching spent lithium-ion batteries by thiobacillus ferrooxidans[J].Environmental Chemistry,2012,31(9):1381-1386.
[41] 辛亚云. 废旧锂离子电池中有价金属离子的生物淋滤及其机理研究[D]. 北京:北京理工大学,2016.XIN Y Y. Study on bioleaching of valuable metals from spent Li-ion batteries and mechanism exploration[D]. Beijing:Beijing Institute of Technology,2016.
[42] ZENG G,DENG X,LUO S,et al. A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries[J]. Journal of Hazardous Materials,2012,199/200:164-169.[43] ZENG G,LUO S,DENG X,et al. Influence of silver ions on bioleaching of cobalt from spent lithium batteries[J]. Minerals Engineering,2013,49:40-44.
[44] 常伟,满瑞林,尹晓莹,等. 电化学还原技术从废旧锂离子电池中浸出LiCoO2[J]. 中国有色金属学报,2014,24(3):787-792.CHANG W,MAN R L,YI X Y,et al. Leaching LiCoO2from spent lithium-ion batteries by electrochemical reduction[J]. The Chinese Journal of Nonferrous Metals,2014,24(3):787-792.
[45] 张建,满瑞林,徐筱群,等. 电解浸出废旧锂电池中钴的热力学和动力学[J]. 中国有色金属学报,2014,24(4):993-1000.ZHANG J,MAN R L,XU X Q,et al. Thermodynamic and kinetic of electrolytic leaching cobalt from waste lithium-ion battery[J]. The Chinese Journal of Nonferrous Metals,2014,24(4):993-1000.
[46] KANG J,SENANAYAKE G,SOHN J,et al. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272[J]. Hydrometallurgy,2010,100(3/4):168-171.
[47] ZHAO J M,SHEN X Y,DENG F L,et al. Synergistic extraction and separation of valuable metals from waste cathodic material of lithium ion batteries using Cyanex272 and PC-88A[J]. Separation and Purification Technology,2011,78(3):345-351.
[48] 潘晓勇,彭玲,陈伟华,等. 废旧锂离子电池中钴和锂的回收及综合利用[J]. 中国有色金属学报,2013,23(7):2047-2054.PAN X Y,PENG L,CHEN H W,et al. Recovery of Co and Li from spent lithium-ion batteries and their comprehensive utilization[J]. The Chinese Journal of Nonferrous Metals,2013,23(7):2047-2054.[49] PRANOLO Y,ZHANG W,CHENG C Y. Recovery of metals from spent lithium-ion battery leach solutions with a mixed solvent extractant system[J]. Hydrometallurgy,2010,102(1/2/3/4):37-42.
[50] 谭海翔,胡启阳,李新海,等. 钴酸锂废极片中钴回收新工艺研究[J]. 电源技术,2007(4):288-290.TAN H X,HU Q Y,LI X H,et al. Study on the new process for the recovery of cobalt from spent lithium cobalt oxide [J]. Chinese Journal of Power Source,2007(4):288-290.
[51] 孟洋. 利用废旧锂离子电池制备超细Co3O4粉体材料[D]. 锦州:辽宁工业大学,2015.MENG Y. Preparation of ultrafine Co3O4powder materials using waste lithium-ion battery[D]. Jinzhou:Liaoning University of Technology,2015.
[52] HUANG Y,HAN G,LIU J,et al. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leachingflotation-precipitation process[J]. Journal of Power Sources,2016,325:555-564.
[53] FREITAS M B J G,GELANTE V G,PIETRE M K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits[J]. Journal of Power Sources,2010,195(10):3309-3315.
[54] FREITAS M B J G,GARCIA E M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries[J]. Journal of Power Sources,2007,171(2):953-959.
[55] GARCIA E M,SANTOS J S,PEREIRA E C,et al. Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique[J]. Journal of Power Sources,2008,185(1):549-553.
Recovery of Co and Li from spent lithium ion batteries
MENG Qi,ZHANG Yingjie,DONG Peng,LIANG Feng
(National and Local Joint Engineering Laboratory for Lithium-ion Batteries and Materials Preparation Technology,Key Laboratory of Advanced Battery Materials of Yunnan Province,Faculty of Metallurgical and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,Yunnan,China)
With the development of lithium ion batteries industry,some issues related to the spent lithium ion batterie(sLIBs) such as environment pollution and resource recovery have gradually been significant.How to efficiently recover and reuse valuable metals in spent LIBs in a harmless way has become one of the focused issues across the world. In order to obtain a green and efficient recovery of valuable metals such as cobalt and lithium,the recovery processes of valuable metals in spent LIBs are introduced,including pretreatment,cathode material treatment and recovery of cobalt and lithium from leaching solution. New methods and technologies for each process are reviewed with emphasis as well as their advantages/disadvantages. Recently,the hydrometallurgical leaching process with a typical model of acid- reductant was the main method for recovery of spent LIBs. However,little work has been made on the leaching mechanism such as kinetic control and ions transfer path. The research prospects on the methods are also put forward. In order to promote the industrialization for recovery of spent LIBs,the research of organic acid leaching and product precipitating should be paid more attention,with the focus on enhancing leaching efficiency,increasing precipitation index and simplifying process condition.
spent lithium ions batteries;cobalt;lithium;recovery;regeneration
TF11
:A
:1000-6613(2017)09-3485-07
10.16085/j.issn.1000-6613.2016-2390
2016-12-22;修改稿日期:2017-04-12。
孟奇(1989—),男,博士研究生。E-mail:mengqi315117@126.com。联系人:董鹏。E-mail:dongpeng2001@ 126.com。
