E ff ects of visual information regarding tactile stimulation on the somatosensory cortical activation: a functional MRI study
2017-08-07HyeokGyuKwonSungHoJangMiYoungLee
Hyeok Gyu Kwon, Sung Ho Jang, Mi Young Lee
1 Department of Physicalerapy, College of Health Sciences, Catholic University of Pusan, Pusan, Republic of Korea
2 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea
3 Department of Physicalerapy, College of Health anderapy, Daegu Haany University, Gyeongsansi, Republic of Korea
E ff ects of visual information regarding tactile stimulation on the somatosensory cortical activation: a functional MRI study
Hyeok Gyu Kwon1, Sung Ho Jang2, Mi Young Lee3,*
1 Department of Physicalerapy, College of Health Sciences, Catholic University of Pusan, Pusan, Republic of Korea
2 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea
3 Department of Physicalerapy, College of Health anderapy, Daegu Haany University, Gyeongsansi, Republic of Korea
How to cite this article:Kwon HG, Jang SH, Lee MY (2017) E ff ects of visual information regarding tactile stimulation on the somatosensory cortical activation: a functional MRI study. Neural Regen Res 12(7):1119-1123.
Many studies have investigated the evidence for tactile and visual interactive responses to activation of various brain regions. However, few studies have reported on the e ff ects of visuo-tactile multisensory integration on the amount of brain activation on the somatosensory cortical regions.e aim of this study was to examine whether coincidental information obtained by tactile stimulation can a ff ect the somatosensory cortical activation using functional MRI. Ten right-handed healthy subjects were recruited for this study. Two tasks (tactile stimulation and visuotactile stimulation) were performed using a block paradigm during fMRI scanning. In the tactile stimulation task, in subjects with eyes closed, tactile stimulation was applied on the dorsum of the right hand, corresponding to the proximal to distal directions, using a rubber brush. In the visuotactile stimulation task, tactile stimulation was applied to observe the attached mirror in the MRI chamber re fl ecting their hands being touched with the brush. In the result of SPM group analysis, we found brain activation on the somatosensory cortical area. Tactile stimulation task induced brain activations in the leprimary sensory-motor cortex (SM1) and secondary somatosensory cortex (S2). In the visuo-tactile stimulation task, brain activations were observed in the both SM1, both S2, and right posterior parietal cortex. In all tasks, the peak activation was detected in the contralateral SM1. We examined the effects of visuo-tactile multisensory integration on the SM1 and found that visual information during tactile stimulation could enhance activations on SM1 compared to the tactile unisensory stimulation.
*< class="emphasis_italic">Correspondence to: Mi Young Lee, Ph.D., mykawai@hanmail.net.
orcid: 0000-0002-8858-9360 (Mi Young Lee)
Accepted: 2017-06-19
nerve regeneration; functional MRI; somatosensory cortex; somatosensory cortical activation; visuotactile stimulation; neural regeneration
Introduction
Many recent studies have reported that multisensory integration such as visuo-tactile, audio-tactile, and audio-visual integration leads to enhanced behavioral response, resulting in improvement of discrimination threshold and reduced reaction time (McGurk and MacDonald, 1976; Frens et al., 1995; Kennett et al., 2001; Johnson et al., 2006; Schaefer et al., 2006; Haggard et al., 2007; Hotting and Roder, 2009; Pasalar et al., 2010; Mahoney et al., 2014; Sekiyama et al., 2014). Among these multisensory integrations, visuo-tactile multisensory integration is known to be an important technique for use in the fi eld of behavioral neuroscience and rehabilitation (Banati et al., 2000; Haggard et al., 2007; Kim and James, 2010; Gentile et al., 2011; Mahoney et al., 2014). Therefore, many studies have investigated the evidence for tactile and visual interactive responses to activation of various brain regions (Banati et al., 2000; Nakashita et al., 2008; Gentile et al., 2011; Martinez-Jauand et al., 2012; Schaefer et al., 2012). However, few studies have reported on the e ff ects of visuo-tactile multisensory integration on the amount of brain activation on the somatosensory cortical regions (Kim and James, 2010; Gentile et al., 2011).
The aim of the current study was to determine whether coincidental visual information obtained by tactile stimulation can affect the somatosensory cortical activation using functional MRI (fMRI).
Participants and Methods
Participants
Ten right-handed healthy subjects (five males; mean age25.20 ± 2.49 years, range 22–29 years) with no history of neurological, physical, or psychiatric illness were recruited for this studyviabulletin board notices. All subjects understood the purpose of the study and provided written, informed consent prior to participation.e study protocol was conducted in accordance with the ethical principles stated in theDeclaration of Helsinkiand approved by the Institutional Review Board of Daegu Oriental Hospital of Daegu Haany University (DHUMC-D-14001).
Functional MRI
All subjects were examined in a supine position and fi rmly secured with an immobilizing frame. Two tasks (tactile stimulation and visuo-tactile stimulation) were performed using a block paradigm (20-second rest, 20-second stimulation). In the tactile stimulation task, in subjects with eyes closed, tactile stimulation was applied on the dorsum of the right hand, corresponding to the proximal to distal direction, using a rubber brush at a frequency of 1 Hz under metronome guidance. Rest block was not applied tactile stimulation. In the visuo-tactile stimulation task, tactile stimulation was applied in the same manner. In addition, subjects were instructed to watch the attached mirror in the MRI chamber re fl ecting their own hand being touched by the brush. Rest block was seeing the untouched hand. All stimulations were performed by the same experimenter. Each task was repeated three times and the sequences of tasks were assigned randomly.
A 1.5-T Philips Gyroscan Intera scanner (Hoffma n-La-Roche, Best, the Netherlands) and a standard head coil were used in performance of blood oxygenation level dependent (BOLD) fMRI. BOLD-weighted Echo Planar Imaging (EPI) parameters were as follows: repetition time /echo time = 2 seconds/60 ms, fi eld of view = 210 mm, fl ip angle = 90°, matrix size = 64 × 64, and slice thickness = 5 mm. In addition, T1-weighted anatomical reference images were obtained using the following parameters: 20 axial, 5 mm-thick, spin echo images were acquired with a matrix size of 128 × 128, and a fi eld of view of 210 mm. A total of 2,400 images were acquired parallel to the bicommissure line of the anterior commissure-posterior commissure.
fMRI data analysis was performed using statistical parametric mapping soware (SPM 8, Wellcome Department of Cognitive Neurology, London, UK) implemented in the MATLAB environment (e Mathworks, USA). All images were realigned, co-registered, and normalized. The data were then smoothed spatially with a Gaussian kernel at a full width at half maximum (FWHM) of 8 mm to improve signal to noise ratio. First level analysis for each subject was conducted to investigate individual brain activation maps. A second level analysis was performed using a random e ff ect model with one-samplet-tests for group analysis.en, images were registered to the standard stereotaxic space of Talairach coordinates for creation of statistical parametric maps documenting the group average. Activations were based on clusters larger than fi ve voxels. Quantitative comparisons between stimulations were made by comparison of changes in BOLD signals. Also, di ff erences in brain activation during each condition in subjects were compared using pairedt-test within the SPM. An uncorrected threshold ofP< 0.001 was considered statistically signi fi cant.
To analyze volume data mapped to the cortical surface, we projected fMRI group analysis results onto the left and right hemispheres of the Human Colin surface-based atlas mapped to the PALS-B12 surface (“Population-Average Landmark- and Surface-Based” atlas) using version 5.61 of the computerized anatomical reconstruction and editing toolkit (CARET: Washington University, St. Louis, MO, USA) soware (Nakahara et al., 2001; Van Essen et al., 2001; Van Essen, 2002, 2005). Data values in voxels that intersected the cortical surface were directly mapped to the vertices of each participant-specific fiducial cortical surface using the intersections of enclosing voxels and nodes. Nodes representing an individual hemisphere were deformed to the standard PALS-B12 atlas sphere with 73,730 nodes using selective landmarks and spherical alignment (Van Essen, 2005). Regions of interest were drawn around the primary sensory-motor cortex (SM1: Brodmann area (BA) 1, 2, 3, 4), posterior parietal cortex (PPC: BA 5, 7), and secondary somatosensory cortex (S2: BA 43), which are known for their contribution to somatosensory processing (Forss et al., 1999; Cramer st al., 2000; Jang et al., 2010). Voxel counts were used as a measure of amounts of cortical activation in response to each tactile stimulation in each region of interest.
Results
Comparison of brain activations between stimulation and rest conditions
In the result of one samplet-test for group analysis, the cortical activated clusters were found on the various areas related to somatosensory function. In the tactile stimulation task, brain activations were observed on the lepostcentral gyrus and thalamus and right insular, fusiform gyrus, caudate, limbic lobe, and cerebellum (P< 0.001, uncorrected).e peak activation of whole brain areas was detected in the leSM1 (x= –38,y= –24,z= 54; Brodmann area 3). In the visuo-tactile stimulation task, brain activations were observed on leprecentral gyrus, occipital gyrus, and medial frontal gyrus, right precuneus, fi ngulate, superior and inferior temporal gyrus, caudate, meddle and inferior frontal gyrus, and cerebellem, and both postcentral gyrus and insula (P< 0.001, uncorrected).e peak activation of whole brain was also seen in the leSM1 (x=P38,y=P24,z= 58; Brodmann area 4) (Figure 1 and Table 1).
We identi fi ed the voxel count for the amount of brain activation on the somatosensory cortical regions. Visuo-tactile stimulation task was performed to induce cortical activation on the leSM1 (voxel count: 2434) and S2 (voxel count: 12). On the other hand, visuo-tactile stimulation task had brain activations on the left SM1 (voxel count: 4203), right SM1 (voxel count: 154), left S2 (voxel count: 86), right S2 (voxel count: 178), and right PPC (voxel count: 337) (Table 2).
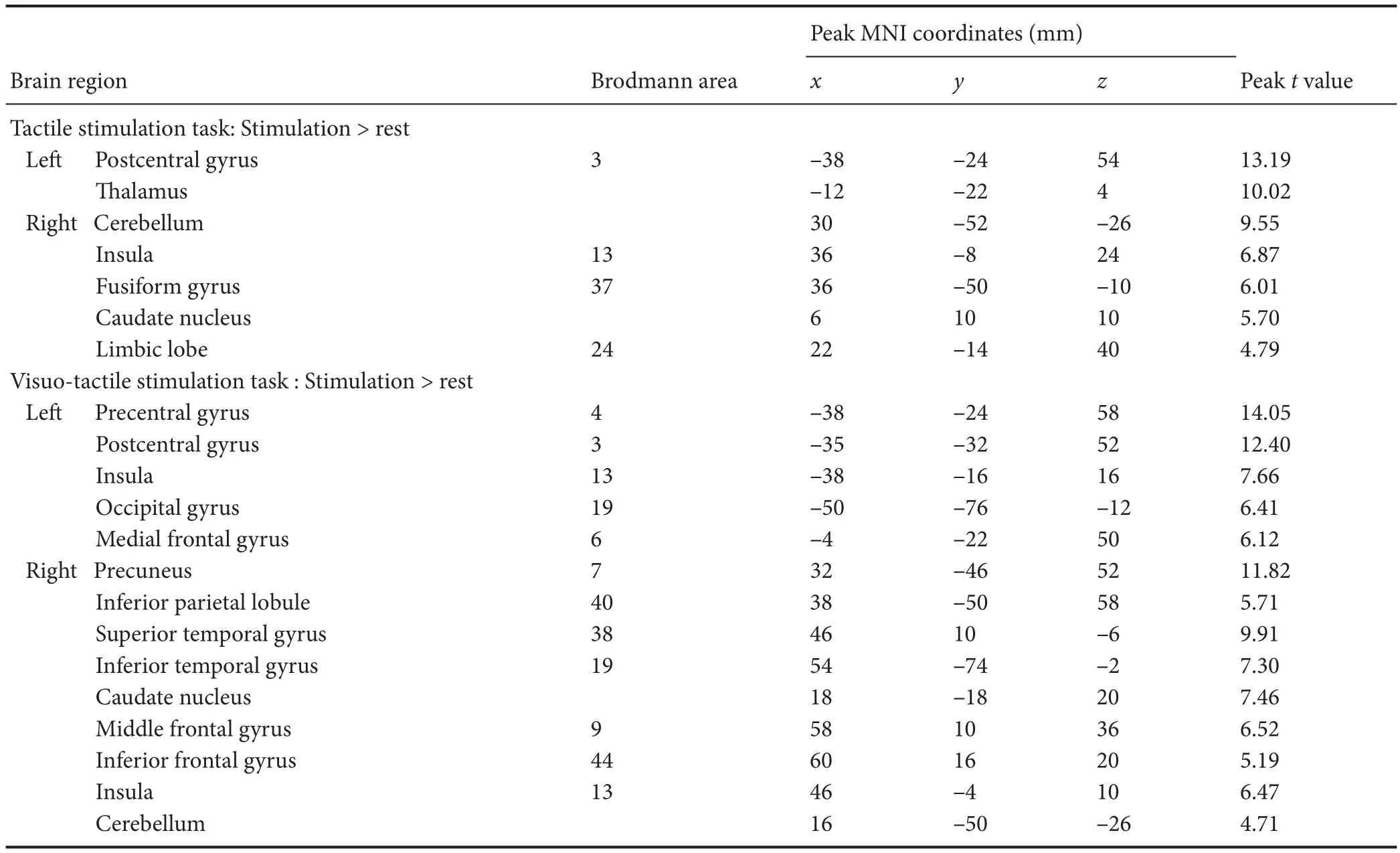
Table 1 Signi fi cant brain activation area during tactile and visuo-tactile stimulations relative to rest in right-handed healthy participants

Table 2 Voxel counts of activation related to somatosensory cortical regions during tactile and visuo-tactile stimulations in right-handed healthy participants
Comparison of brain activations between visuo-tactile and tactile stimulation tasks
Visuo-tactile stimulation task induced significant higher activation in both SM1 and PPC, lemeddle frontal gyrus and fusiform, right inferior frontal gyrus, inferior temporal gyrus, and anterior cingulate than tactile stimulation task (P< 0.001, uncorrected) (Table 3).
Discussion
In the current study, we investigated whether observation of being touched the body during tactile stimulation can a ff ect the brain activation on the somatosensory cortical regions. As a result, we found that visuo-tactile stimulation induced extended somatosensory cortical activations in both hemispheres of the SM1, S2, and the right hemisphere of the PPC compared with those of only tactile stimulation. In addition, higher peak activated response to SM1 was detected in visuo-tactile stimulation compared with that of only tactile stimulation. Our results appeared to indicate that integration of visual information during tactile stimulation would facilitate activation in the cortical area related to the somatosensory response.
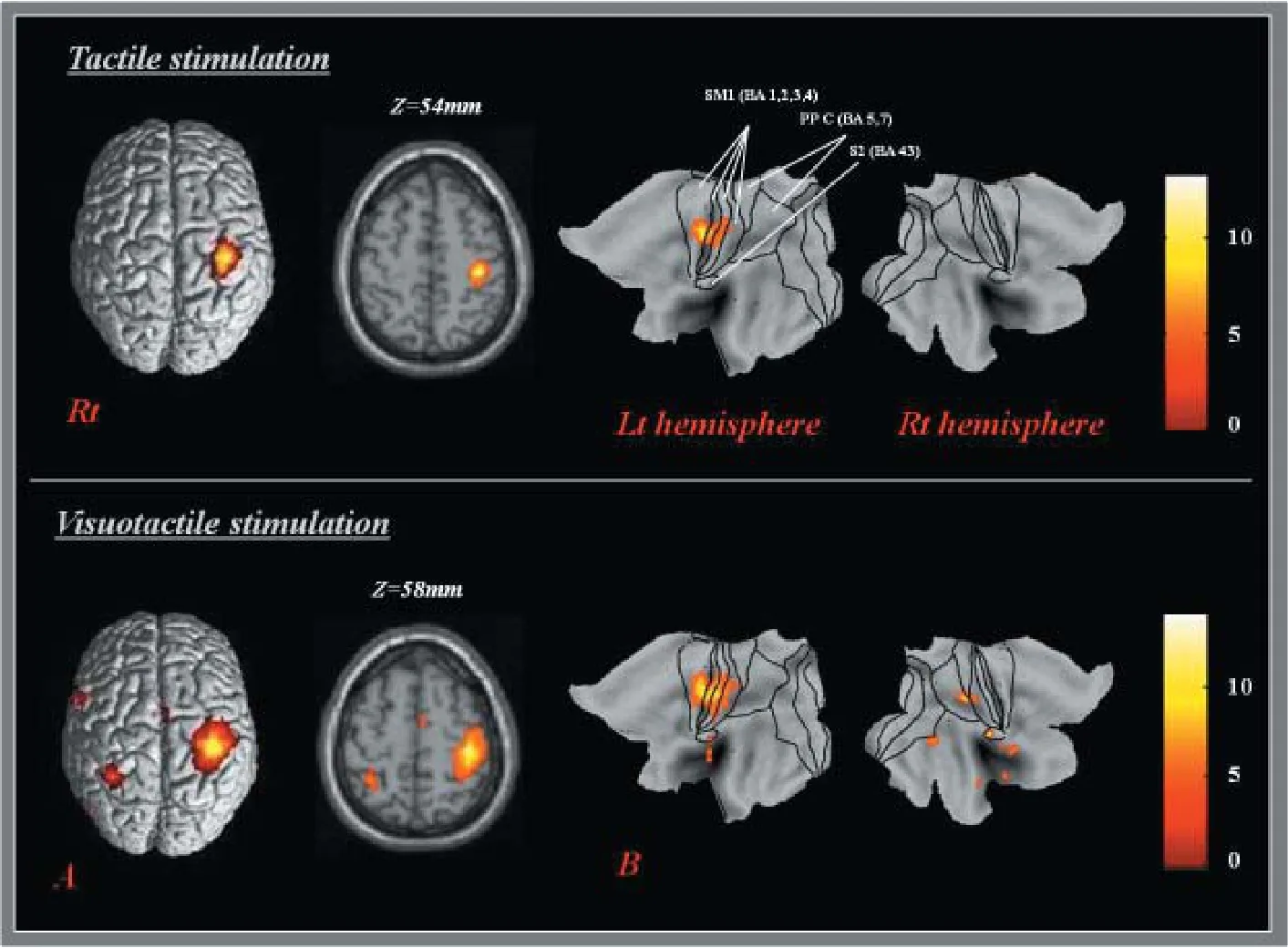
Figure 1 3D rendering of SPM (A) and projecting PALS-B12 atlas (B) for cortical activation in a right-handed healthy participant.

Table 3 Signi fi cant brain activation area between visuo-tactile and tactile stimulation tasks in right-handed healthy participants
Visuo-tactile multisensory integration is known to be involved in various brain regions, particularly the PPC, premotor regions, and putamen. In addition, many studies have reported the e ff ects of concurrent visual stimulation and that tactile stimulation improved both motor function and brain activations using various techniques including TMS, fMRI (Banati et al., 2000; Haggard et al., 2007; Kim and James, 2010; Gentile et al., 2011; Mahoney et al., 2014). In a behavior study, Haggard et al. (2007) reported that discrimination performance was improved when providing visuo-tactile stimulation compared to that of tactile stimulation in 10 healthy subjects. Mahoney et al. (2014) reported that the visuo-tactile stimulation reduced reaction time than simple visual or tactile stimulation in 147 healthy older subjects. In a neuroimaging study, using positron emission tomography, Banati et al. (2000) observed higher cerebral blood fl ow in the inferior parietal lobules, including S2, aer visuo-tactile stimulation than visual stimulation in eight healthy subjects. Subsequently, Kim and James (2010) reported that visual in combination with haptic stimulation induced higher activations on the contralateral occipital, fusiform gyrus, and intraparietal sulcus than simple visual or haptic stimulation in seven healthy subjects using fMRI. Gentilte et al. (2011) compared brain activation regions between simple visual or tactile stimulation and visuo-tactile stimulation in 24 healthy subjects and found that visuo-tactile stimulation induced greater BOLD activation in various brain regions including the contralateral ventral and dorsal premotor cortex, anterior part of the intraparietal sulcus, and inferior parietal cortex than simple visual or tactile stimulation.
Our results are comparable with those of previous studies. Previous studies (Banati et al., 2000; Kim and James, 2010; Gentile et al., 2011) focused on the distribution of brain activation regions rather than the SM1, which is an important region for sensori-motor function. Although our results showed that visuo-tactile stimulation led to greater activation in SM1 than tactile stimulation in healthy subjects, we believe that visuo-tactile stimulation could contribute to recovery of injured sensorimotor cortical area in patients who need sensorimotor rehabilitation. In addition, our results could provide evidence for research in the fi eld of neural regeneration and therapies.
In conclusion, we investigated the effects of visuo-tactile multisensory stimulation on the SM1 and found that visual information during tactile stimulation could enhance activations on the SM1 compared to the tactile unisensory stim-ulation. However, this study recruited only healthy subjects, which is a major limitation. Future studies addressing clinical significance in relation to brain activation or recovery of sensorimotor function following injury of sensorimotor cortical area should be encouraged.
Author contributions:MYL designed this study. HGK collected experimental data. SHJ provided technical assistance and supervised the study. HGK and MYL wrote the paper and provided critical revision of the paper for intellectual content. All authors approved the fi nal version of this paper.
Con fl icts of interest:None declared.
Research ethics:All subjects provided informed consent for participation and the study was approved by Institutional Review Board of Daegu Oriental Hospital of Daegu Haany University (approval number: DHUMC-D-14001). The study was performed in accordance with the Declaration of Helsinki and relevant ethical principles.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal.e participants understand that their names and initials will not be published and due e ff orts will be made to conceal their identity, but anonymity cannot be guaranteed.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Banati RB, Goerres GW, Tjoa C, Aggleton JP, Grasby P (2000) The functional anatomy of visual-tactile integration in man: a study using positron emission tomography. Neuropsychologia 38:115-124.
Bogousslavsky J, Caplan LR (2001) Stroke syndromes. 2nded. New York. Cambridge University Press.
Borich MR, Brodie SM, Gray WA, Ionta S, Boyd LA (2015) Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia 79(Pt B):246-255.
Cramer SC, Moore CI, Finklestein SP, Rosen BR (2000) A pilot study of somatotopic mapping aer cortical infarct. Stroke 31:668-671.
Forss N, Hietanen M, Salonen O, Hari R (1999) Modi fi ed activation of somatosensory cortical network in patients with right-hemisphere stroke. Brain 122:1889-1899.
Frens MA, Van Opstal AJ, Van der Willigen RF (1995) Spatial and temporal factors determine auditory-visual interactions in human saccadic eye movements. Percept Psychophys 57:802-816.
Gentile G, Petkova VI, Ehrsson HH (2011) Integration of visual and tactile signals from the hand in the human brain: an FMRI study. J Neurophysiol 105:910-922.
Haggard P, Christakou A, Serino A (2007) Viewing the body modulates tactile receptive fi elds. Exp Brain Res 180:187-193.
Hotting K, Roder B (2009) Auditory and auditory-tactile processing in congenitally blind humans. Hear Res 258:165-174.
Jang SH, Ahn SH, Lee J, Cho YW, Son SM (2010) Cortical reorganization of sensori-motor function in a patient with cortical infarct. NeuroRehabilitation 26:163-166.
Johnson RM, Burton PC, Ro T (2006) Visually induced feelings of touch. Brain Res 1073-1074:398-406.
Kennett S, Taylor-Clarke M, Haggard P (2001) Noninformative vision improves the spatial resolution of touch in humans. Curr Biol 11:1188-1191.
Kim S, James TW (2010) Enhanced effectiveness in visuo-haptic object-selective brain regions with increasing stimulus salience. Hum Brain Mapp 31:678-693.
Mahoney JR, Holtzer R, Verghese J (2014) Visual-somatosensory integration and balance: evidence for psychophysical integrative di ff erences in aging. Multisens Res 27:17-42.
Martinez-Jauand M, Gonzalez-Roldan AM, Munoz MA, Sitges C, Cifre I, Montoya P (2012) Somatosensory activity modulation during observation of other’s pain and touch. Brain Res 1467:48-55.
McGurk H, MacDonald J (1976) Hearing lips and seeing voices. Nature 264:746-748.
Nakahara H, Doya K, Hikosaka O (2001) Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences–a computational approach. J Cogn Neurosci 13:626-647.
Nakashita S, Saito DN, Kochiyama T, Honda M, Tanabe HC, Sadato N (2008) Tactile-visual integration in the posterior parietal cortex: a functional magnetic resonance imaging study. Brain Res Bull 75:513-525.
Pasalar S, Ro T, Beauchamp MS (2010) TMS of posterior parietal cortex disrupts visual tactile multisensory integration. Eur J Neurosci. 31:1783-1790.
Schaefer M, Heinze HJ, Rotte M (2012) Embodied empathy for tactile events: Interindividual di ff erences and vicarious somatosensory responses during touch observation. NeuroImage 60:952-957.
Schaefer M, Flor H, Heinze HJ, Rotte M (2006) Dynamic modulation of the primary somatosensory cortex during seeing and feeling a touched hand. Neuroimage 29:587-592.
Sekiyama K, Soshi T, Sakamoto S (2014) Enhanced audiovisual integration with aging in speech perception: a heightened McGurk e ff ect in older adults. Front Psychol 5:323.
Sommerfeld DK, von Arbin MH (2004)e impact of somatosensory function on activity performance and length of hospital stay in geriatric patients with stroke. Clin Rehabil 18:149-155.
Sullivan JE, Hedman LD (2008) Sensory dysfunction following stroke: incidence, significance, examination, and intervention. Top Stroke Rehabil 15:200-217.
Van Essen DC (2002) Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol 12:574-579.
Van Essen DC (2005) A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage 28:635-662.
Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH (2001) An integrated soware suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 8:443-459.
Copyedited by Li CH, Song LP, Zhao M
10.4103/1673-5374.211191
Mi Young Lee, Ph.D., mykawai@hanmail.net.
杂志排行
中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
