RACK1 regulates neural development
2017-08-07LeahKershnerKristyWelshhans
Leah Kershner, Kristy Welshhans,
1 Department of Biological Sciences, Kent State University, Kent, OH, USA
2 School of Biomedical Sciences, Kent State University, Kent, OH, USA
RACK1 regulates neural development
Leah Kershner1, Kristy Welshhans1,2,*
1 Department of Biological Sciences, Kent State University, Kent, OH, USA
2 School of Biomedical Sciences, Kent State University, Kent, OH, USA
How to cite this article:Kershner L, Welshhans K (2017) RACK1 regulates neural development. Neural Regen Res 12(7):1036-1039.
Funding: Research in the author's laboratory that is related to this review article is supported by a grant from NIH (NINDS; grant number R15NS098389 to KW).
Receptor for activated C kinase 1 (RACK1) is an evolutionarily conserved sca ff olding protein within the tryptophan-aspartate (WD) repeat family of proteins. RACK1 can bind multiple signaling molecules concurrently, as well as stabilize and anchor proteins. RACK1 also plays an important role at focal adhesions, where it acts to regulate cell migration. In addition, RACK1 is a ribosomal binding protein and thus, regulates translation. Despite these numerous functions, little is known about how RACK1 regulates nervous system development. Here, we review three studies that examine the role of RACK1 in neural development. In brief, these papers demonstrate that (1) RACK-1, theC. eleganshomolog of mammalian RACK1, is required for axon guidance; (2) RACK1 is required for neurite extension of neuronally di ff erentiated rat PC12 cells; and (3) RACK1 is required for axon outgrowth of primary mouse cortical neurons.us, it is evident that RACK1 is critical for appropriate neural development in a wide range of species, and future discoveries could reveal whether RACK1 and its signaling partners are potential targets for treatment of neurodevelopmental disorders or a therapeutic approach for axonal regeneration.
RACK1; RACK-1; neural development; neurite outgrowth; axon outgrowth; axon guidance
Accepted: 2017-06-26
Introduction
Receptor for activated C kinase 1 (RACK1) is an evolutionarily conserved scaffolding protein that can interact with multiple signaling molecules concurrently through its seven WD repeats. Although RACK1 interacts with many proteins, its most studied binding partners are protein kinase C (PKC) and Src tyrosine kinase. RACK1 binds and stabilizes the active conformation of PKC, and is both a substrate and inhibitor of Src. Other known binding partners of RACK1 include transmembrane receptors, receptor tyrosine kinases, ion channels, ribosomes, and integrins. Because RACK1 interacts with a wide variety of signaling molecules, it can regulate many functions within cells.ese include apoptosis, cell migration, circadian rhythms, and development (Adams et al., 2011). More recent studies have shown that RACK1 is also involved in feeding behaviors (Fang et al., 2015) and memory maintenance (Liu et al., 2016). Alterations in RACK1 expression and signaling are implicated in various diseases including neural tube defects, cardiac dysfunction, renal failure, cancer, addiction, muscle atrophy, and faulty sperm development (Adams et al., 2011). In addition, changes in RACK1 expression levels have been observed in cases of bipolar disorder (Wang and Friedman, 2001), Down syndrome (Peyrl et al., 2002), Alzheimer’s disease (Battaini et al., 1999; Battaini and Pascale, 2005), epilepsy (Xu et al., 2015), and addiction (McGough, 2004). Furthermore, changes in RACK1 localization have been observed in amyotrophic lateral sclerosis (ALS) patients (Russo et al., 2017) and there is increased interaction between huntingtin protein and RACK1 in a mouse model of Huntington’s disease (Culver et al., 2012).ough, how RACK1 contributes to these conditions is still unclear.
Little is also known about the role of RACK1 in nervous system development. However, RACK1 is crucial for development, as mice lacking RACK1 are embryonic lethal (Volta et al., 2013) and RACK1 interacts with PTK7 to control neural tube closure (Wehner et al., 2011). RACK1 is also a member of messenger ribonucleoprotein complexes, which are involved in the transport and translational regulation of mRNAs. Specifically, RACK1 forms a complex with the mRNA binding protein, zipcode binding protein 1 (ZBP1), andβ-actinmRNA. Within axonal growth cones, ZBP1 and RACK1 both regulate the local translation ofβ-actinmRNA following brain derived neurotrophic factor (BDNF) stimulation (Figure 1, Ceci et al., 2012). Local translation ofβ-actinmRNA is necessary for appropriate axon guidance (Yao et al., 2006).us, RACK1 may also regulate neural development via this mechanism (Ceci et al., 2012), as well as through its many other signaling functions (Adams et al., 2011).
RACK1 Regulates Axon Growth and Guidance
Demarco and Lundquist (2010) provided the first indication that RACK1 is involved in axon guidance. In this study, they investigated potential binding partners of UNC-115, which is homologous to the vertebrate actin binding LIM protein (abLIM) and a known regulator of lamellipodia and fi lopodia formation inC. elegansgrowth cones. A yeast two-hybrid screen revealed that UNC-115 binds to RACK-1, and subsequent co-immunoprecipitation experiments substantiated this result. Because RACK-1 interacts with Src, PKC, and UNC-115, all of which regulate axon path fi nding, it was hypothesized that RACK-1 would also regulate axon path fi nding.
The nervous system ofC. elegansis well-characterized; thus, any defects in axon path fi nding are easily detected. Demarco and Lundquist (2010) used RNA interference (RNAi) torack-1, and found that it caused defects in axon path fi nding of DD and VD motor neurons. These defects, which include aberrant axon guidance, branching, and premature termination, are very similar to those seen withunc-115perturbation. In addition to RNAi, an in-frame deletion of therack-1locus, which eliminates part of the fi rst, all of the second, and most of the third WD repeat (which contains the PKC binding site) was used. Similar to the RNAi experiments, the deletion allele also results in path fi nding defects of DD and VD motor neurons.
Finally, Demarco and Lundquist (2010) determined where RACK-1 is expressed by using a reporter transgene (RACK-1::GFP). RACK-1::GFP is present in most tissues, and is predominantly in the cytoplasm. Further, it is also expressed in the growth cones, axons, and cell bodies of VD commissural axons. As RACK-1 is expressed in most tissues, the next question was whether RACK-1 regulates axon path fi nding in a cell-autonomous manner. Expression of rack-1 was driven in DD and VD neurons using the GABAergic neuron-speci fi cunc-25promoter. Expression of this transgene signi fi cantly rescued lateral asymmetry and axon wandering defects in therack-1deletion animals, con fi rming that RACK-1 cell-autonomously regulates axon path fi nding.e pathway by which RACK-1 regulates axon path fi nding was also investigated. RACK-1 acts downstream of CED-10/Rac and upstream of UNC-115 to regulate lamellipodia and fi lopodia formation as well as axon path fi nding.ese results show that RACK-1 is an important regulator ofC. elegansneural development.
A recent study investigated the role of RACK1 in a rat PC12 cell line, which can be di ff erentiated into neuron-like cells (Dwane et al., 2014). Because RACK1 regulates cell protrusion via FAK in non-neuronal cells, it was suspected that a similar interaction may occur in neurons. Indeed, RACK1 coimmunoprecipitates with FAK in both rat hippocampal cells and PC12 cells. Next, PC12 cells were transfected with a plasmid containing RACK1-Y52F, a construct that prevents the interaction of FAK and RACK1. Cells expressing this construct have signi fi cantly shorter neurites, suggesting the interaction between FAK and RACK1 is necessary for neurite outgrowth. Dwane et al. (2014) then investigated whether other RACK1 binding partners might be part of this pathway. Mass spectrometry of RACK1 immunoprecipitates from rat hippocampus identi fi ed AGAP2 as a RACK1 interacting protein. Interestingly, AGAP2 is a known regulator of FAK activity and focal adhesion assembly. Further experiments demonstrated that following RACK1 knockdown, the interaction of AGAP2 to FAK is decreased. AGAP2 knockdown was also shown to cause a 3-fold decrease in neurite length.us, RACK1, at least in part through its interactions with FAK and AGAP2, is a regulator of neurite extension in PC12 cells (Dwane et al., 2014).
We recently showed that RACK1 directly regulates axon growth of primary mouse cortical neurons. First, we performed a knockdown of RACK1 using shRNA (Kershner and Welshhans, 2017). Knockdown of RACK1 results in signi fi cantly shorter axons versus non-silencing control shRNA. Further, knockdown of RACK1 eliminates a BDNF-induced increase in growth cone area, suggesting that RACK1 plays an important role in the regulation of growth cone morphology. Next, we overexpressed wild-type RACK1. Interestingly, RACK1 overexpression also results in significantly shorter axonsversuscontrol conditions, suggesting that appropriate levels of RACK1 expression are necessary for axon outgrowth. Overexpression of RACK1 results in aberrant growth cone morphology and spreading as well. Under basal conditions, the growth cone area of RACK1 overexpressing neurons is significantly higher than control. Moreover, BDNF does not result in an increase in growth cone area.us, it appears that overexpression of RACK1 creates a ceiling e ff ect whereby growth cones no longer respond to BDNF. In summary, we fi nd that RACK1 is essential to appropriate axon length and growth cone morphology under both basal and BDNF-stimulated conditions.
RACK1 Regulates Local Translation and Point Contacts in Growth Cones
Taken together, these studies show a clear role for RACK1 in the regulation of neural development. RACK1 is a multifunctional sca ff olding protein, and therefore could regulate neural development through multiple pathways. We focused our studies on the translational aspect of RACK1. RACK1 binds directly to ribosomes and is a member of the local translation complex (Ceci et al., 2012), thus it is highly likely that RACK1 mediated local translation regulates neural development. We further investigated the role of RACK1 in local translation, speci fi cally examining where in the growth cone local translation occurs.
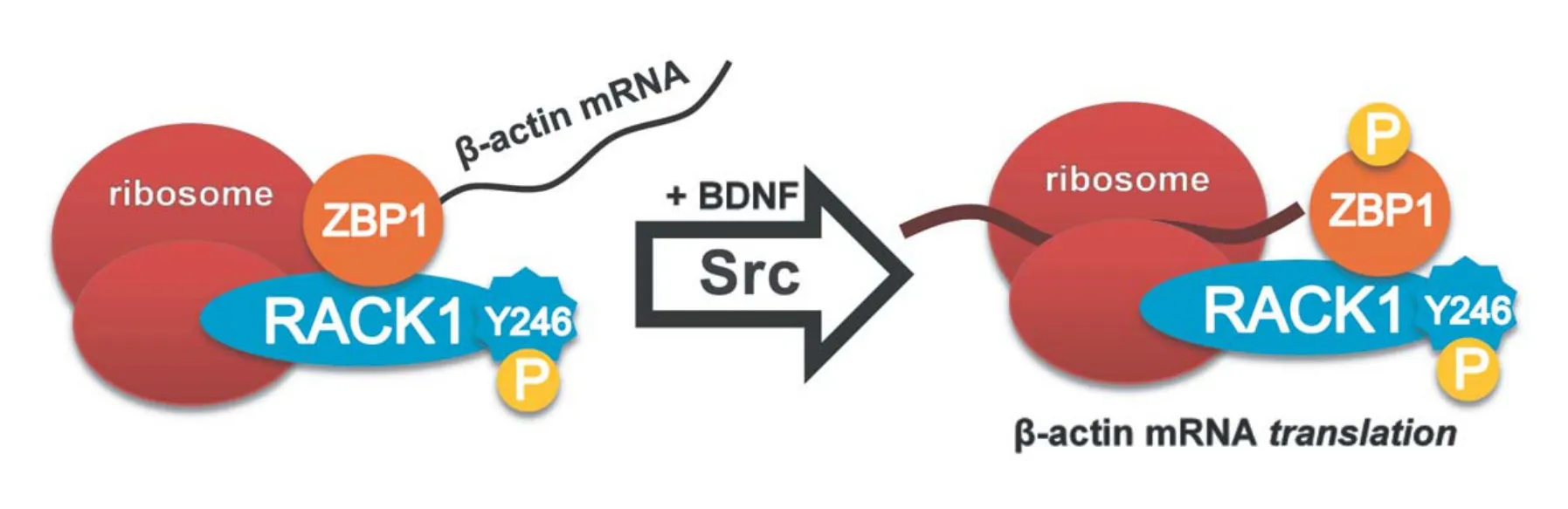
Figure 1 Model for the probable mechanism by which RACK1 facilitates local translation aer BDNF induced phosphorylation by Src kinase.

Figure 2 Appropriate levels of RACK1 are required for optimal point contact density and axon outgrowth.
Multiple studies have demonstrated that point contacts, structures which adhere growth cones to the extracellular matrix, contribute to guidance cue mediated axon path fi nding (Short et al., 2016). Point contacts are somewhat similar to focal adhesions seen in other cell types, and are composed of adhesion proteins that link the extracellular matrix to the actin cytoskeleton. Within growth cones, retrograde fl ow is a facet of actin treadmilling whereby actin fi laments fl ow from the distal (barbed) end back towards the proximal (pointed) end. “Clutching” of retrograde flow via focal adhesions in non-neuronal cells provides the force necessary for membrane protrusion and extension. A recent study has demonstrated that this “clutching” is accomplished in growth cones directly by point contact regulation of retrograde fl ow (Nichol et al., 2016).us, point contacts are essential to guidance cue mediated axon outgrowth and path fi nding, and appropriate neural development.
Because point contacts respond dynamically to guidance cues to regulate growth cone protrusion (Myers and Gomez, 2011; Nichol et al., 2016) and thus would be an optimal site for local translation, we investigated whether local translation occurs at point contacts. To address this question, we fi rst examined whether RACK1, which is known to directly bind ribosomes, localizes at point contacts in growth cones (Kershner and Welshhans, 2017). We stained for two markers of point contacts, paxillin and vinculin, and are the fi rst to show that RACK1 localizes at point contacts. However, this does not necessarily indicate that local translation occurs at point contacts.erefore, we stained for other members of the local translation complex, ribosomes andβ-actinmRNA, and found that both of them reside at point contacts. A previous study demonstrated that BDNF simulation results in an increase in the local translation ofβ-actinin growth cones (Yao et al., 2006). In our most recent study, we demonstrate that BDNF stimulation significantly increases the localization of RACK1 andβ-actinmRNA at point contacts (Kershner and Welshhans, 2017).is provides additional support that point contacts are a site of local translation.us, point contacts act not only as adherence sites, but also as signaling centers for local translation within developing neurons.
Next, we examined how RACK1 expression levels affect point contact formation (Kershner and Welshhans, 2017). We found, in line with previous fi ndings (Myers and Gomez, 2011), that BDNF results in an increase in point contact density in growth cones (Kershner and Welshhans, 2017). We also found that knockdown of RACK1 signi fi cantly decreases point contact density in growth cones under basal conditions and eliminates the BDNF-induced increase in paxillin-containing point contacts. Further, overexpressionof RACK1 causes a “ceiling effect” whereby point contact density is signi fi cantly increased under basal conditions and there is no additional increase following BDNF stimulation.erefore, we conclude that appropriate RACK1 expression levels are required for point contact formation and, as a result, axon outgrowth (Figure 2). It is possible that separate pathways regulate point contact formation and axon outgrowth; however, the retrograde fl ow experiments by Nichol et al. (2016) demonstrate that point contact formation and growth cone protrusion are linked.us, we hypothesize it is the same pathway, and future experiments will help elucidate this mechanism.
Conclusions and Future Directions
From these studies, it is clear that RACK1 is vital for neurodevelopment given its regulation of axon growth and guidance, point contacts and local translation. RACK1 can regulate both point contact formation and local translation because it is a multi-functional ribosomal scaffolding protein. Thus, it can bind to components of the extracellular matrix, signaling molecules, RNA binding proteins, and ribosomes. Future studies in live cells using total internal reflection fluorescence (TIRF) microscopy, which permits visualization of proteins only at the membrane-substrate interface, will allow us to determine how RACK1 regulates aspects of point contact dynamics, such as formation, lifetime, and turnover. Further, the use of photoconvertible translation reporters in live cells will conclusively reveal whether local translation ofβ-actinmRNA occurs at point contacts. Increasing our understanding of this topic will not only provide foundational knowledge of the mechanism by which RACK1 regulates neural development, but could potentially reveal how disrupted RACK1 signaling may contribute to neurodevelopmental de fi cits.us, targeting RACK1 signaling may be a promising treatment for neurodevelopmental disorders and a therapeutic approach to axonal regeneration.
Author contributions:LK and KW both contributed to the conception, writing, and revision of this manuscript.
Con fl icts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Open peer reviewer:Madhu G. Tapadia, Banaras Hindu University, India.
Adams DR, Ron D, Kiely PA (2011) RACK1, A multifaceted sca ff olding protein: Structure and function. Cell Commun Signal 9:22.
Battaini F, Pascale A (2005) Protein kinase C signal transduction regulation in physiological and pathological aging. Ann N Y Acad Sci 1057:177-192.
Battaini F, Pascale A, Lucchi L, Pasinetti GM, Govoni S (1999) Protein kinase C anchoring de fi cit in postmortem brains of Alzheimer’s disease patients. Exp Neurol 564:559-564.
Ceci M, Welshhans K, Ciotti MT, Brandi R, Parisi C, Paoletti F, Pistillo L, Bassell GJ, Cattaneo A (2012) RACK1 is a ribosome sca ff old protein for β-actin mRNA/ZBP1 complex. PLoS One 7:e35034.
Culver BP, Savas JN, Park SK, Choi JH, Zheng S, Zeitlin SO, Yates JR, Tanese N (2012) Proteomic analysis of wild-type and mutant huntingtin-associated proteins in mouse brains identi fi es unique interactions and involvement in protein synthesis. J Biol Chem 287:21599-21614.
Demarco RS, Lundquist EA (2010) RACK-1 acts with Rac GTPase signaling and UNC-115/abLIM in Caenorhabditis elegans axon pathf i nding and cell migration. PLoS Genet 6:e1001215.
Dwane S, Durack E, O’Connor R, Kiely PA (2014) RACK1 promotes neurite outgrowth by sca ff olding AGAP2 to FAK. Cell Signal 26:9-18.
Fang L, Zhou J, Cheng S, Ying J, Yang Z, Yin L, Li S, Hou W, Wang Z (2015) High orexin-A neuron activity and RACK1 expression might be involved in the restricted feeding-entrained behaviors in mice. Biol Rhythm Res :1-11.
Kershner L, Welshhans K (2017) RACK1 is necessary for the formation of point contacts and regulates axon growth. Dev Neurobiol doi:10.1002/dneu.22491.
Liu L, Zhu J, Zhou L, Wan L (2016) RACK1 promotes maintenance of morphine-associated memory via activation of an ERK-CREB dependent pathway in hippocampus. Sci Rep 6:20183.
McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Kharazia V, Janak PH, Ron D (2004) RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci 24:10542-10552.
Myers JP, Gomez TM (2011) Focal adhesion kinase promotes integrin adhesion dynamics necessary for chemotropic turning of nerve growth cones. J Neurosci 31:13585-13595.
Nichol IV RH, Hagen KM, Lumbard DC, Dent EW, Go TM, Nichol RH, Hagen KM, Lumbard DC, Dent EW, Gomez TM (2016) Guidance of axons by local coupling of retrograde fl ow to point contact adhesions. J Neurosci 36:2267-2282.
Peyrl A, Weitzdoerfer R, Gulesserian T, Fountoulakis M, Lubec G (2002) Aberrant expression of signaling-related proteins 14-3-3 gamma and RACK1 in fetal Down syndrome brain (trisomy 21). Electrophoresis 23:152-157.
Russo A, Scardigli R, La Regina F, Murray ME, Romano N, Dickson DW, Wolozin B, Cattaneo A, Ceci M (2017) Increased cytoplasmic TDP-43 reduces global protein synthesis by interacting with RACK1 on polyribosomes. Hum Mol Genet 26:1407-1418.
Short CA, Suarez-Zayas EA, Gomez TM (2016) Cell adhesion and invasion mechanisms that guide developing axons. Curr Opin Neurobiol 39:77-85.
Volta V, Beugnet A, Gallo S, Magri L, Brina D, Pesce E, Calamita P, Sanvito F, Bi ff o S (2013) RACK1 depletion in a mouse model causes lethality, pigmentation deficits and reduction in protein synthesis e ffi ciency. Cell Mol Life Sci 70:1439-1450.
Wang HY, Friedman E (2001) Increased association of brain protein kinase C with the receptor for activated C kinase-1 (RACK1) in bipolar a ff ective disorder. Biol Psychiatry 50:364-370.
Wehner P, Shnitsar I, Urlaub H, Borchers A (2011) RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development 138:1321-1327.
Xu X, Yang X, Xiong Y, Gu J, He C, Hu Y, Xiao F, Chen G, Wang X (2015) Increased expression of receptor for activated C kinase 1 in temporal lobe epilepsy. J Neurochem 133:134-143.
Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ (2006) An essential role for β-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci 9:1265-1273.
Kristy Welshhans, Ph.D., kwelshha@kent.edu.
10.4103/1673-5374.211175
*< class="emphasis_italic">Correspondence to: Kristy Welshhans, Ph.D., kwelshha@kent.edu.
orcid: 0000-0001-6624-4719 (Kristy Welshhans)
杂志排行
中国神经再生研究(英文版)的其它文章
- Mitochondrial quality control in amyotrophic lateral sclerosis: towards a common pathway?
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- SoxC transcription factors in retinal development and regeneration
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
