BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
2017-08-07ToshiyukiMizuiKojiOhiraMasamiKojima
Toshiyuki Mizui, Koji Ohira, Masami Kojima,
1 Biomedical Research Inst. (BMD), National Institute of Advanced Industrial Science and Technology (AIST), Osaka, Japan
2 Laboratory of Nutritional Brain Science, Department of Food Science and Nutrition, Mukogawa Women’s University, Hyogo, Japan
3 Core Research for Evolutional Science and Technology (CREST), Science and Technology Agency (JST), Kawaguchi, Japan
4 Graduate School of Frontier Bioscience, Osaka University, Suita, Japan
BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
Toshiyuki Mizui1,3, Koji Ohira2, Masami Kojima1,3,4,*
1 Biomedical Research Inst. (BMD), National Institute of Advanced Industrial Science and Technology (AIST), Osaka, Japan
2 Laboratory of Nutritional Brain Science, Department of Food Science and Nutrition, Mukogawa Women’s University, Hyogo, Japan
3 Core Research for Evolutional Science and Technology (CREST), Science and Technology Agency (JST), Kawaguchi, Japan
4 Graduate School of Frontier Bioscience, Osaka University, Suita, Japan
How to cite this article:Mizui T, Ohira K, Kojima M (2017) BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing. Neural Regen Res 12(7):1024-1027.
Most growth factors are initially synthesized as precursors and it was cleaved into bioactive mature domain and pro-domain. However, compared with the expression and function of bioactive mature domain, the biological role of the pro-domain is poorly understood. Unexpectedly, we found that the pro-domain (or pro-peptide) of brain-derived neurotrophic factor (BDNF), which is well-known neurotrophic factor in brain, has a potential ability to facilitate hippocampal long-term depression. Furthermore, a BDNF polymorphism Val66Met, which substitute valine into methionine at 66 amino acid, impacted the biological activity of the BDNF pro-peptide. We lastly discuss the possible roles of BDNF and its pro-peptide in the generation of neural stem cells and progress of ischemia.
BDNF; growth factor; neural stem cells; ischemia; peptide
Accepted: 2017-07-05
Introduction
Brain-derived neurotrophic factor (BDNF) is a well-studied growth hormone that controls numerous complex physiological processes in the nervous system. Herein, we briefly review the role of BDNF, and focus on the novel bioactive BDNF pro-peptide that is generated by proteolytic processing of the proBDNF precursor. Interestingly, the pro-peptide facilitates hippocampal long-term depression, while BDNF reportedly enhances long-term potentiation. Thus, these peptides appear to have opposing biological roles in the nervous system. Recent and future research in this area could provide new insight into the neurobiology and neuropathology of BDNF and related proteins.
BDNF Functions and Cellular Mechanisms
BDNF is a neurotrophin that is widely distributed in the central nervous system (CNS) and exerts its biological actions by binding to tyrosine kinase receptor TrkB. In developing neurons, BDNF mainly promotes survival and di ff erentiation. During development, however, the biological actions of BDNF extend beyond neuronal survival and differentiation. In mature neurons, BDNF reportedly enhances synaptic transmission and modulates synaptic plasticity.is observation was further supported by genetic and behavioral mouse studies, the results of which indicate that the primary function of BDNF in the adult brain is to control synaptic transmission and plasticity, rather than cell survival. In line with these fi ndings, BDNF expression and secretion is elicited by neuronal activity, a fundamental driving force of neuronal networks. Furthermore, the transcriptional mechanism underlying the activity-dependent expression of BDNF has been thoroughly investigated. Numerous reports implicate impaired BDNF function in cellular mechanisms related to brain diseases.us, the molecular and cellular knowledge concerning BDNF, accumulated since its discovery, has gradually illuminated its physiological and pathological roles. For more detailed information, please see a recent review article by Castren and Antila (2017).
BDNF Pro-peptide is Localized at Presynaptic Dense Core Vesicles in Hippocampal Mossy Fibers
Post-translational mechanisms are known to specify and diversify the actions of growth factors. However, compared with transcription and cell signaling, post-translational mechanisms related to BDNF are less well understood. Like other growth factors, BDNF is initially synthesized as a precursor protein (proBDNF, ~270 amino acids), which is composed of a signal sequence, pro-domain, and mature domain (Figure 1A). To produce bioactive BDNF, the N-terminalpro-domain (~120 amino acids) is cleaved from proBDNF by intracellular and/or extracellular peptidase enzymes (Lessmann and Brigadski, 2009). In theory, BDNF and its pro-peptide (pro-domain) are produced in equivalent amounts aer processing, but the existence of the endogenous pro-peptide had not been detected until recently. Dieni et al. (2012) fi rst demonstrated the endogenous presence of the BDNF pro-peptide by western blotting and immunocytochemistry. Interestingly, in their electro-microscopic study, both BDNF and its pro-peptide were localized in dense core vesicles in excitatory presynaptic terminals of the adult mouse hippocampus, suggesting that they function in the synapse region and are secreted in an anterograde manner.
BDNF Pro-peptide is a Novel Facilitator of Long-Term Depression (LTD)
Although the BDNF pro-peptide corresponds to the N-terminal fragment of proBDNF, we found that it functions as a modulator of synaptic plasticity by enhancing hippocampal LTD (Mizui et al., 2015). In our study, we applied a sequence of low-frequency stimulation (LFS) pulses (900 pulses, 1 Hz, 15 minutes) to Scha ff er collaterals of hippocampal slices prepared from 3–4-week-old juvenile mice, and measured fi eld excitatory postsynaptic potential (fEPSP) slopes in the CA1 area. We showed that a 30-minute treatment with the BDNF pro-peptide (10 ng/mL) enhances LTD without a ff ecting basal synaptic transmission, and a sub-nanomolar concentration of BDNF pro-peptide is sufficient for LTD enhancement (Figure 1B, Val-BDNF pro-peptide + LFS). Additionally, application of the BDNF pro-peptide to Bdnf–/–hippocampal slices facilitates LTD, confirming the role of the pro-peptide as a ligand. This work also revealed mechanistic details; BDNF pro-peptide-dependent LTD facilitation requires the activation of GluN2B-containing N-methyl-D-aspartate (NMDA) receptors and the pan-neurotrophin receptor p75NTR. In a study using Banker-style primary cultures of hippocampal neurons, BDNF pro-peptide promoted NMDA-induced α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor endocytosis, a critical mechanism for LTD expression. More recently, it was demonstrated that exposure of mature hippocampal neurons to the BDNF pro-peptide dramatically reduced dendritic spine densityin vitro(Guo et al., 2016). Interestingly, this effect was mediated by caspase-3, and BDNF pro-peptide increased the number of elongated mitochondria and the amount of cytosolic cytochrome c, indicating the involvement of the mitochondrial-caspase-3 pathway in the BDNF pro-peptide-induced decrease in spine density. BDNF pro-peptide has also recently been linked to growth cones (Anastasia et al., 2013) and LTD (Mizui et al., 2015), suggesting that it may act as a negative regulator of neuronal structure and function at distinct developmental stages.
Molecular Role of the Common Val66Met BDNF Polymorphism
There is a body of evidence speculating on the impact of the common Val66Met BDNF polymorphism in human brain functions and diseases (Notaras et al., 2015). Several reports focused on the molecular outcome of this amino acid substitution. We previously showed that the Val66Met polymorphism impairs intracellular trafficking of BDNF (Egan et al., 2003). It was next reported that the Vps10p protein sortilin interacts with the BDNF pro-domain, and the Val66Met mutation stimulated the interaction of the BDNF pro-domain with BDNF rather than with sortilin (Chen et al., 2005). Furthermore, we recently quantitatively demonstrated that the Val66Met mutation enhanced the stability of the complex between BDNF and its pro-peptide using surface plasmon resonance, and found that the interaction is stronger in acidic conditions (Uegaki et al., 2017). In another report, hippocampal slices prepared from mice with the Val66Met mutation revealed defective NMDAR-dependent synaptic plasticity (Ninan et al., 2010). Interestingly, our recent report showed that the BDNF pro-peptide-induced LTD and its mechanism endocytosis of GluA2, was reversed by the mutated BDNF pro-peptide (Figure 1B, Met-BDNF pro-peptide + LFS) (Mizui et al., 2015). These findings together suggest that the Val66Met polymorphism impacts the cellular activity of BDNF and the BDNF pro-peptide. In the future, it will be interesting to investigate the impact of the Val66Met BDNF polymorphism at the level of neuronal circuits, and probe the mechanistic in fl uence in brain diseases.
Development of Potential Newerapies for Brain Diseases Based on BDNF and Related Proteins
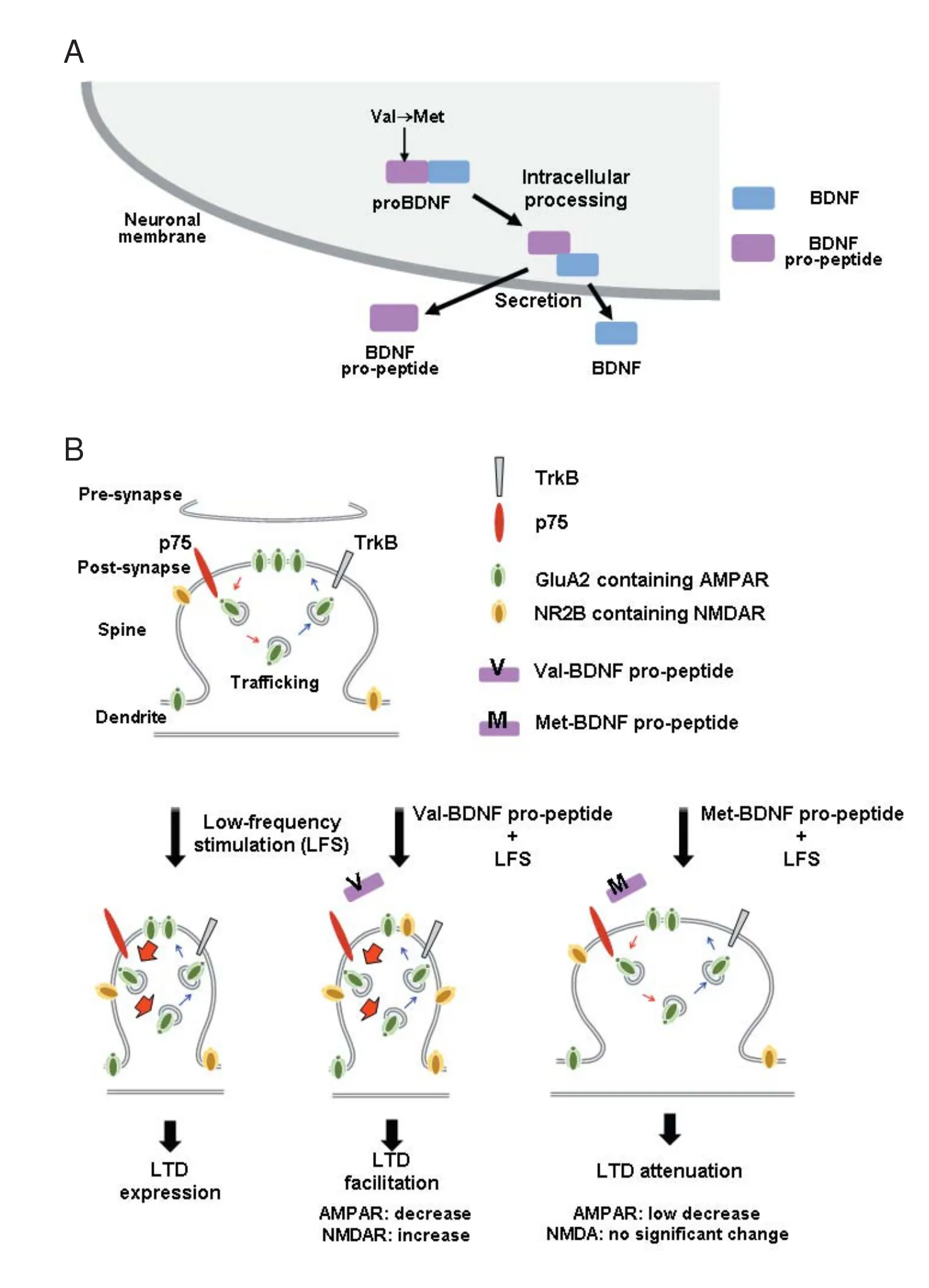
Figure 1 BDNF pro-peptide is a novel synaptic modulator generated from the BDNF precursor and Val66Met BDNF polymorphism located in the BDNF pro-peptide-dependent exerts distinct functions in LTD.
Adult neurogenesis in the subventricular zone (SVZ) and hippocampal dentate gyrus has been demonstrated in studies going back to the 1960s. In these regions, there are neural stem cells (NSCs) and neural progenitor cells (NPCs), which might be the cellular basis for endogenous regenerative therapy for brain damage. It was demonstrated that NPCs were detectable in the cortex, and their ability to produce new neurons was activated after brain ischemia (Ohira et al., 2010) and antidepressant treatments (Ohira et al., 2013). Additionally, new-born neurons possessed neuroprotective activity by inhibiting neuronal cell deathviaglutamate excitotoxicity in ischemia (Ohira et al., 2013).
Previously, it was demonstrated that brain ischemia elicits BDNF expression (Lindvall et al., 1994) and adult neurogenesis (Liu et al., 1998). A recent report shows that NSCs and NPCs express neurotrophin receptors, p75NTRand Trk receptors, and neurotrophin signaling could control proliferation and di ff erentiation of NSCs and NPCs (Vilar and Mira, 2016; Castren and Antila, 2017). Taken together, BDNF signaling might undergo neuroprotective and neuroregerative actions of NPCs against ischemia, while the BDNF pro-peptide (Anastasia et al., 2013; Mizui et al., 2015; Guo et al., 2016) may exert pathological action on NPCs and progressive role on ischemia.
Herein, we reviewed the role of the BDNF pro-peptide that corresponds to the N-terminus of proBDNF following cleavage by proteolytic processing. The BDNF pro-peptide is detectable in multiple cell and tissue types and elicits hippocampal LTD and spine pruning. Moreover, an interesting recent study showed that the BDNF pro-peptide is more abundant in the brain during development, and appears to control growth cone collapse (Anastasia et al., 2013).us, a new ligand-based model, in which BDNF and its pro-pep-tide exert distinct biological functions, could provide novel insight into the biology of neurotrophins and support the development of new therapies for brain diseases.
Author contributions:TM and MK made equal contributions to this paper as co-first authors. KO made contributions to the discussion on neural stem cells and brain diseases.
Con fl icts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Open peer review report:
Reviewer: Giovanni Casini, Universita degli Studi di Pisa, Italy.
Comments to author:is paper is a concise, very clear and up-to-date commentary on the physiology of BDNF pro-peptide.e research focusing on this peptide is relatively new and this paper makes sets the state of the art of such research fi eld.
Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, Hempstead BL, Bracken C (2013) Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun 4:2490.
Castren E, Antila H (2017) Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry doi:10.1038/mp.2017.61.
Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS (2005) Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25:6156-6166.
Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundel fi nger ED, Kojima M, Nestel S, Frotscher M, Barde YA (2012) BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol 196:775-788.
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003)e BDNF val66met polymorphism a ff ects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257-269.
Guo J, Ji Y, Ding Y, Jiang W, Sun Y, Lu B, Nagappan G (2016) BDNF pro-peptide regulates dendritic spines via caspase-3. Cell Death Dis 7:e2264.
Lessmann V, Brigadski T (2009) Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res 65:11-22.
Lim JY, Reighard CP, Crowther DC (2015)e pro-domains of neurotrophins, including BDNF, are linked to Alzheimer’s disease through a toxic synergy with Aβ. Hum Mol Genet 24:3929-3938.
Lindvall O, Kokaia Z, Bengzon J, Elmer E, Kokaia M (1994) Neurotrophins and brain insults. Trends Neurosci 17:490-496.
Liu J, Solway K, Messing RO, Sharp FR (1998) Increased neurogenesis in the dentate gyrus aer transient global ischemia in gerbils. J Neurosci 18:7768-7778.
Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T, Yamawaki S, Takahashi M, Shiosaka S, Itami C, Uegaki K, Saarma M, Kojima M (2015) BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci U S A 112:E3067-3074.
Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV (2010)e BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci 30:8866-8870.
Notaras M, Hill R, van den Buuse M (2015)e BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatry 20:916-930.
Ohira K, Takeuchi R, Shoji H, Miyakawa T (2013) Fluoxetine-induced cortical adult neurogenesis. Neuropsychopharmacology 38:909-920.
Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S (2010) Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci 13:173-179.
Uegaki K, Kumanogoh H, Mizui T, Hirokawa T, Ishikawa Y, Kojima M (2017) BDNF binds its pro-peptide with high affinity and the common Val66Met polymorphism attenuates the interaction. Int J Mol Sci 18:E1042.
Vilar M, Mira H (2016) Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front Neurosci 10:26.
Yao XQ, Jiao SS, Saadipour K, Zeng F, Wang QH, Zhu C, Shen LL, Zeng GH, Liang CR, Wang J, Liu YH, Hou HY, Xu X, Su YP, Fan XT, Xiao HL, Lue LF, Zeng YQ, Giunta B, Zhong JH, et al. (2015) p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-beta toxicity in the brain of Alzheimer’s disease. Mol Psychiatry 20:1301-1310.
Masami Kojima, Ph.D., m-kojima@aist.go.jp.
10.4103/1673-5374.211173
*< class="emphasis_italic">Correspondence to: Masami Kojima, Ph.D., m-kojima@aist.go.jp.
orcid: 0000-0001-7769-9604 (Masami Kojima)
杂志排行
中国神经再生研究(英文版)的其它文章
- Mitochondrial quality control in amyotrophic lateral sclerosis: towards a common pathway?
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- SoxC transcription factors in retinal development and regeneration
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- Impact of glucocorticoid on neurogenesis
