Serum prealbumin as an e ff ective prognostic indicator for determining clinical status and prognosis in patients with hemorrhagic stroke
2017-08-07ShenqiZhangBinPengCreedStaryZhihongJianXiaoxingXiongQianxueChen
Shen-qi Zhang, Bin Peng, Creed M. Stary, Zhi-hong Jian, Xiao-xing Xiong,, Qian-xue Chen
1 Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
3 Department of Anesthesiology, Stanford University School of Medicine, Palo Alto, CA, USA
Serum prealbumin as an e ff ective prognostic indicator for determining clinical status and prognosis in patients with hemorrhagic stroke
Shen-qi Zhang1, Bin Peng2, Creed M. Stary3, Zhi-hong Jian1, Xiao-xing Xiong1,*, Qian-xue Chen1
1 Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
2 Department of Neurology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, China
3 Department of Anesthesiology, Stanford University School of Medicine, Palo Alto, CA, USA
How to cite this article:Zhang SQ, Peng B, Stary CM, Jian ZH, Xiong XX, Chen QX (2017) Serum prealbumin as an e ff ective prognostic indicator for determining clinical status and prognosis in patients with hemorrhagic stroke. Neural Regen Res 12(7):1097-1102.
Graphical Abstract
Xiao-xing Xiong, Ph.D., M.D., xiaoxingxiong@whu.edu.cn.
orcid:
0000-0001-6983-8547 (Xiao-xing Xiong)
Serum prealbumin is a recognized marker of malnutrition, but its prognostic role in patients with hemorrhagic stroke remains unclear. In this study, we retrospectively reviewed the records of 105 patients with hemorrhagic stroke admitted to Renmin Hospital of Wuhan University, China, from January to December 2015. We collected demographic and radiological data, and recorded serum prealbumin levels at admission and on days 1, 3, 6, 9, and 14–21.e existence of infections and gastrointestinal hemorrhage, and clinical condition at discharge were also recorded. Serum prealbumin levels during hospitalization were signi fi cantly lower in patients with infections compared with those without infections, and also signi fi cantly lower in patients with gastrointestinal hemorrhage compared with those without. Serum prealbumin levels at discharge were signi fi cantly higher in patients with good recovery than in those with poor recovery. We conclude that regular serum prealbumin measurements in patients with hemorrhagic stroke may be a useful indicator for determining clinical status and prognosis, which may therefore help to guide clinical decision-making.
nerve regeneration; prealbumin; hemorrhagic stroke; infection; gastrointestinal hemorrhage; prognostic indicator; prognosis; neural regeneration
Introduction
Hemorrhagic stroke is estimated to be the leading cause of disability and the second most common cause of death globally.e global burden of hemorrhagic stroke increased signi fi cantly between 1990 and 2010 in terms of the absolute number of people with incident hemorrhagic stroke (47% increase), number of deaths (20% increase), and disability-adjusted life years lost (14% increase) (Hemphill et al., 2015).e presentations of hemorrhagic stroke vary in terms of their severity and mortality, but a third of patients die within 1 month after onset, and most survivors are lewith some permanent disability (Schrader et al., 2003; Hemphill et al., 2015). In addition to the direct e ff ects of the initial bleeding event and secondary neurologic complications, patients with aneurysmal subarachnoid or intracerebral hemorrhage are predisposed to other medical complications with direct impacts on outcome, length of hospital and intensive care unit stay, and associated increased care costs (Dennis et al., 2008; Urra et al., 2009; Sharma et al., 2014). Furthermore, patients with hemorrhagic stroke have been shown to experience alterations in the levels of acute phase proteins, an acute surge of sympathetic activity, and changes in vascular tone (Fluri et al., 2012), fl uid status and administration, cardiac output, and major organ blood fl ow, resulting in a hyperdynamic state (Gariballa et al., 1998; Gaudiani et al., 2014).
Infections, gastrointestinal hemorrhage, and fluid and electrolyte disorders are the most frequent medical complications that occur aer hemorrhagic stroke (Unosson et al., 1994; Steiner et al., 2014; Codullo et al., 2016). Hemorrhagic stroke-associated infection (HSAI) is one of the major complications and has been shown to worsen the clinical course and outcome of stroke patients (Steiner et al., 2014), while gastrointestinal hemorrhage was observed in 91% of patients undergoing endoscopy within 24 hours following hemorrhagic stroke (Unosson et al., 1994; O’Malley et al., 1995; Ohwaki et al., 2008; Codullo et al., 2016). In experimental studies, HSAI was shown to cause severe in fl ammatory responses (such as fever, acidosis, and coagulation function disturbance) and lead to further deterioration of the central nervous system. HSAI and gastrointestinal hemorrhage may delay rehabilitation, prolong hospitalization, and even cause death in patients with hemorrhagic stroke (Ingenbleek and Young, 1994; May et al., 2015). However, early detection and intervention may prevent these complications and improve patient prognosis and clinical outcome (Fleming et al., 2007; Rambod et al., 2008; Cheng et al., 2015). The identification of e ff ective biomarkers for the early prediction of HSAI and gastrointestinal hemorrhage may thus be clinically relevant in this context.
Prealbumin is a 55-kDa protein that is synthesized in the liver (Tempel et al., 2015) and serves as a carrier protein for thyroxine and retinol-binding protein. Prealbumin possesses the shortest biological half-life (1–2 days) of all serum proteins (Wartenberg and Mayer, 2010; Davis et al., 2012). Recent studies identi fi ed copeptin, procalcitonin, leukocytes, C-reactive protein, interleukin-6, and heart rate variability measured on admission as possible predictors of stroke (Davis et al., 2012; Caccialanza et al., 2013; Kwan et al., 2013b). However, there are currently no relevant interventional measurements for these indicators. Serum prealbumin is a negative acute-phase reactant that decreases during inflammation, malignancies, and liver cirrhosis (Gocmen et al., 2010; Kwan et al., 2013a; Ye et al., 2015; Lin et al., 2017). Importantly, serum prealbumin concentrations fall rapidly as a result of decreased synthesis following reprioritization of the synthesis of acute-phase proteins such as C-reactive protein (Bourguignat et al., 1996; Bramer et al., 2014). Several studies have con fi rmed that in fl ammation is associated with decreased serum prealbumin (Ingenbleek and Young, 2002; Kopple et al., 2002; Rambod et al., 2008; Gurlek Gokcebay et al., 2015; Liu et al., 2015). Low serum prealbumin levels have also been associated with malnutrition, impaired functional status, poor outcome, and mortality (Yovita et al., 2004; Chrysostomou et al., 2010). To provide a theoretical basis for clinical treatment, the present investigation aimed to determine the value of serum prealbumin as a meaningful predictor of clinical status and prognosis in patients with hemorrhagic stroke.
Subjects and Methods
Subjects
Consecutive patients with hemorrhagic stroke admitted to Renmin Hospital of Wuhan University, China from January to December 2015 were enrolled in the study. All patients experiencing a new focal or global neurological event were admitted within 48 hours. A diagnosis of hemorrhagic stroke was verified by skull computed tomography (CT) and/or magnetic resonance imaging (MRI) on admission (Hemphill et al., 2015). Patients with transient ischemic attack, ischemic stroke, traumatic brain injury, brain tumor, chronic in fl ammatory disease, connective tissue or autoimmune diseases, malignant tumor, or deficient liver or renal function were excluded.
A total of 105 patients with hemorrhagic stroke met the inclusion criteria. The clinical characteristics of the study population are presented in Table 1.
Detection method
The severity of the cerebrovascular event was classified according to the Glasgow Coma Scale (GCS) (Hemphill et al., 2015). All patients were assigned to one of two catego-ries using the Glasgow Outcome Scale (GOS) at discharge: good-outcome group (GOS = 4–5) and poor-outcome group (GOS = 1–3).
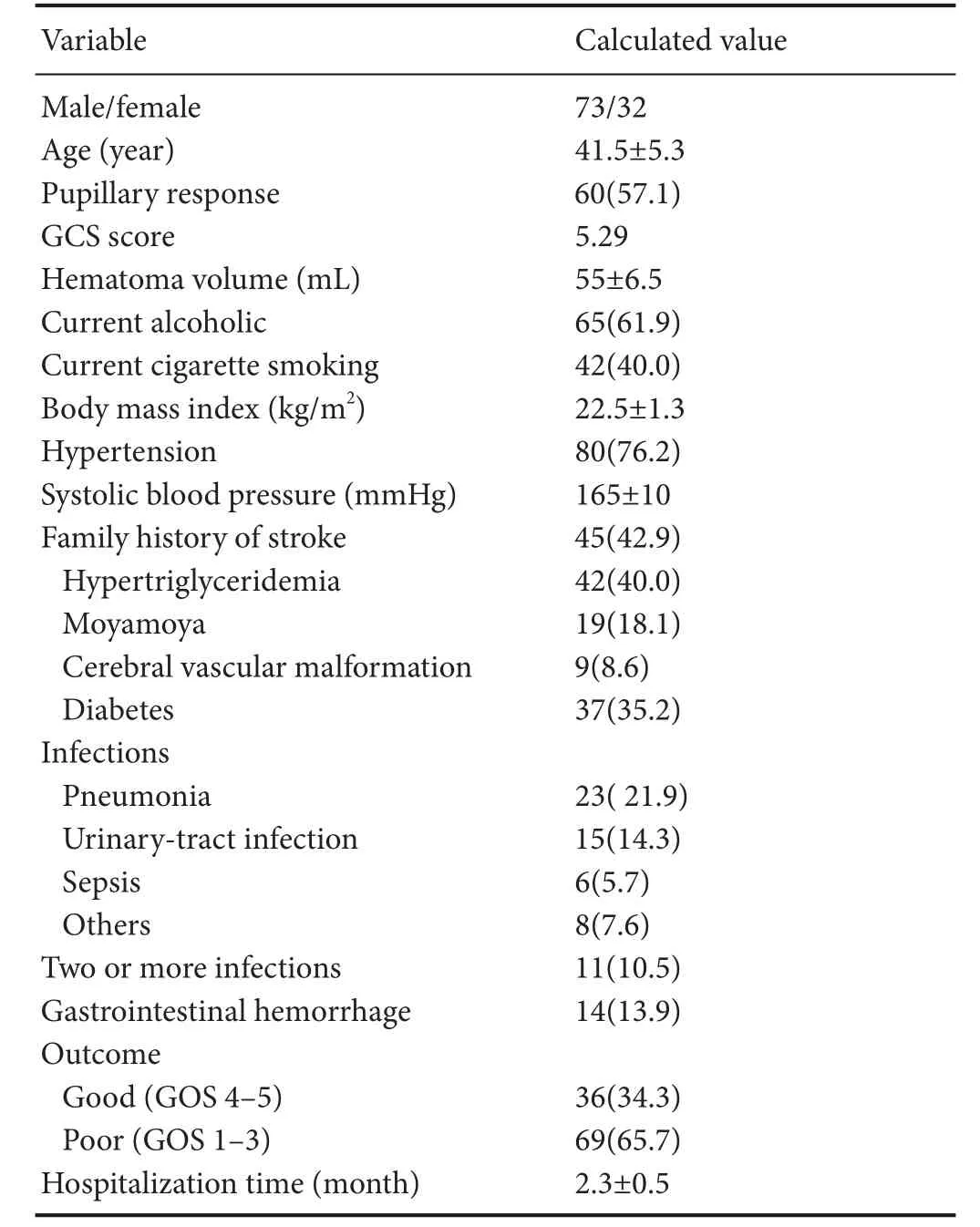
Table 1 Clinical characteristics of the study population
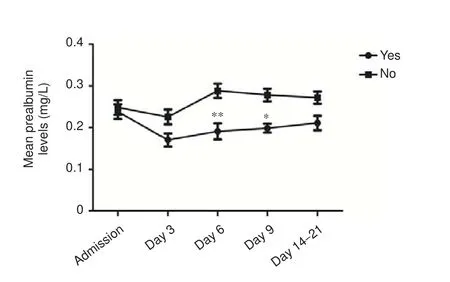
Figure 1 Serum prealbumin levels in hemorrhagic stroke patients with and without evidence of infection.
In addition to collecting demographic and radiological data, we also recorded serum prealbumin levels at admission and on days 3, 6, 9, 14–21. Fasting peripheral venous blood samples were obtainedviathe median cubital vein or basilic vein, and serum prealbumin was measured in the hospital biochemistry department using an automatic biochemical analyzer (AU5800, Beckman, CA, USA). Serum prealbumin levels ≤ 170 mg/L were de fi ned as abnormal.
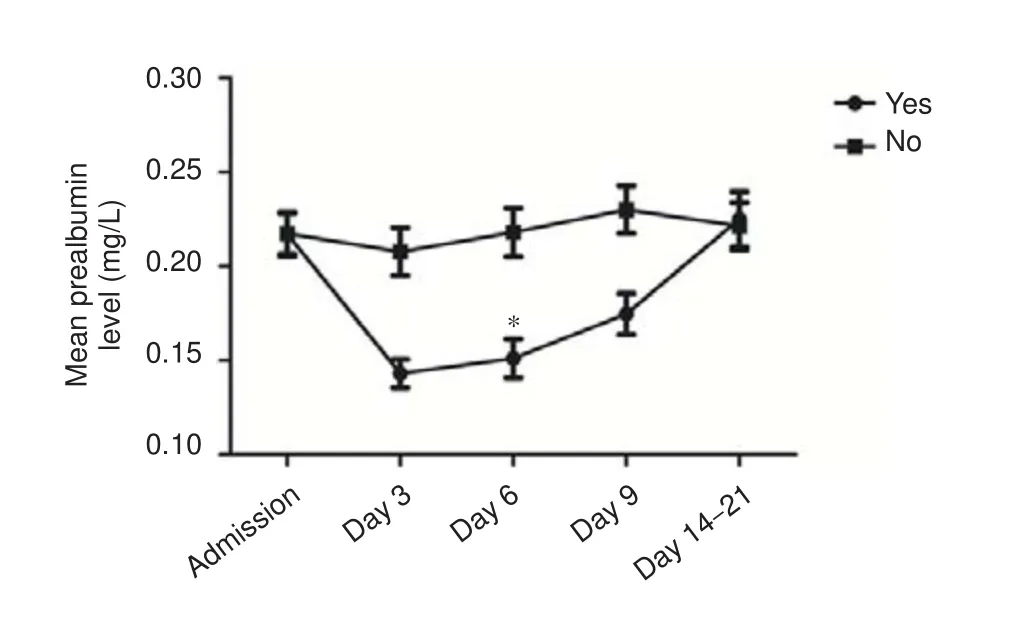
Figure 2 Serum prealbumin levels in hemorrhagic stroke patients with and without gastrointestinal hemorrhage.
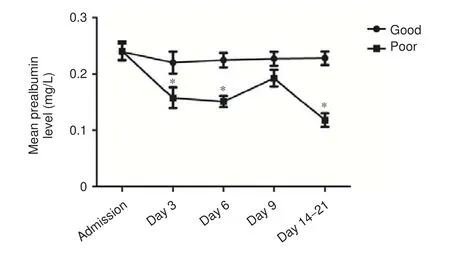
Figure 3 Serum prealbumin levels in hemorrhagic stroke patients with good and poor outcomes.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using SPSS for Windows 17.0 software (SPSS, Chicago, IL, USA). A probability value < 0.05 was considered statistically significant. Statistical analyses were performed using chi-square tests for binary and categorical data and Mann-WhitneyUtests for continuous variables.
Results
Value of serum prealbumin for predicting infection in hemorrhagic stroke patients
Infections were observed in 51 patients (48.8%), of which pneumonia accounted for 45.1% (n= 23). Serum prealbumin levels were significantly lower in patients with evidence of infection during hospitalization comparedwith non-infected patients on days 6 and 9 aer admission (P= 0.001,P= 0.039, respectively). As shown in Figure 1, serum prealbumin levels in patients with evidence of infection were signi fi cantly reduced on day 6, followed by gradual normalization, while serum prealbumin levels in non-infected patients were typically within the normal reference range (200–400 mg/L).
Value of serum prealbumin for predicting gastrointestinal hemorrhage in hemorrhagic stroke patients
Serum prealbumin levels on day 6 aer admission were signi fi cantly lower in patients with compared with those without gastrointestinal hemorrhage (P= 0.026). As shown in Figure 2, serum prealbumin levels in patients with gastrointestinal hemorrhage fell to a minimum on day 6 aer onset, followed by a gradual return to baseline levels. In contrast, serum prealbumin levels in patients without gastrointestinal hemorrhage were signi fi cantly higher than average (180.25 mg/L), with a gradually increasing trend.
Value of serum prealbumin for predicting clinical outcome in hemorrhagic stroke patients
Serum prealbumin levels in patients with poor outcomes at discharge were significantly lower compared with patients with good outcomes on days 3, 6, and 14–21 aer admission (P= 0.041,P= 0.02,P= 0.02, respectively). As shown in Figure 3, serum prealbumin levels in patients with good recovery at discharge were higher than average (180.25 mg/L); levels declined to a minimum on day 3 aer onset, with a gradual recovery to baseline levels after the sixth day. In contrast, serum prealbumin levels in patients with poor recovery were signi fi cantly lower.
Discussion
In fl ammation, infection, trauma, and neoplasms may result in significant changes in plasma concentrations of acutephase reactive proteins, including C-reactive protein, transferrin, amyloid protein A, and prealbumin. Acute-phase reactive proteins, as markers of clinical inflammation, have been used as important prognostic indicators after surgery and following stroke (Fleming et al., 2007; Rocha et al., 2010; Muangchan et al., 2012). C-reactive protein is commonly used as clinical marker of infection and infl ammation (Dalrymple et al., 2013; Upadhyay et al., 2013; Lourenco et al., 2014; Lee et al., 2015; Qin et al., 2015; Gu et al., 2016), and serum prealbumin is a highly sensitive marker of nutrition and survival in dialysis patients (Lin et al., 2011; Chen et al., 2014; Fujii et al., 2014). A variety of inflammatory mediators induce tissue and organ damage during the acute phase response to injury and infection, including damage to liver sinusoidal endothelial cells, resulting in a decline in serum prealbumin synthesis. Decreased serum prealbumin levels have been reported in various diseases, such as kidney injury, systemic inflammation, liver cirrhosis, and brain trauma (Cruse et al., 1992; Hilker et al., 2003; Dziedzic et al., 2006).e results of the present study provide the fi rst evidence for an association between decreased serum prealbumin and risk in acute ischemic stroke patients (Yeun and Kaysen, 1997; Shenkin, 2006).
Hemorrhagic stroke patients demonstrate differing levels of consciousness, commonly associated with an inability to protect the airway and thus e ff ectively discharge respiratory secretions. In addition, the immune response can be impaired in relation to helper T-cell function, lymphokine-activated killer cell cytotoxicity, and other immune-cell dysfunctions (Phang and Aeberhardt, 1996; Chertow et al., 2005). Surgery and indwelling catheters predispose the tissue mucosa to infection. In the present study, infections were observed in 51 patients (65.4%), of which pneumonia accounted for 45.1% (23 patients). There was a significant difference in serum prealbumin levels on days 6 and 9 after admission between patients with and without evidence of infection during hospitalization. Hemorrhagic stroke is associated with a significant increase in basal metabolic rate, and alterations in energy consumption are thus likely to contribute to a rapid prealbumin de fi ciency. Serum prealbumin levels may therefore serve as an indicator of metabolic and nutritional states during the acute phase of the clinical course. Infection is a major complication of acute stroke, with an incidence rate of 21–62% (Zahuranec et al., 2014), leading to prolonged hospitalization and poorer outcomes.e fi ndings of the current study suggest that low serum prealbumin levels might be an early biomarker of infection risk. Given that serum prealbumin can be detected rapidly and monitored easily, measurement of this marker may help hemorrhagic stroke patients at high risk of infection to be identi fi ed and treated appropriately.
Gastrointestinal hemorrhage is an additional co-morbid complication associated with hemorrhagic stroke, mainly secondary to acute post-traumatic stress ulcers (Wang et al., 2009; Sikora Newsome et al., 2015).e present study included 14 patients (14%) with gastrointestinal hemorrhage, and demonstrated a signi fi cant correlation between gastrointestinal hemorrhage and serum prealbumin levels. Furthermore, serum prealbumin levels in patients with poor recovery at discharge demonstrated greater variability compared with patients with better outcomes. Monitoring of serum prealbumin levels should thus pay attention to variability from baseline, particularly on post-injury day 3.
In summary, the results of the present study provide the first evidence for correlations between serum prealbumin levels and post-injury infection, gastrointestinal hemorrhage, and clinical outcome at discharge in patients with hemorrhagic stroke. Early detection of serum prealbumin levels during the acute phase of hemorrhagic stroke may therefore reflect the severity and prognosis of the disease, and may provide a rapid guide for appropriate interventions to prevent deterioration. Further research is required to determine if nutritional supplementation might increase serum prealbumin levels and thus reduce the risk of nosocomial infection and gastrointestinal hemorrhage in patients with hemorrhagic stroke.
Author contributions:SQZ and XXX conceived and designed the study. SQZ, ZHJ and QXC collected the clinical data. SQZ and BP analyzed the data. SQZ, CMS and XXX participated in the design and coordination of this study and helped to drathe paper. All authors approved the fi nal version of the paper.
Con fl icts of interest:None declared.
Research ethics:
Registration:This trial was registered with the Chinese Clinical Trial Register (registration number: ChiCTR-ROC-16008562).
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal.e patients understand that their names and initials will not be published and due e ff orts will be made to conceal their identity, but anonymity cannot be guaranteed.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Bourguignat A, Ferard G, Jenny JY, Gaudias J, Kempf I (1996) Diagnostic value of C-reactive protein and transthyretin in bone infections of the lower limb. Clin Chim Acta 255:27-38.
Bramer D, Hoyer H, Gunther A, Nowack S, Brunkhorst FM, Witte OW, Hoyer D (2014) Study protocol: prediction of stroke associated infections by markers of autonomic control. BMC Neurol 14:9.
Caccialanza R, Palladini G, Klersy C, Cereda E, Bonardi C, Quarleri L, Vadacca G, Albertini R, Merlini G (2013) Serum prealbumin: an independent marker of short-term energy intake in the presence of multiple-organ disease involvement. Nutrition 29:580-582.
Chen D, Bao L, Lu SQ, Xu F (2014) Serum albumin and prealbumin predict the poor outcome of traumatic brain injury. PLoS One 9:e93167.
Cheng V, Inaba K, Haltmeier T, Gutierrez A, Siboni S, Benjamin E, Lam L, Demetriades D (2015) Serum transthyretin is a predictor of clinical outcomes in critically ill trauma patients. Surgery 158:438-444.
Chertow GM, Goldstein-Fuchs DJ, Lazarus JM, Kaysen GA (2005) Prealbumin, mortality, and cause-speci fi c hospitalization in hemodialysis patients. Kidney Int 68:2794-2800.
Chrysostomou S, Stathakis C, Petrikkos G, Daikos G, Gompou A, Perrea D (2010) Assessment of prealbumin in hemodialysis and renal-transplant patients. J Ren Nutr 20:44-51.
Codullo V, Cereda E, Klersy C, Cavazzana I, Alpini C, Bonardi C, Turri A, Franceschini F, Caccialanza R, Montecucco C, Caporali R (2016) Serum prealbumin is an independent predictor of mortality in systemic sclerosis outpatients. Rheumatology (Oxford) 55:315-319.
Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E (1992) Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res 11:104-116.
Dalrymple LS, Johansen KL, Chertow GM, Grimes B, Anand S, Mc-Culloch CE, Kaysen GA (2013) Longitudinal measures of serum albumin and prealbumin concentrations in incident dialysis patients: the comprehensive dialysis study. J Ren Nutr 23:91-97.
Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S (2012)e use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr 36:197-204.
Dennis RA, Johnson LE, Roberson PK, Heif M, Bopp MM, Cook J, Sullivan DH (2008) Changes in prealbumin, nutrient intake, and systemic inflammation in elderly recuperative care patients. J Am Geriatr Soc 56:1270-1275.
Dziedzic T, Pera J, Klimkowicz A, Turaj W, Slowik A, Rog TM, Szczudlik A (2006) Serum albumin level and nosocomial pneumonia in stroke patients. Eur J Neurol 13:299-301.
Fleming CE, Saraiva MJ, Sousa MM (2007) Transthyretin enhances nerve regeneration. J Neurochem 103:831-839.
Fluri F, Morgenthaler NG, Mueller B, Christ-Crain M, Katan M (2012) Copeptin, procalcitonin and routine in fl ammatory markers-predictors of infection aer stroke. PLoS One 7:e48309.
Fujii T, Yajima R, Takada T, Sutoh T, Morita H, Yamaguchi S, Tsutsumi S, Kuwano H (2014) Serum albumin and prealbumin do not predict recurrence in patients with breast cancer. Anticancer Res 34:3775-3779.
Gariballa SE, Parker SG, Taub N, Castleden CM (1998) In fl uence of nutritional status on clinical outcome aer acute stroke. Am J Clin Nutr 68:275-281.
Gaudiani JL, Sabel AL, Mehler PS (2014) Low prealbumin is a signi fi -cant predictor of medical complications in severe anorexia nervosa. Int J Eat Disord 47:148-156.
Gocmen H, Ediger D, Uzaslan E, Doganay S, Guney NA, Ege E (2010)e relationships of serum prealbumin levels with parameters that indicate severity of disease and emphysema pattern in patients with stable chronic obstructive pulmonary disease. Eurasian J Med 42:105-110.
Gu LN, Zhang M, Zhu H, Liu JY (2016) Higher frequency of brain abnormalities in neuromyelitis optica spectrum disorder patients without primary Sjögren’s syndrome. Neural Regen Res 11:1633-1637.
Gurlek Gokcebay D, Emir S, Bayhan T, Demir HA, Gunduz M, Tunc B (2015) Assessment of nutritional status in children with cancer and e ff ectiveness of oral nutritional supplements. Pediatr Hematol Oncol 32:423-432.
Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46:2032-2060.
Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD (2003) Nosocomial pneumonia aer acute stroke: implications for neurological intensive care medicine. Stroke 34:975-981.
Ingenbleek Y, Young V (1994) Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr 14:495-533.
Ingenbleek Y, Young VR (2002) Signi fi cance of transthyretin in protein metabolism. Clin Chem Lab Med 40:1281-1291.
Kopple JD, Mehrotra R, Suppasyndh O, Kalantar-Zadeh K (2002) Observations with regard to the National Kidney Foundation K/DOQI clinical practice guidelines concerning serum transthyretin in chronic renal failure. Clin Chem Lab Med 40:1308-1312.
Kwan J, Hors fi eld G, Bryant T, Gawne-Cain M, Durward G, Byrne CD, Englyst NA (2013a) IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly aer an ischemic stroke. Exp Gerontol 48:960-965.
Kwan J, Pickering RM, Kunkel D, Fitton C, Jenkinson D, Perry VH, Ashburn AM; Stroke Association Rehabilitation Research Centre (2013b) Impact of stroke-associated infection on long-term survival: a cohort study. J Neurol Neurosurg Psychiatry 84:297-304.
Lee JL, Oh ES, Lee RW, Finucane TE (2015) Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med 128:1023.e1-22.
Lin MY, Liu WY, Tolan AM, Aboulian A, Petrie BA, Stabile BE (2011) Preoperative serum albumin but not prealbumin is an excellent predictor of postoperative complications and mortality in patients with gastrointestinal cancer. Am Surg 77:1286-1289.
Lin SP, Long Y, Chen XH, Lin PY, Jiang HL (2017) STAF score is a new simple approach for diagnosing cardioembolic stroke. Int J Neurosci 127:261-266.
Liu M, Yang J, Yu X, Huang X, Vaidya S, Huang F, Xiang Z (2015)e role of perioperative oral nutritional supplementation in elderly patients aer hip surgery. Clin Interv Aging 10:849-858.
Lourenco P, Silva S, Frioes F, Alvelos M, Amorim M, Couto M, Torres-Ramalho P, Guimaraes JT, Araujo JP, Bettencourt P (2014) Low prealbumin is strongly associated with adverse outcome in heart failure. Heart 100:1780-1785.
May CC, Arora S, Parli SE, Fraser JF, Bastin MT, Cook AM (2015) Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care 23:374-379.
Muangchan C, Harding S, Khimdas S, Bonner A, Canadian Scleroderma Research group, Baron M, Pope J (2012) Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 64:1405-1414.
O’Malley T, Langhorne P, Elton RA, Stewart C (1995) Platelet size in stroke patients. Stroke 26:995-999.
Ohwaki K, Yano E, Nagashima H, Nakagomi T, Tamura A (2008) Impact of infection on length of intensive care unit stay aer intracerebral hemorrhage. Neurocrit Care 8:271-275.
Phang PT, Aeberhardt LE (1996) E ff ect of nutritional support on routine nutrition assessment parameters and body composition in intensive care unit patients. Can J Surg 39:212-219.
Qin CS, Li XY, Jiang XJ, Feng GK (2015) Micro-in fl ammatory state and calci fi cation. Zhongguo Zuzhi Gongcheng Yanjiu 19:4721-4725.
Rambod M, Kovesdy CP, Bross R, Kopple JD, Kalantar-Zadeh K (2008) Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am J Clin Nutr 88:1485-1494.
Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhäupl K, Diener HC, Dominiak P; Acute Candesartan Cilexetilerapy in Stroke Survivors Study Group (2003)e ACCESS Study: evaluation of Acute Candesartan Cilexetilerapy in Stroke Survivors. Stroke 34:1699-1703.
Sharma A, Giraddi G, Krishnan G, Shahi AK (2014) E ffi cacy of serum prealbumin and CRP levels as monitoring tools for patients with fascial space infections of odontogenic origin: A clinicobiochemical study. J Maxillofac Oral Surg 13:1-9.
Shenkin A (2006) Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin Chem 52:2177-2179.
Sikora Newsome A, Casciere BC, Jordan JD, Rhoney DH, Sullivan KA, Morbitzer KA, Moore JD, Durr EA (2015)e role of statin therapy in hemorrhagic stroke. Pharmacotherapy 35:1152-1163.
Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, et al. (2014) European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 9:840-855.
Tempel Z, Grandhi R, Maserati M, Panczykowski D, Ochoa J, Russavage J, Okonkwo D (2015) Prealbumin as a serum biomarker of impaired perioperative nutritional status and risk for surgical site infection aer spine surgery. J Neurol Surg A Cent Eur Neurosurg 76:139-143.
Unosson M, Ek AC, Bjurulf P, von Schenck H, Larsson J (1994) Feeding dependence and nutritional status aer acute stroke. Stroke 25:366-371.
Upadhyay K, Uniyal A, Guleria R, Prasad D, Sharma A, Pandey R, Mohan A (2013) Serum prealbumin is a useful marker of in fl ammation and monitoring tool in patients with AECOPD. Chest 144:708A.
Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A (2009) Monocytes are major players in the prognosis and risk of infection aer acute stroke. Stroke 40:1262-1268.
Wang Q, Ding H, Tang JR, Zhang L, Xu YJ, Yan JT, Wang W, Hui RT, Wang CY, Wang DW (2009) C-reactive protein polymorphisms and genetic susceptibility to ischemic stroke and hemorrhagic stroke in the Chinese Han population. Acta Pharmacol Sin 30:291-298.
Wartenberg KE, Mayer SA (2010) Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am 21:325-338.
Ye F, Liu J, Yang S, Guo FQ (2015) Higher apolipoprotein B levels are associated with earlier onset of fi rst-ever atherosclerotic stroke. Int J Neurosci 125:186-190.
Yeun JY, Kaysen GA (1997) Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin in peritoneal dialysis patients. Am J Kidney Dis 30:923-927.
Yovita H, Djumhana A, Abdurachman SA, Saketi JR (2004) Correlation between anthropometrics measurements, prealbumin level and transferin serum with Child-Pugh classi fi cation in evaluating nutritional status of liver cirrhosis patient. Acta Med Indones 36:197-201.
Zahuranec DB, Lisabeth LD, Sanchez BN, Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Morgenstern LB (2014) Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology 82:2180-2186.
Copyedited by Furness S, Norman C, Yu J, Li CH, Qiu Y, Song LP, Zhao M
10.4103/1673-5374.211188
admission, all patients
routine treatments and nursing care in accordance with clinical guidelines. Routine blood and biochemical tests, electrocardiograms, and baseline brain CT/MRI scans were performed in all patients on admission. Laboratory investigations for vascular risk factors, brain CT angiography, and a thorough cardiac investigation were also performed.
Accepted: 2017-05-27
*Correspondence to:
杂志排行
中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
