Impact of dietary supplementation of one-carbon metabolism on neural recovery
2017-08-07JoshuaT.Emmerson,LaurenK.Murray,NafisaM.Jadavji
Impact of dietary supplementation of one-carbon metabolism on neural recovery
In the cell, one-carbon metabolism modulates nucleotide synthesis, DNA repair, as well as methylation through the reduction of homocysteine (Figure 1). High levels of plasma homocysteine have been associated with negative health outcomes in humans (Murray et al., 2017). Folates, B-vitamins, are a major component of one-carbon metabolism and play an important role in brain function. Speci fi cally, they are involved in nucleotide synthesis, DNA repair, methylation, second messenger systems, ion channels, protein, and neurotransmitter synthesis, as well as the metabolism of homocysteine (Murray et al., 2017). Folate is the natural form found in foods, whereas folic acid is the chemically synthesized and often found in supplements. In 1998, the importance of folates was noted in the prevention of neural tube defects by both the Canadian and US governments and mandatory folic acid forti fi cation laws were put into place.e neuroprotective properties of folic acid during development suggest that it may modulate growth and di ff erentiation in the brain. Another component of one-carbon metabolism is the nutrient choline. In the brain, choline’s primary role is in the synthesis of the neurotransmitter, acetylcholine, and lipid metabolism. In the rest of the body, choline also generates a methyl group to remethylate homocysteine to methionine.is reaction is done to a lesser extent in the brain.
Dietary supplementation using components of one-carbon metabolism in patients diagnosed with di ff erent neurological diseases might be effective in managing symptoms or reducing disease progression. A study published in 2010 reported that B-vitamin supplementation for 2 years reduced brain atrophy in patients with mild cognitive impairment within the UK (Smith et al., 2010). More recently, a clinical study in China showed that hypertensive patients treated with Enalapril and folic acid for 4.5 years had a reduced risk of developing a stroke compared to those treated with enalapril alone (Huo et al., 2015). Both these studies were conducted in countries that do not have mandatory folic acid forti fi cation laws in place. It is important to note that not all individuals bene fi t from mandatory folic acid forti fi cation. For example, individuals with a polymorphism in methylenetetrahydrofolate reductase (MTHFR) cannot reduce folic acid as e ff ectively as someone without the polymorphism and therefore have increased levels of homocysteine. Interestingly, there are alternative ways to reduce homocysteine levels. For example, cytidine 5′-diphosphocholine (CDP-choline), a metabolite of choline, has been reported to aid in neural repair in the central and peripheral nervous systems as well as increase levels of acetylcholine (Arenth et al., 2011). The mechanisms through which one-carbon supplementation may change the brain to reduce disease severity are not well understood.
We recently reported that supplementation with components of one-carbon metabolism after ischemic damage to the sensorimotor cortex increased neuroplasticity and anti-oxidant activity at the damage site as well as reduced sensorimotor impairment in a mouse model (Jadavji et al., 2017). The mice in our study were maintained on a folic acid de fi cient diet prior to damage to increase levels of plasma homocysteine. Aer ischemic damageviaphotothrombosis we supplemented the diet of these mice with folic acid, vitamins B12, B2, and choline for 4 weeks aer which motor function of animals was assessed and tissue and blood was collected. In the supplemented mice, we observed reduced impairment on the accelerating rotarod and ladder beam task, as well as increased use of impaired forelimb on cylinder task aer ischemic damage.ese behavioral changes were mirrored with increases in neuronal brain derived neurotrophic factor (BDNF) and immediate early gene, FosB, levels, as well as increased phospho-AKT (pAKT) expression within the damage cortex. We also reported reduced p53 levels in supplemented mice. Additionally, both stroke and increased levels of homocysteine result in oxidative stress, so we evaluated the impact of one-carbon metabolism on antioxidant activity. We observe increased levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and superoxide dismutase 2 (SOD2) at the damage site.e results from this study show that supplementation with one-carbon metabolism may be bene fi cial for recovery.
Damage to the spinal cord allows for detailed analysis of neuronal regeneration and dissection of potential mechanisms.e impact of folic acid on regeneration was shown in a study by Iskander et al. (2004). Adult rats underwent damage to the spinal cord using the spinal cord regeneration model.ree days prior to and aer damage animals were administered folic acid.e study reported a daily dose of 80 μg/kg resulted in 54 labeled neurons per ganglion which was similar to regenerating axons on the ipsilateral side to peripheral nerve injury in untreated mice. Using the Basso, Beattie, and Breshahan (BBB) scoring system, neurological recovery was assessed in the animals with spinal cord injury. Folic acid treatment produced signi fi cant improvement in the BBB starting at 7 days aer injury and was maintained throughout the study period (42 days after injury). Additionally, folic acid supplementation aer optic nerve injury increased the number of retinal ganglion cells (RGCs) per retina to 1,373 ± 73.42 compared to controls 913.4 ± 11.83. Another study from the same group reports that increased expression of the folate receptor 1 (Folr1) enables increased folic acid to enter the cell which then leads to positive benefits. Additionally, folic acid regulates Folr1 activation in a dose-dependent fashion (Iskandar et al., 2010). The interaction of folic acid and Folr1 is governed by dehydrate folate reductase (DHFR) andde novomethylation (Figure 1).
Recent research demonstrates a strong rationale to investigate one-carbon supplementation as an epigenetic therapeutic to counteract neurodegenerative disease including Alzheimer’s disease (AD). In primary neuronal cells cultured with amyloid beta (Aβ) oligomers, cell viability and methylation status increased when folic acid was present in the media (Li et al., 2015). Methylation status in the 40 μM folic acid treatment group showed activity similar with controls including an increase in S-adenosylmethionine (SAM): S-adenosylhomocysteine (SAH) ratio, as well as reduced mRNA and protein expression of amyloid precursor protein (APP) and Presenilin-1 (PS-1) to levels observed at baseline.In vivo, 7-monthold APP/PS1 (APPswe/PS1dE9) mice supplemented with 600 μg/ kg folic acid showed consistently lower expression of APP, PS1, and Aβ (Li et al., 2015). SAM only treatment did not alter PS-1 nor Aβ expression of mRNA or proteins in brain tissue of mice. Whereas, a combined treatment of folic acid and SAM had a similar e ff ect as only the folic acid treatment, highlighting the key role of folic acid. Increased methylation status for both the APP and PS1 genes in thesupplemented diet compared to folate de fi cient diet was reported bothin vitroandin vivo. Overall, these data suggest that supplementation with folic acid may regulate gene expression thereby reducing the magnitude of AD pathology.
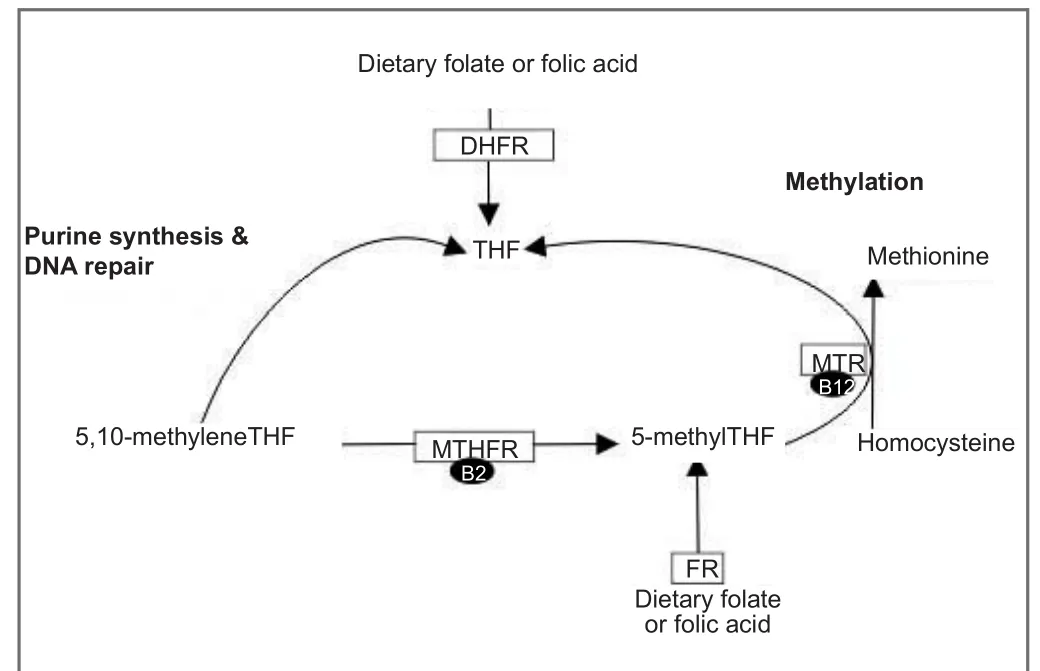
Figure 1 Simpli fi ed folate metabolism in the cell.
The effects of folate supplementation have also been examined in animal models of Parkinson’s disease (PD). In a study by Haghdoost-Yazdi et al. (2012), the e ff ect of supplementation with several B vitamin doses and combinations was examined both behaviourally and on levels of homocysteine. A model for PD-associated dopaminergic degeneration using the neurotoxin 6-hydroxydopamine (6-OHDA) was stereotaxically administered into the striatum of mice. The group that received 10× the amount of folic acid normally found in the diet performed better on rotational behaviour testing, with 60% fewer rotations post-surgery at both test points compared to control group.e same pattern was observed for rotarod, with this group as well as the 5× folic acid and the B complex groups performing at close to control levels. Contrary to what was expected, levels of homocysteine were not reduced in these or any group, and were in fact elevated compared to control levels. This finding suggests that the mechanism by which folic acid supplementation limits the e ff ect of 6-OHDA is not through decreasing amounts of homocysteine, as was theorized. Unfortunately, authors did not quantify dopaminergic cells in either the striatum or substantia nigra. Srivastav et al. (2015) studied the e ff ect of supplemental folate in aDrosophilamodel of early-onset familial PD. A novel recessive allele for theParkingene was used, producing reduced mRNA and null amounts of the Parkin protein, which is involved in the degradation of unfolded proteins and is also linked to proper mitochondrial function.is resulted in several de fi ciencies in homozygous fl ies, including increased lethality at the pupal stage, decreased transition rates to later life stages, and impaired motor function. It also increased oxidative stress, reduced antioxidant activity, and a ff ected mitochondrial functionality, as evidenced by lower levels of ATP. In fl ies given a 10 to 250 μM e ff ective dose of folic acid, these deficiencies were at least partially reversed. Lethality was reduced, more fl ies transitioned to later life stages, and motor function improved. In addition, levels of oxidative stress decreased and the amount of ATP present increased, suggesting improved mitochondrial function. Based on these results, folic acid supplementation may be useful for attenuating some of the e ff ects of dopaminergic degeneration associated with PD.
Animal models have been used to describe mechanisms through which dietary supplementation with one-carbon metabolism components has a beneficial role in regeneration within the central nervous system. Functional benefits from supplementation has been reported in both animal models and humans. However, the regenerative properties of folate may not be apparent in populations with mandatory folic acid forti fi cation laws in place. It is important to note that the aging process reduces the ability to absorb many needed nutrients and vitamins from our diet. Therefore, dietary supplementation with one-carbon metabolism components could be considered for this population even in countries with mandatory folic acid forti fi cation.
JTE was funded by Graduate Award for Ontario Students for Research in Dementia, LKM was funded by Canadian Institutes of Health Research (CIHR) Studentship and NMJ was funded by the Natural Sciences and Engineering Research Council (NSERC) grant.
Joshua T. Emmerson, Lauren K. Murray, Na fi sa M. Jadavji*
Department of Neuroscience, Carleton University, Ottawa, ON, Canada
*Correspondence to: Na fi sa M. Jadavji, Ph.D., na fi sa.jadavji@mail.mcgill.ca.
orcid: 0000-0002-3557-7307 (Na fi sa M. Jadavji)
Accepted:2017-07-03
How to cite this article:Emmerson JT, Murray LK, Jadavji NM (2017) Impact of dietary supplementation of one-carbon metabolism on neural recovery. Neural Regen Res 12(7):1075-1076.
Open access statement:is is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:is paper has been checked twice with duplication-checking soware ienticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and signi fi cance of this paper.
Arenth PM, Russell KC, Ricker JH, Zafonte RD (2011) CDP-choline as a biological supplement during neurorecovery: a focused review. PM R 3:S123-31.
Haghdoost-Yazdi H, Fraidouni N, Faraji A, Jahanihashemi H, Sarookhani M (2012) High intake of folic acid or complex of B vitamins provides anti-Parkinsonism e ff ect: No role for serum level of homocysteine. Behav Brain Res 233:375-381.
Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, et al. (2015) E ffi cacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China. JAMA 313:1325-1335.
Iskandar BJ, Nelson A, Resnick D, Skene JHP, Gao P, Johnson C, Cook TD, Hariharan N (2004) Folic acid supplementation enhances repair of the adult central nervous system. Ann Neurol 56:221-227.
Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee R V, Skene JHP, Nelson A, Patel N, Gherasim C, Simon K, Cook TD, Hogan KJ (2010) Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest 120:1603-1616.
Jadavji N, Emmerson J, Willmore WG, MacFarlane AJ, Smith P (2017) B-vitamin and choline supplementation increases neuroplasticity and recovery aer stroke. Neurobiol Dis 103:89-100.
Li W, Liu H, Yu M, Zhang X, Zhang M, Wilson JX, Huang G (2015) Folic acid administration inhibits amyloid β-peptide accumulation in APP/PS1 transgenic mice. J Nutr Biochem 26:883-889.
Liu H, Li W, Zhao S, Zhang X, Zhang M, Xiao Y, Wilson JX, Huang G (2016) Folic acid attenuates the e ff ects of amyloid β oligomers on DNA methylation in neuronal cells. Eur J Nutr 55:1849-1862.
Murray L, Emmerson J, Jadavji N (2017) Roles of folate in neurological function. In: Folic Acid: Sources, Health E ff ects and Role in Disease. Nova Publishers Science Inc.
Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H (2010) Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 5:e12244.
Srivastav S, Singh SK, Yadav AK, Srikrishna S (2015) Folic acid supplementation ameliorates oxidative stress, metabolic functions and developmental anomalies in a novel fl y model of Parkinson’s disease. Neurochem Res 40:1350-1359.
Yu L, Chen Y, Wang W, Xiao Z, Hong Y (2016) Multi-Vitamin B supplementation reverses hypoxia-induced tau hyperphosphorylation and improves memory function in adult mice. J Alzheimers Dis 54:297-306.
10.4103/1673-5374.211183
杂志排行
中国神经再生研究(英文版)的其它文章
- SoxC transcription factors in retinal development and regeneration
- Umbilical cord: an unlimited source of cells di ff erentiable towards dopaminergic neurons
- Targeting 14-3-3 adaptor protein-protein interactions to stimulate central nervous system repair
- RACK1 regulates neural development
- Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways
- BDNF pro-peptide: a novel synaptic modulator generated as an N-terminal fragment from the BDNF precursor by proteolytic processing
