Syntheses,Characterization and Antitumor Activity of Three Mononuclear Ruthenium(Ⅱ)Complexes
2017-07-05ZHANGYanYANGYanWENYanZhenJIAShiFang
ZHANG YanYANG YanWEN Yan-ZhenJIA Shi-Fang
(School of Chemical and Biological Engineering,Tai Yuan Science and Technology University,Taiyuan 030024,China)
Syntheses,Characterization and Antitumor Activity of Three Mononuclear Ruthenium(Ⅱ)Complexes
ZHANG Yan*YANG YanWEN Yan-ZhenJIA Shi-Fang*
(School of Chemical and Biological Engineering,Tai Yuan Science and Technology University,Taiyuan 030024,China)
Three new mononuclear ruthenium complexes[Ru(bpy)2(paH)]PF6(1),[Ru(dmb)2(paH)]PF6(2)and[Ru (phen)2(paH)]PF6(3)(bpy=2,2′-bipyridine,dmb=4,4′-dimethyl-2,2′-bipyridine,phen=phenanthroline,paH= pyridinecarboxylic acid)were synthesized and characterized using elemental analysis,infrared,nuclear magnetic spectroscopy and electrospray ionization mass spectrometry.Their photophysical and electrochemical properties were also studied.The complexes 1~3 undergo metal centered oxidation and the Ru(Ⅱ)/Ru(Ⅲ)redox couple peak are in 0.7~1.0 V versus a saturated calomel electrode.The cytotoxicity of these complexes in vitro was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT)assay.The results indicated that 1~3 exhibited significant dose-dependent cytotoxicity to human breast cancer(MCF-7),gastric cancer(AGS)and lung cancer(A549)tumour cell lines.It is worth noting that 2 showed excellent antitumor effects in a cellular study (IC50value of 2.85 μmol·L-1for human breast cancer cells in vitro).
mononuclear ruthenium complexes;spectroscopy property;electrochemistry property;cytotoxicity
0 Introduction
Development of more efficient anticancer drugs with better selectivity but less toxic side effects is currently an area of intense research in bioinorganic chemistry[1-2].Since the discovery of cisplatin by Rosenberg in 1964,more and more attention has been paid to the metal complexes as potential anticancerdrugs[3-5].Cisplatin is still used today and is highly effective against testicular and ovarian carcinoma as well as bladder,head and neck tumours[6].However, platinum-based anticancer chemotherapy is associated with severe side effects because of poor specificity[7]. In the case of cisplatin,systemic toxicities like nephrotoxicity,neurotoxicity andototoxicityinflict serious disorders or injuries on the patients during the treatment,which badly restrict its efficacy[8].Many transition metal complexes and small-molecule-based antitumoragentshavebeendevelopedasnew drugs[9-15].Amongthedifferentmetalcomplexes generating interests,ruthenium complexes have shown great potential and remain the subject of extensive drug discovery efforts,because ruthenium has low energy barrier between its oxidation states and low atomic radius in the transition metal series(0.125 nm) and soluble in water,which are beneficial for its accumulation in cancer tissues[16].In the year 1999, NAMI-A(ImH[trans-RuCl4(DMSO)(Im)])and in the year 2003,KP1019(InH[trans-RuCl4(In)2])was successfully entered into phase I clinical trials for the treatment of metastatic tumors and colon cancers[17-18]. Now NAMI-A was successfully entered into phaseⅡclinical trials for the treatment of non-small cell lung cancer[19].Both of them have their own limitations but in order to promote them as drugs,the way for development of new Ru(Ⅱ)anticancer complexes is paved.Now the journey of new anticancer drugs starts with ruthenium polypyridyl complexes by overcoming limitations.Recently,someRu(Ⅱ)polypyridine complexes as antitumor agents were reported by our group[20-21].A large number of ruthenium(Ⅱ)complexes containing a{Ru(bpy)2}(bpy=2,2′-bipyridine)moiety with a variety of ancillary bidentate ligands have been reported[22-27].Among these ligands a class belongs to the carboxylic acid ligands.Such ligands are of particular interest as they can play important roles in decidingthephysicalpropertiesandchemical properties of the complex due to the possibility of redox electron delocalization between the metal ion and the ligand.
In this study,three new ruthenium(Ⅱ)complexes containing the ancillary ligand pyridinecarboxylic acid were synthesized and characterized using elemental analysis,IR,1H NMR spectroscopy and electrospray ionization mass spectrometry(ESI-MS).The UV-Vis absorptionspectroscopy,fluorescencespectroscopy andelectrochemicalbehaviorofthemwerealso studied.Moreover,theseantitumoreffectswere investigated in vitro,and 2 exhibited the best inhibit ory effect among three complexes.
1 Experimental
1.1 Materials and instrument
All reagents and solvents were of commercial origin and were used without further purification unless otherwise noted.Ultrapure Milli-Q water was used in all experiments.Dimethyl sulfoxide(DMSO) and RPMI 1640 were purchased from Sigma.MCF-7 (humanbreastcancer),AGS(humangastric carcinoma)and A549(human lung cancer)cell lines were purchased from the American Type Culture Collection.RuCl3·3H2O,2,2′-bipyridyl(bpy),4,4′-dimethyl-2,2′-bipyridine(dmb),phenanthroline(phen) and 2-pyridinecarboxylic acid(paH)were purchased from the Wuhan Shenshi Huagong,[Ru(L)2Cl2]·2H2O (L=bpy,dmb and phen)were synthesized and purified according to literature methods[28].
Microanalysis(C,H,and N)was conducted with a PerkinElmer 240Q elemental analyzer.Electrospray ionization mass spectra were recorded with an LCQ system(Finnigan MAT,USA)using CH3OH as the mobilephase.300MHz1HNMRspectroscopic measurements were performed on a Bruker AM-300 NMR spectrometer,using DMSO-d6as solvent and TMS(SiMe4)as an internal reference at 25℃. Infrared spectra were recorded on a Perkin-Elmer SpectrumFTIRspectrometerusingKBrpellets. Solution electronic absorption and emission spectra were recorded on Shimadzu 3100 spectrophotometer in acetonitrileandShimadzuRF-5301PC spectrofluorometer,respectively.Differentialpulse voltammetry(DPV)was done with a CHI 630E instrument in a three-electrode cell with a pure Ar gas inlet and outlet.The working electrode and counterelectrodewerePtelectrode,andthereference electrode was a saturated calomel electrode(SCE). The experiments were carried out in the presence of CH3CN.DPV experiments were performed with a scan rate of 20 mV·s-1.
1.2 Synthesis of Ru complexes
[Ru(bpy)2(paH)]PF6(1):AgNO3(0.17 g,1.0 mmol) was added to an ethanol solution of[Ru(bpy)2Cl2]· 2H2O(0.26 g,0.5 mmol).After refluxing for 30 min and filtering to remove the deposited AgCl,the filtrate was added to an ethanol solution(30 mL)of 2-pyridinecarboxylic acid(0.06 g,0.5 mmol)and NaOH (0.02 g,0.5 mm).The mixture was refluxed for 12 h under N2,and the resulting brown red solution was evaporated to 5 mL.To this solution,excess KPF6was added and a brown red solid precipitated.This solid was collected by filtration,washed thoroughly with water and dried in vacuum over P2O5.The purification of the complex was performed on a neutral aluminium oxide column.The first moving brown red band was elutedwithdichloromethanecontaining5%(V/V) acetone.This was collected and evaporated.The solid thusobtainedwasrecrystallizedfromacetonediethylether(2∶1).Yield:62%.Anal.Calcd.for C26H20N5O2PF6Ru(%):C,45.88;H,2.94;N,10.29. Found(%):C,45.83;H,2.97;N,10.27.1H NMR(300 MHz,DMSO-d6):δ 7.36(2H,d,J=9.0 Hz),7.50(2H, m),7.60(2H,d,J=6.0 Hz),7.78(1H,m),7.90(1H,d, J=3.0 Hz),7.95~7.99(4H,m),8.17(2H,m),8.70~8.78 (4H,m),8.81(2H,d,J=9.0 Hz).IR(KBr,cm-1):1 605, 1 580,1 508,1 441,1 341,1 263,1 240,1 182, 1 148,1 130,1 020,901,839,760,727,658,556. ESI-MS:m/z 443.6([Ru(bpy)2·CH3O]+),536.0([MPF6]+).
[Ru(dmb)2(paH)]PF6(2):The method used to synthesize 2 was exactly similar to the 1,except that [Ru(dmb)2Cl2]·2H2O was used.Yield:78%.Anal. Calcd.for C30H28N5O2PF6Ru(%):C,48.91;H,3.80;N, 9.51.Found(%):C,48.93;H,3.77;N,9.52.1H NMR (300 MHz,DMSO-d6):δ 2.45(6H,s),2.56(6H,s),7.19 (2H,m),7.40~7.49(3H,m),7.61(2H,d,J=6.0 Hz), 7.69(1H,d,J=6.0 Hz),7.96~8.01(3H,m),8.49(1H,d, J=6.0 Hz),8.58(2H,m),8.64(2H,d,J=6.0 Hz).IR (KBr,cm-1):1 708,1 621,1 486,1 371,1 228,1 122, 1 016,843,766,690,555.ESI-MS:m/z 592.1([MPF6]+).
[Ru(phen)2(paH)]PF6(3):The methodused to synthesize 3 was similar to the 1,except that[Ru (phen)2Cl2]·2H2O was used.Yield:67%.Anal.Calcd. for C30H20N5O2PF6Ru(%):C,49.45;H,2.75;N,9.62. Found(%):C,49.48;H,2.69;N,9.65.1H NMR(300 MHz,DMSO-d6):δ 7.37(1H,m),7.47(1H,d,J=3.0 Hz),7.59(2H,m),7.86(1H,d,J=6.0 Hz),7.94(2H,m), 8.01~8.06(3H,m),8.12(2H,m),8.33~8.38(4H,m), 8.57(2H,m),8.81(2H,d,J=9.0 Hz).IR(KBr,cm-1): 1 620,1 420,1 324,1 141,834,719,555.ESI-MS: m/z 584.1([M-PF6]+).
1.3 Methods
Standard MTT assay procedures were used[29]. Cells were placed in 96-well microassay culture plates (8×103cells per well)and grown overnight at 37℃in a 5%(V/V)CO2incubator.The complexes tested were then added to the wells to achieve final concentrations ranging from 0.39 to 100 μmol·L-1.Control wells were prepared by addition of culture medium(200 μL).The plates were incubated at 37℃in a 5%(V/V)CO2incubator for 48 h.On completion of the incubation, stock MTT dye solution(20 μL,5 mg·mL-1)was addedtoeachwell.After4h,150mL dimethylsulfoxide(DMSO)was added to solubilize the MTT formazan.The optical density of each well was then measured with a microplate spectrophotometer at a wavelength of 490 nm.The IC50values were determined by plotting the percentage viability versus the concentration and reading off the concentration at which 50%of the cells remained viable relative to the control.Each experiment was repeated at least three times to obtain the mean values.Three different tumor cell lines were the subjects of this study:MCF-7 (humanbreastcarcinoma),AGS(humangastric carcinoma),and A549(human lung carcinoma).
2 Results and discussion
2.1 Design and synthesis
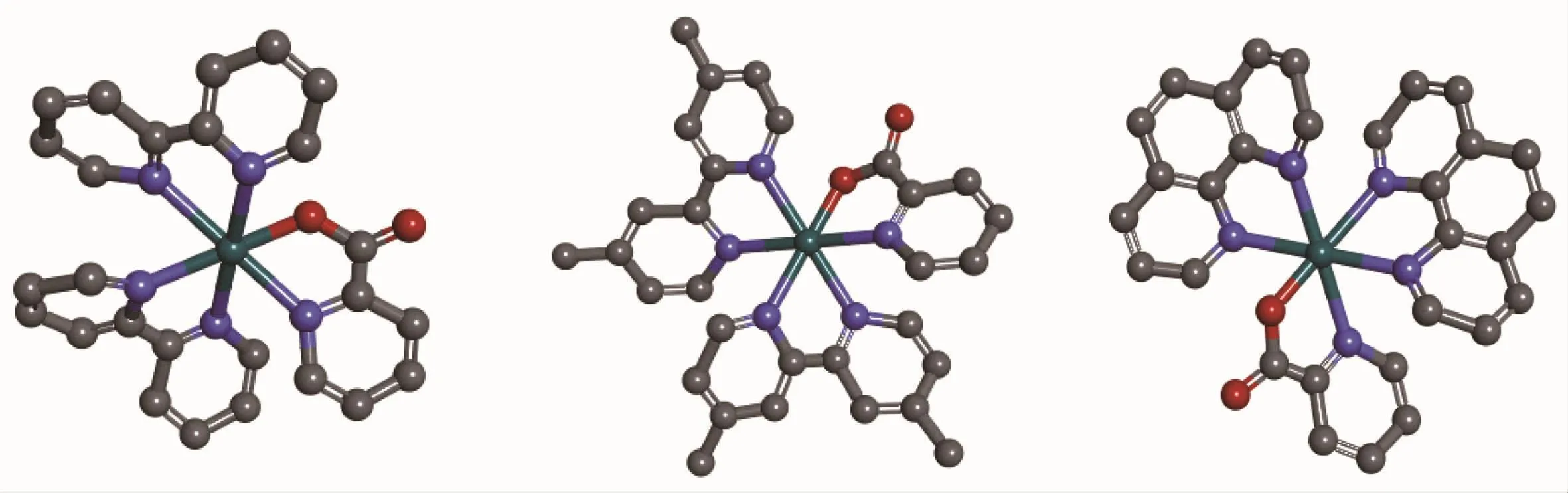
Scheme 1Structures of the complexes 1,2 and 3 omitting the anion PF6-
All three complexes(Scheme 1)were synthesized using the general,previously reported method.In order to obtain the pure products,chromatographic purification was necessary on a neutral aluminum oxide column,and the complexes were afforded in good yields.The crystals of the complexes were not obtained,however,ESI-MS and1H NMR helped us to determine their structure in solution.
In the ESI-MS of the complexes 1,2 and 3,the peaks at m/z 536.0,592.1 and 584.1 were designated to the species[Ru(bpy)2(paH)]+,[Ru(dmb)2(paH)]+and [Ru(phen)2(paH)]+,respectively.Arepresentative spectrum for 2 was shown in Fig.1(ESI-MS spectra of 1 and 3 are in supporting information).The observed peaks revealed that coordination cations of the three complexes were formed by one Ru(Ⅱ)center,one paH, and two bpy(or dmb,or phen),and the coordination cations were stable in the solution.
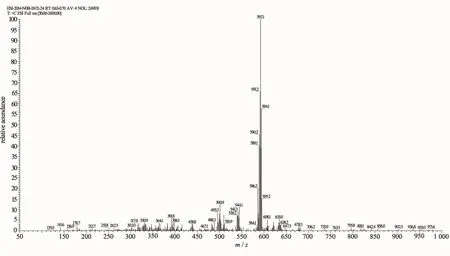
Fig.1Electrospray mass spectrum of complex 2 in CH3OH
The1H NMR spectra of the three complexes were recorded in DMSO-d6.The absence of free carboxyl proton(δ>10)in all spectra confirm the complexation through the CO-O.The aromatic region of each spectrum was very complicated due to their similar electronic environments of many aromatic hydrogen atoms,and their signals were in a narrow regions shift range(δ=7.3~8.9).The number of aromatic proton signals was consistent with the number of protons of the molecular formula.However,it is difficult toassign all the individual signals,respectively.A representative1H NMR spectrum of 2 was shown in Fig.2(1H NMR spectra of 1 and 3 are in supporting information).In their solid state,elemental analysis dataweresatisfactorywiththegeneralformula including the PF6-anion.
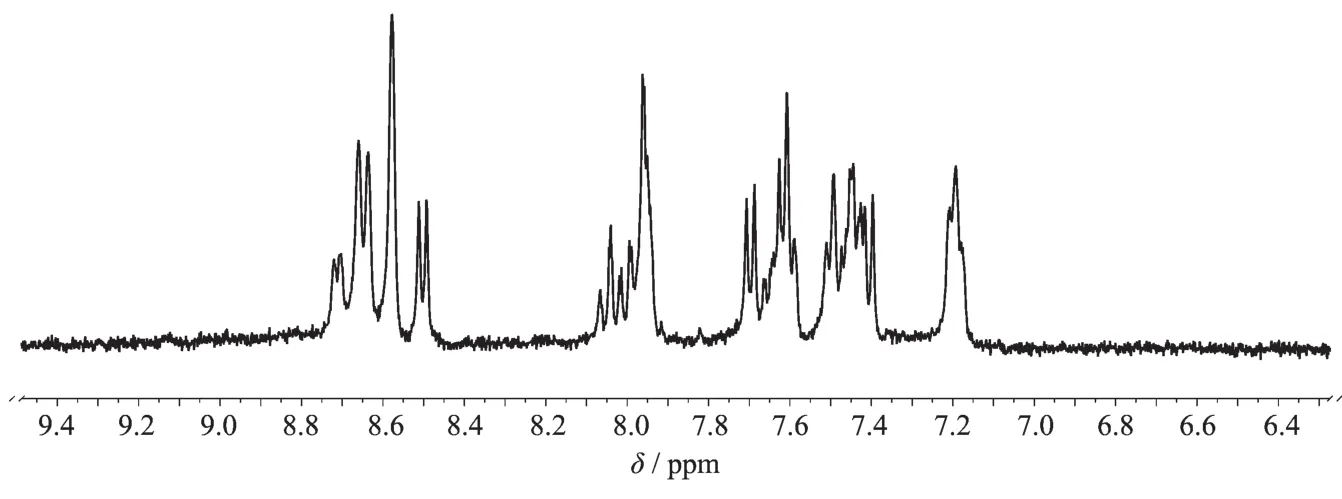
Fig.21H NMR spectrum in DMSO-d6of complex 2
2.2 Infrared spectroscopy
Infrared spectra of the complexes do not display any band near 3 300 cm-1,which suggested that the -COOH of paH was deprotonated in the complexes. The free carboxylic acid displayed a peak near 1 650 cm-1that was assigned to the C=O group.A medium to strong peak was observed in the range of 1 590~1 640 cm-1for all the three complexes.This peak might originated from the metal-coordinated C=O group of paH.The strong and sharp peak displayed by the complexes in the range of 1 420~1 440 cm-1is likely to be associated with the C=N fragments of the ancillary ligands.The presence of PF6-in each complex is indicated by a strong peak at 840 cm-1[30](IR spectra of 1 and 3 are in supporting information).
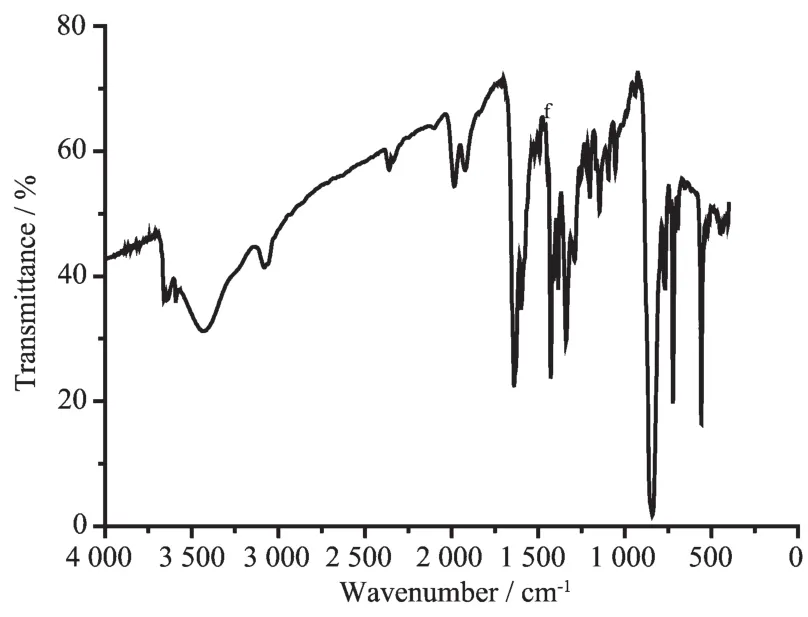
Fig.3Infrared spectrum(KBr pellet)of complex 2
2.3 Electronic absorption spectra
The absorption spectra of the three complexes wererecordedinacetonitrile(Fig.4).Withthe exception of 3,the spectral profiles of the other complexes were very similar.Complexes 1 and 2 displayed three absorptions peaks at 490~495 nm, 360~365 nm and 285~295 nm,while 3 displayed two absorptions peaks at 435~445 nm and 260~265 nm. These absorption peaks for 3 were significantly blue shifted compared with the other complexes.The bands at 260,290 and 360 nm are attributed to intraligand π→π*transitions.Thelowestenergybands, approximately 440 and 490 nm,are assigned to metalligand charge transfer(MLCT)(dRu→π*)[31].
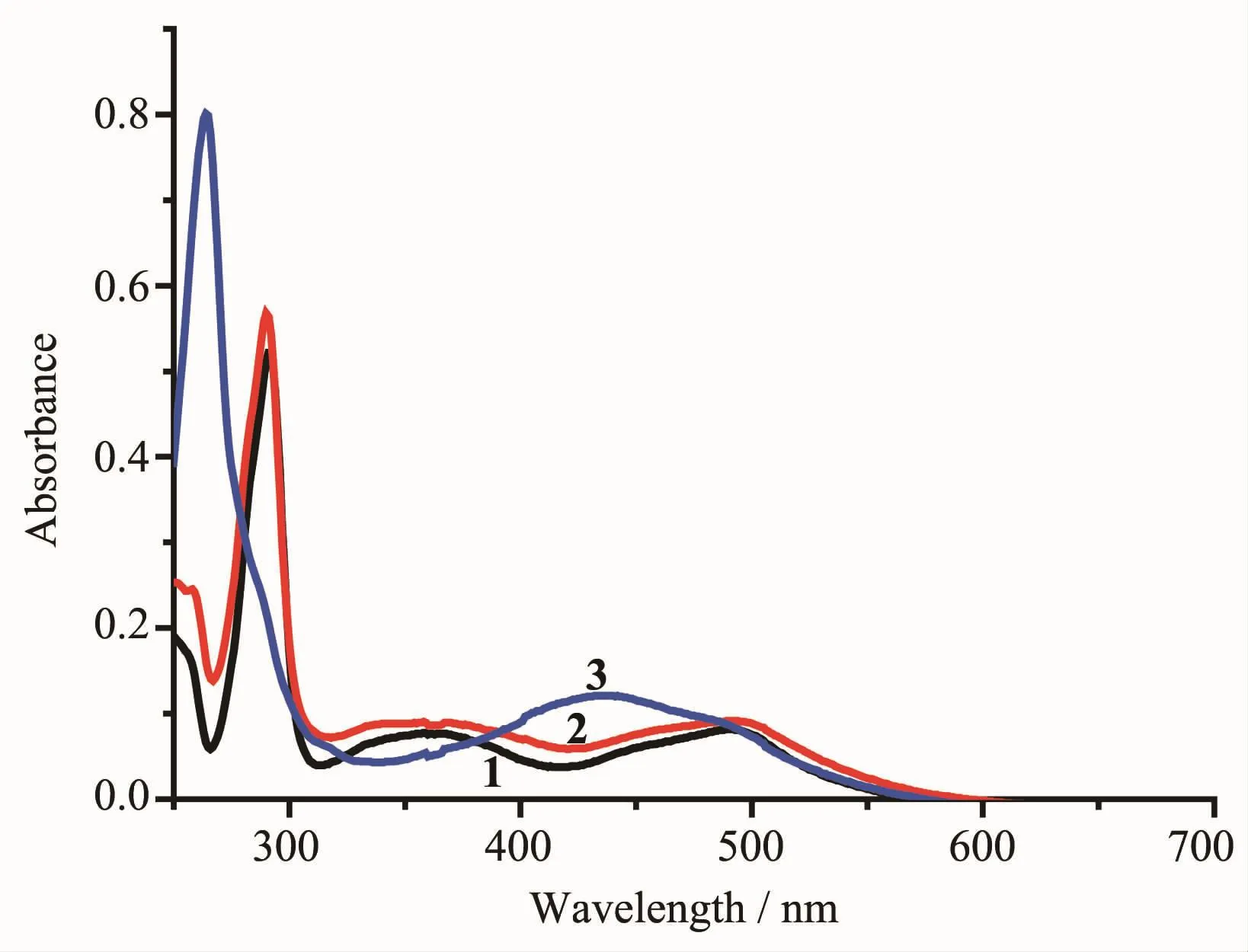
Fig.4Absorption spectra of 1~3 in acetonitrile (10 μmol·L-1)
2.4 Luminescence spectra studies
Theemissionspectraldata(wavelengthof excitation and emission)are presented in Table 1.All themeasurementsweremadewithsolutions deoxygenated by purging Ar.The excitation spectra of 1~3 were monitored at the emission maxima of each complex at room temperature.Complexes 1~3 upon excitation onto their excitation maxima exhibited an emission band at 602,599 and 587 nm,respectively. The emission profileandemissionmaximawere similar and independent of the excitation wavelength. What is noteworthy is that 3 has a shoulder peak at 661nm.The electronic emission spectra of the 1~3 are presented in Fig.5.
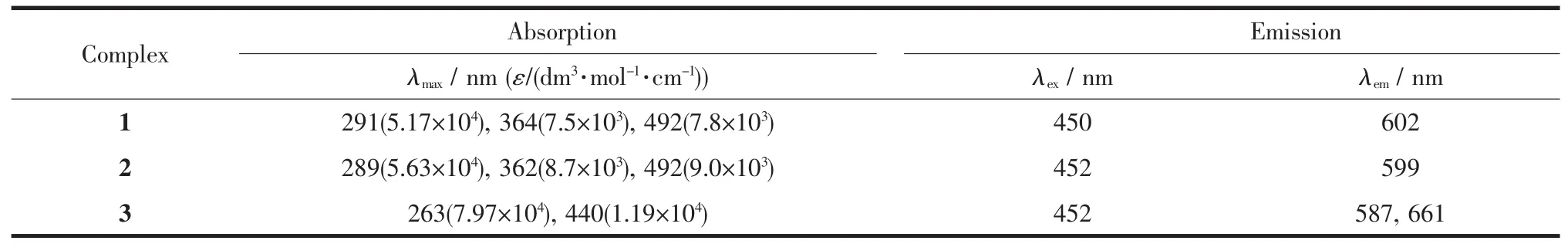
Table 1Electronic spectroscopic data of complexes 1~3 in CH3CN
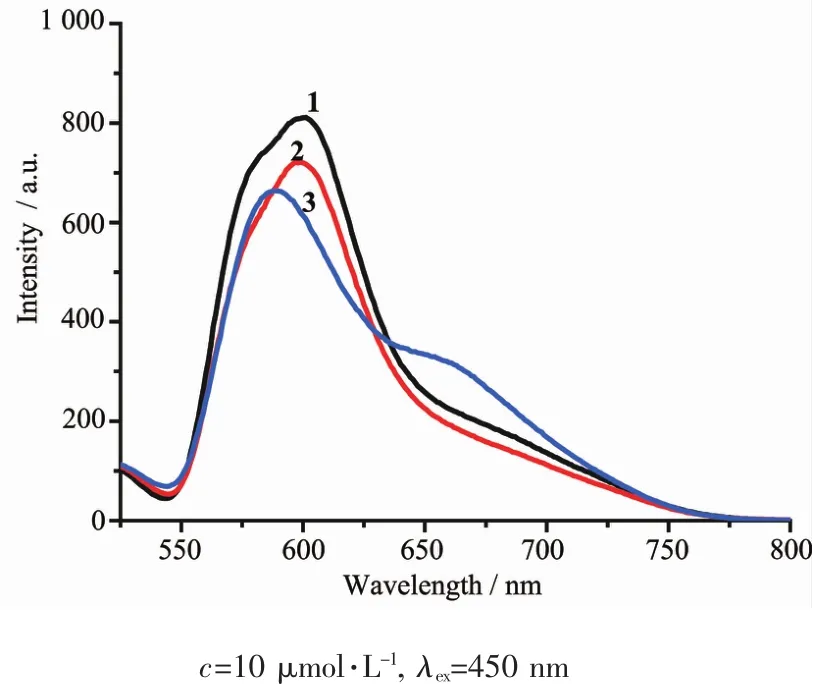
Fig.5Emission spectra of 1~3 in acetonitrile
2.5 Electrochemistry analysis
Thedifferentialpulsevoltammetry(DPV) techniqueisemployedtoobtainedwell-resolved potentialinformation,whiletheindividualredox processes for the multinuclear complexes are poorly resolved in the cyclic voltammetry experiment,in which individual E1/2potentials cannot be easily or accurately extracted from the data.Differential pulse voltammetry (DPV)of the 1~3 were recorded in acetonitrile,the voltammograms were shown in Fig.6.All the three complexes exhibit one oxidations in the range of 0.7~1.0 V,which assigned to Ru(Ⅱ)/Ru(Ⅲ)couple[32-33].
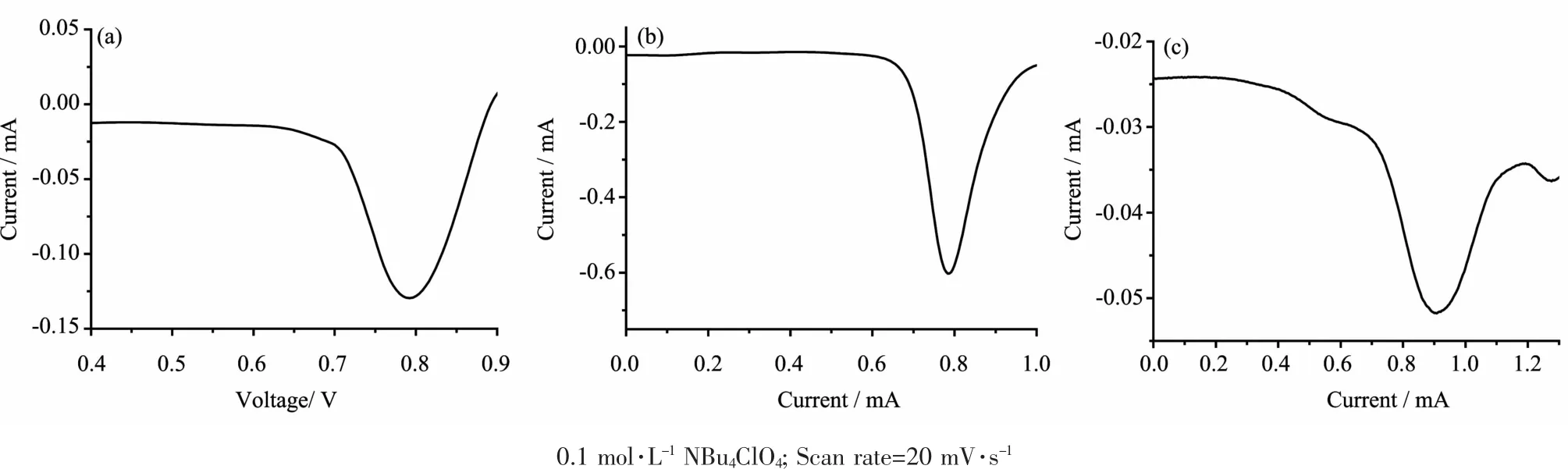
Fig.6DPV of 1(a),2(b)and 3(c)in CH3CN solution
2.6 In vitro cytotoxicity

Fig.7Cell viability of 1~3 toward proliferation of MCF-7(a),AGS(b)and A549(c)cells in vitro
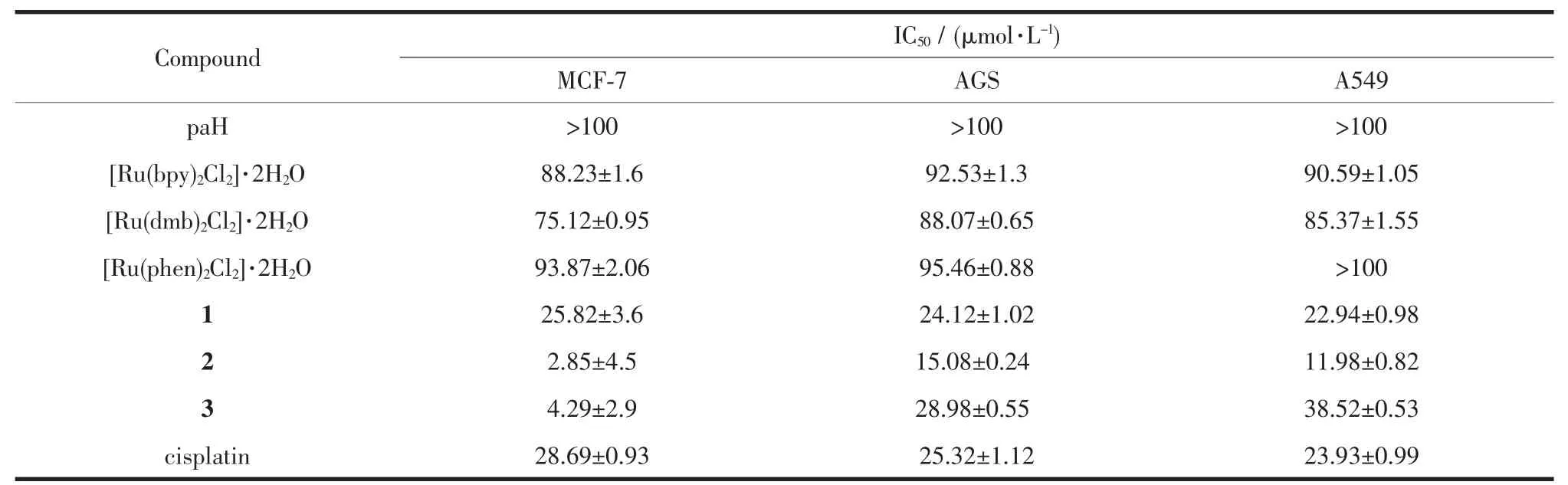
Table 2IC50value of Ru-complexes,paH and cisplatin against selected cell lines
The MCF-7(human breast cance cell line),AGS (human gastric cancer cell line)and A549(human gastric cancer cell line)cells were treated with different concentrationsofpaH,[Ru(bpy)2Cl2]·2H2O,[Ru(dmb)2Cl2] ·2H2O,[Ru(phen)2Cl2]·2H2O,cisplatin,1,2 and 3 for 48 h.The cytotoxicity of these compounds towards the aforementioned cell lines was evaluated using the MTT method.Culture medium containing 0.05%DMSO was used as the negative control.After treatment of three cell lines for 48 h with these compounds in a range of concentrations(0.39~100 μmol·L-1),the percentage inhibition of growth of the cancer cells was determined. The cell viabilities(%)vs concentrations obtained with continuous exposure for 48 h are depicted in Fig.7,and the IC50values are listed in Table 2.It was found that the IC50value of paH was more than 100 μmol·L-1,the IC50values of[Ru(bpy)2Cl2]·2H2O,[Ru(dmb)2Cl2]·2H2O, [Ru(phen)2Cl2]·2H2O were greater than 1~3,respectively.The cytotoxicity of the complexes was found to beconcentrationdependent.Thecellviability decreasedwithincreasingconcentrationsofthe complexes.As shown in Table 2,it was clear that 2 was generally the most active complex and exhibited low IC50values,particularly at the concentration of 2.85 μmol·L-1for the MCF-7 cells at 48 h,compared with that of cisplatin,28.69 μmol·L-1.The cytotoxicity of 1 was higher than that of 3 towards AGS and A549 cells,and there was an opposite phenomenon in the MCF-7 cells,but these IC50values were obviously higher than those of 2 in all three cell lines.Overall, amongthesecomplexes,2exhibitedthehighest cytotoxicity in MCF-7,AGS and A549 cells.A series of mononuclear ruthenium complexes[Ru(bpy)2(salH)] PF6(Ru1),[Ru(dmb)2(salH)]PF6(Ru2)and[Ru(phen)2(salH)]PF6(Ru3),where salH=salicylaldehyde,were reported by our group[34].The A549,BGC823 and MDA-MB-231cellsweretreatedwithdifferent concentrations of Ru1,Ru2 and Ru3.It was found that Ru2 was generally the most active complex and exhibitedlowIC50values,particularlyatthe concentrations of 7.97 and 3.60 μmol·L-1for the A549 and BGC823 cells respectively for 48 h.The activity of these two series of complexes as anti-cancer drugs is consistent.When the ancillary ligand is dmb (dmb=4,4′-dimethyl-2,2′-bipyridine),the cytotoxicity of complex is the largest.
2.7 Octanol-water partition coefficients
The activity of anticancer drugs is often related totheirlipophiliccharacter,andtheresulting hydrophobicity may contribute to an increased uptake of the complex by the cells,thereby enhancing the antiproliferative activity[35-39].The standard octanolwater partition coefficient(lgP)was determined for 1~3:-1.5 for 1;-1.2 for 2;-1.9 for 3.From the results, complex 2 is more lipophilic than 1 and 3.For the ruthenium complexes,the anticancer activity directly related to the lgP,because of activity increasing with theincreaseinlipophilicity.Thissuggeststhat lipophilicity is an important determinant of activity, but only to the level that allows the ruthenium complex to easily diffuse across the cellularmem brane[40].
3 Conclusions
A series of new mononuclear Ru(Ⅱ)complexes were synthesized and characterized using elemental analysis,IR,ESI-MS and1H NMR techniques.Absorption spectra,luminescent properties and electrochemical behavior of the complexes were also studied. Complexes 1~3 exhibit the spin-allowed singlet metalto-ligand charge transfer transition at approximate 490 and 440 nm and Ru(Ⅱ)metal centered emission at around 600 nm in solution at room temperature. Moreover,1~3 undergo metal centered oxidation and the Ru(Ⅱ)/Ru(Ⅲ)redox couple are 0.7~1.0 V(vs SCE). In vitro cytotoxic assays of the complexes were studied,and the results indicate that Ru(Ⅱ)complexesshow significant dose-dependent cytotoxicity to breast cancer(MCF-7),gastric cancer(AGS)and lung cancer (A549)tumour cell lines,and 2 shows excellent antitumor effects in a cellular study(IC50value of 2.85 μmol·L-1for MCF-7 in vitro).This result reveals that 2 might be a potential anticancer agent that could improve on the efficacy of common anticancer therapies,such as platinum-based drugs.
Supporting information is available at http://www.wjhxxb.cn
[1]Makhubela B C E,Meyer M,Smith G S.J.Organomet. Chem.,2014,772:229-241
[2]Alagesan M,Sathyadevi P,Krishnamoorthy P,et al.Dalton Trans.,2014,43:15829-15840
[3]Nguyen T D,Duong T T,Le T P Q,et al.Med.Chem.Res., 2011,20:790-793
[4]Li G Y,Du K J,Wang J Q,et al.J.Inorg.Biochem.,2013, 119:43-53
[5]Pinato O,Musetti C,Farrell N P,et al.J.Inorg.Biochem., 2013,122:27-37
[6]Reedijk J.Chem.Commun.,1996,801-806
[7]McWhinney S R,Goldberg R M,McLeod H L.Mol.Cancer Ther.,2009,8:10-16
[8]Liu Y J,Zeng C H,Liang Z H,et al.Eur.J.Med.Chem., 2010,45:3087-3095
[9]Morais T S,Santos F,Côrte-Real L,et al.J.Inorg.Biochem., 2013,122:8-17
[10]Liu Y J,Liang Z H,Li Z Z,et al.J.Organomet.Chem., 2011,696:2728-2735
[11]Jaividhya P,Dhivya R,Akbarsha M A,et al.J.Inorg. Biochem.,2012,114:94-105
[12]Abdi K,Hadadzadeh H,Weil M,et al.Polyhedron,2012, 31:638-648
[13]Li M J,Lan T Y,Cao X H,et al.Dalton Trans.,2014,43: 2789-2798
[14]Govender P,Edafe F,Makhubela B C E,et al.Inorg.Chim. Acta,2014,409:112-120
[15]Kalidasan M,Forbes S,Mozharivskyj Y,et al.Inorg.Chim. Acta,2014,421:349-358
[16]Rademaker-Lakhai J M,Bongard D,Pluim D.Clin.Cancer Res.,2004,10:3717-3727
[17]Hartinger C G,Jakupeca M A,Zorbas-Seifrieda S,et al. Chem.Biodivers.,2008,5:2140-2155
[18]Lentz F,Drescher A,Lindauer A,et al.Anti-Cancer Drugs., 2009,20:97-103
[19]Leijen S,Burgers S A,Baas P,et al.Invest.New Drugs., 2015,33:201-214
[20]Zhang Y,Lai L,Cai P,et al.New J.Chem.,2015,39:5805-5812
[21]ZhangY,HuPC,CaiP,etal.RSCAdv.,2015,5:11591-11598
[22]Roy S,Maheswari P U,Golobic A,et al.Inorg.Chim.Acta, 2012,393:239-245
[23]Karki S S,Thota S,Darj S Y,et al.Bioorg.Med.Chem., 2007,15:6632-6641
[24]Srishailam A,Kumar Y P,Reddy P V,et al.J Photochem. Photobiol.B,2014,132:111-123
[25]Stringer T,Therrien B,Hendricks D T,et al.Inorg.Chem. Commun.,2011,14:956-960
[26]Garza-Ortiz A,Maheswari P U,Siegler M,et al.New J.Chem., 2013,37:3450-3460
[27]Lin G J,Jiang B,Xie Y Y,et al.J.Biol.Inorg.Chem.,2013, 18:873-882
[28]Sullivan B P,Salmon D J,Meyer T J.Inorg.Chem.,1978, 17:3334-3341
[29]Mosmann T.J.Immunol.Methods,1983,65:55-63
[30]Pal S.Z.Anorg.Allg.Chem.,2002,628:2091-2098
[31]Constable E C,Housecro C E,Kopecky P,et al.Polyhedron, 2013,64:38-44
[32]Pal S,Pal S.Inorg.Chem.,2001,40:4807-4810
[33]ZHANG Yan(张燕),XIONG Bi(熊碧),CAI Ping(蔡苹), et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28(11): 2437-2443
[34]Hu P C,Wang Y,Zhang Y,et al.RSC Adv.,2016,6: 29963-29976
[35]Mendoza-Ferri M G,Hartinger C G,Mendoza M A,et al.J. Med.Chem.,2009,52:916-925
[36]Giannini F,Paul L E H,Furrer J,et al.New J.Chem., 2013,37:3503-3511
[37]Mulyana Y,Weber D K,Buck D P,et al.Dalton Trans., 2011,40:1510-1523
[38]Gorle A K,Ammit A J,Wallace L,et al.New J.Chem.,2014, 38:4049-4059
[39]DeBerardinis R J,Lum J J,Hatzivassiliou G,et al.Cell Metab., 2008,7:11-20
[40]Gorle A K,Feterl M,Warner J M,et al.Dalton Trans.,2014, 43:16713-16725
三个单核钌配合物的合成、表征及抗肿瘤活性
张燕*杨艳 温艳珍 贾士芳*
(太原科技大学化学与生物工程学院,太原030024)
对3种单核钌配合物[Ru(bpy)2(paH)]PF6(1),[Ru(dmb)2(paH)]PF6(2),[Ru(phen)2(paH)]PF6(3)(bpy=2,2′-联吡啶,dmb=4,4′-二甲基-2,2′-联吡啶,phen=菲咯啉,paH=2-吡啶甲酸)进行合成,并通过元素分析、红外、核磁和电喷雾质谱对其进行表征。此外,对配合物进行了光谱学和电化学测试,电化学实验表明1~3在0.7~1.0 V范围内均有1个氧化还原峰,说明钌中心发生了氧化还原反应。最后通过MTT法(MTT为3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐)对配合物进行了体外细胞毒性实验,结果表明:1~3对人乳腺癌细胞、胃癌细胞和肺癌细胞都表现出浓度依赖性,即随着配合物浓度的逐渐增大,细胞的存活率逐渐降低。值得注意的是2对乳腺癌细胞表现出非常明显的抑制作用(IC50=2.85 μmol·L-1)。
单核钌配合物;光谱性质;电化学性质;细胞毒性
O614.82+1文献标识码:A
1001-4861(2017)06-1035-08
2016-12-16。收修改稿日期:2017-04-05。
10.11862/CJIC.2017.123
国家自然科学基金(No.21101121)、湖北省自然科学基金(No.2010CDB01301)和太原科技大学博士启动基金(No.20172005)资助项目。
*通信联系人。E-mail:yanzhang872010@163.com,jiashifang@126.com
猜你喜欢
杂志排行
无机化学学报的其它文章
- CoAl2O4/蜂窝陶瓷催化剂的制备及其催化臭氧化性能
- Photocatalytic Hydrogen Production Based on Cobalt-Thiosemicarbazone Complex with the Xanthene Dye Moiety
- 两种金属-有机钙钛矿材料的负热膨胀性质
- Pyrazolate-Based Dipalladium(Ⅱ,Ⅱ)Complexes:Syntheses,Characterization and Catalytical Performance in Suzuki-Coupling Reaction
- 以滤纸为模板合成新型介孔生物活性玻璃微管材料
- Br-掺杂Bi2WO6的水热法合成及其可见光催化性能
