植物MYB44转录因子的功能及其在橡胶树抗逆研究中的应用前景
2017-06-12王立丰陆燕茜王纪坤覃碧张冬
王立丰+陆燕茜+王纪坤+覃碧+张冬


摘 要 MYB44是植物典型的R2R3-MYB转录因子,在不同物种间基因结构保守,可转录调控植物对干旱和盐等胁迫的抵抗能力。笔者总结了MYB44的结构特征,着重阐述MYB44转录因子在逆境响应中的调控机制与应用现状,并对HbMYB44在橡胶树抗逆研究中的应用进行了展望。
关键词 MYB44 ;转录调控 ;逆境 ;橡胶树
中图分类号 S794.1 ;Q291 文献标识码 A Doi:10.12008/j.issn.1009-2196.2017.05.007
Functions of Plant MYB44 Transcript Factor and Its ApplicationProspects
for Stress Resistance Research in Rubber Tree
WANG Lifeng1) LU Yanxi2) WANG Jikun1) QIN Bi1) ZHANG Dong2)
(1 Rubber Research Institute, CATAS / Danzhou Investigation and Experiment Station of
Tropical Crops, Ministry of Agriculture, Danzhou, Hainan 571737;
2 Hainan Key Laboratory for Sustainable Utilization of Tropical Bioresource /
Institute of Tropical Agriculture and Forestry, Hainan University, Haikou, Hainan 570228)
Abstract MYB44 was a typical plant R2R3-MYB transcription factor, and its gene structure was conserved in different plant species. MYB44 could regulate plant resistance to drought and salt stress. The structural characteristics of MYB44 were summarized, with an emphasis on the regulation mechanism and application status of MYB44 transcription factor in stress response, and a prospect for application of HbMYB44 in the study of stress resistance of Hevea brasiliensis was put forward.
Keywords MYB44 ; transcriptional regulation ; stress ; Hevea brasiliensis Muell. Arg
植物R2R3-MYB转录因子通过结合靶标基因启动子MBSI(T/C)AAC(T/G)G和MBSIIG(G/T)T(A/T)G(G/T)T元件来调控植物次生代谢和逆境响应等重要生理生化过程,且其调控过程还受多种激素和环境因子诱导。MYB转录因子主要通过与bHLH、WD等其它转录因子或者其互作蛋白相结合来调控植物多个重要生理生化过程,其自身也受到转录水平和翻译后水平的调节。天然橡胶生物合成是通过异戊二烯合成路径进行的,是典型的植物次生代谢途径,参与橡胶树生长发育和抗逆反应。在模式植物中,已证明MYB调控异戊二烯合成关键酶的表达,但MYB转录因子转录调节天然橡胶生物合成的机制尚不清楚。笔者详细综述了R2R3MYB类转录因子MYB44的结构特征及其在逆境响应中的调控机制和应用现状,并对HbMYB44在橡胶树抗逆研究中的应用进行了展望。
1 MYB转录因子结构与功能概述
MYB转录因子在高等植物中分布广泛,是最大的转录因子家族成员之一,在N端具有高度保守的HTH_MYBDNA结合结构域[1]。MYB转录因子是植物激素信号转导和各种应激反应途径的关键物质,在植物生长发育、代谢及响应生物和非生物胁迫的调控网络中具有重要作用[2]。植物第一个MYB轉录因子是玉米中与色素合成相关的ZmMYBC1基因[3]。迄今为止,在拟南芥中已发现超过198个MYB家族基因[4],棉花中发现大约有200个MYB转录因子[5],玉米中有100个左右MYB 转录因子[6],毛果杨中至少有197个MYB转录因子,葡萄中已发现超过124个MYB转录因子[7]。MYB类转录因子以其结构上都有一段保守的DNA结合区——MYB结构域而得名,由3个保守的结构域组成,即DNA结合结构域、转录激活结构域和一个不完全界定的负调节区[8]。其中DNA结合结构域最为保守,一般包含1~3个不完全重复序列(R),每个重复片段R由51~52个保守的氨基酸残基和间隔序列组成,每隔约18个氨基酸规则间隔1个色氨酸残基,这些氨基酸残基使MYB结构域折叠成一个3D的螺旋-转角-螺旋(Helix-Turn-Helix,HTH)结构[9],对维持HTH的构型有重要意义。根据MYB结构域所含MYB重复个数,把MYB类转录因子分为4种类型,即单一的MYB结构域蛋白(R1/R2)、包含2个重复的2R蛋白(R2R3)、包含3个重复的3R蛋白(R1R2R3)以及4个MYB重复的4R-MYB蛋白[10-11]。
研究表明,MYB转录因子广泛参与植物次生代谢[12]、激素和环境因子应答[13],并对细胞分化、细胞周期以及叶片等器官形态建成[14]具有重要的调节作用。例如,AtMYB23转录因子能调控拟南芥表皮细胞分化、诱导不定根发育及叶子和茎的伸长[15],过表达AtMYB2能增强转基因植株的抗旱能力[16]。GhMYB109转录因子能直接参与调节棉花纤维细胞的发生和延伸[17]。在杨树中过表达BpMYB106转录因子能显著地提高表皮毛密度、净光合速率以及生长速率[18],JcMYB2通过参与茉莉酸和脱落酸信号途径交互来调控麻风树(Jatropha curcas)根系发育的逆境响应[19]。MYB转录因子主要通过下调下游关键基因表达参与防卫反应和植物生长发育。如,金鱼草AmMYB305和其同源的拟南芥AtMYB4转录因子通过抑制编码C4H酶的关键基因的表达来积累紫外线,从而防护物质芥子酸酯[20]。AmMYB308转录因子下调C4H(cinnamate-4-hydroxylase),4CL(coumaroyl-4-Co A ligase),CAD(cinnamyl alcohol dehdrogenase)基因调控苯丙酸和木质素的生物合成[21]。AtMYB32转录因子通过抑制拟南芥中COMT基因的表达来调控花粉管发育[22]。
2 MYB44转录因子研究进展
2.1 MYB44转录因子结构
拟南芥基因组MYB转录因子按结构特性分为22个亚组[23],相同亚组的基因成员具有类似的功能。MYBR1(MYB44)是最早发现的与动物MYB转录因子相似的基因之一[24]。AtMYB44是典型的R2R3-MYB转录因子,属于第22亚家族,这个亚家族中由AtMYB44、AtMYB70、AtMYB73[25]和AtMYB77[26]4个成员组成,有2个保守的基元TGLYMSPxSP和GxFMxVVQEMIxxEVRSYM,此结构含2个分别由50~53个氨基酸序列形成的螺旋-转角-螺旋R2R3基元。该亚组成员具有基因结构保守、功能相近和表达规律相似等特点,与下游基因启动子区MBSI(T/C)AAC(T/G)G和MBSIIG(G/T)T(A/T)G(G/T)T元件结合[23,27-28]。
2.2 MYB44转录因子在逆境胁迫中的功能研究
2.2.1 MYB44在植物激素信号中的作用
植物抗逆、防御病虫害主要与激素信号转导机制有关。研究表明,MYB44是水杨酸(salicylic acid,SA)、脱落酸(abscisic acid,ABA)、茉莉酸(jasmonic acid,JA)和乙烯(ethylene,ET)、生长素(Auxin)和赤霉素(gibberellin,GA)信号途径共同的转录因子,参与上述激素信号途径的交互作用,对微生物、真菌、钙离子信号、盐和干旱等生物和非生物胁迫有所响应。
茉莉酸类是一种植物激素家族,调节生殖发育过程,例如花發育、花粉成熟和衰老[29],茉莉酮酸酯还作为响应于伤口和病原体感染中的激活防御基因的局部或系统的信号分子[30]。AtMYB44是拟南芥中一个多功能的转录激活因子,最初通过微阵列被确定为茉莉酸诱导的基因[31]。茉莉酸和胡萝卜软腐欧文氏菌胡萝卜亚种(Erwinia carotovora subsp. Carotovora, Ecc)可以诱导AtMYB44的表达[32],这说明AtMYB44参与拟南芥防卫胡萝卜软腐欧文氏菌的过程,其机制为AtMYB44抑制JA通路信号传导(图1),并负调控拟南芥对胡萝卜软腐欧文氏菌的抗病性。尽管茉莉酸甲酯(MeJA)和伤害处理能够上调AtMYB44表达,但AtMYB44对茉莉酸激素信号的响应无特异性[32-33]。
用ABA处理拟南芥后,AtMYB44转录水平明显增加,并且在导管和叶片气孔中高效表达。MYB44在ABA信号转导中的作用机制为脱落酸信号受体PYL8与MYB77和MYB44形成蛋白复合体,促进MYB44结合下游靶标基因启动子区的MBSI基序,从而调控ABA响应基因表达(图2)[34]。此外,AtMYB44通过抑制ABA的负调控因子PP2Cs(丝氨酸/苏氨酸蛋白激酶2C家族)来对ABA进行正向调控[35]。Li等[36]2014采用pulldown和酵母双杂交技术证明AtMYB44转录因子N端54-105氨基酸与ABA受体RCAR1具有直接互作作用。
在乙烯信号途径中,MYB44调控乙烯信号途径重要环节EIN2表达,从而调节植株对蚜虫和蛾子的抗性。AtMYB44与EIN2都对芥子油苷合成相关基因中的11个基因转录水平有影响,AtMYB44与EIN2的转录调控作用可能是通过影响芥子油苷合成来达到抗蚜虫和小菜蛾的目的,这一调控过程对诱导抗性和免疫反应都有作用,代表了植物抵抗植食昆虫的一种重要防卫机制[37]。
在激素信号交互中,革兰氏阴性植物病原细菌产生的harpin蛋白HrpNEa在抗虫、抗病中的作用是:通过拟南芥MYB44转录调控ABA和乙烯信号的关键因子ABI2和EIN2蛋白来实现的[38]。AtMYB44通过直接调控WRKY70表达来调节水杨酸和茉莉酸信号在植物防卫反应中的作用[39]。脱落酸和赤霉素在植物种子萌发和幼苗发育过程具有拮抗作用,如赤霉素抑制剂会上调MYB44表达来抑制种子萌发[40]。
2.2.2 MYB44在病虫害防御和植物生长发育中的作用机制
MYB44在氧化胁迫和非生物抗性中具有重要作用,还参与种子成熟、胚的发育等生理过程。MYB44是丝裂原活化蛋白激酶(MPK3)底物,是植物防卫的早期响应因子[41],参与ISR(intergenic spacer region)分子机制,对叶片气孔阻力进行调控[42]。研究表明,过表达MYB44转录因子能提高叶片的抗旱能力,降低叶片水分蒸发几率[34]。王艳红等[43]采用 mRNA差异显示技术(DDRT-PCR)和反向Northern杂交技术分析小麦 Brock在白粉菌诱导下的差异表达基因,发现经白粉菌诱导后MYB44的表达明显上调。AtMYB44可调控拟南芥受丁香假单胞菌侵染后所引起的细胞防卫反应,这种反应依赖SA信号通路PR1基因,AtMYB44通过调控PR1基因的表达来提高对丁香假单胞菌的抗性[44]。拟南芥对蚜虫的抗性与harpin蛋白有关,MYB44是37个响应harpin蛋白的一员,其上调表达量最高[45-46]。植物生长促进真菌Penicillium simplicissimum GP17-2通过调控MYB44介导气孔张开,从而提高植株对丁香假单胞菌Pseudomonas syringae pv. tomato DC3000(Pst)的抗性[42]。但是,灰霉病会导致过表达拟南芥的抗性降低。可见,拟南芥对真菌Penicillium simplicissimum的系统抗性与HbMYB44调控气孔开关有关。
在拟南芥种子萌发过程中,MPK3和MPK6激酶分别作用于MYB44的Ser53和Ser145,从而调控种子萌发[40]。AtMYB44还具有促进拟南芥抗病、耐热及抑制开花的作用。过表达MYB44会使细胞变小,但不影响细胞数量[47]。
2.3 MYB44在其它作物中的应用
以小麦幼胚诱导的愈伤组织为转化受体,以磷酸甘露糖异构酶基因作为选择标记,通过基因枪介导法将带有AtMYB44基因的表达质粒AF234296-44导入小麦品种扬麦158,证明拟南芥转录因子AtMYB44基因对小麦抗病能力的影响[48]。在番茄中的研究证明番茄激酶SpMPKs通过调控MYB44转录因子蛋白,进而调控番茄对非生物胁迫的响应。将拟南芥AtMYB44转入水稻后,分别对经不同脅迫处理后的水稻各组织进行RT-PCR 分析,结果表明AtMYB44基因在干旱和高盐的胁迫下存在过量表达。生理功能分析结果表明,转基因水稻中MYB44基因的过量表达明显提高了水稻对低温胁迫的耐受性[49]。将番茄的SpMPK3基因在拟南芥中过表达,也发现它能通过提高AtMYB44表达量来提高植株对渗透胁迫的抗性[50]。在转基因大豆中,也证明了MYB44在大豆对干旱和盐胁迫抗性中的作用[51]。
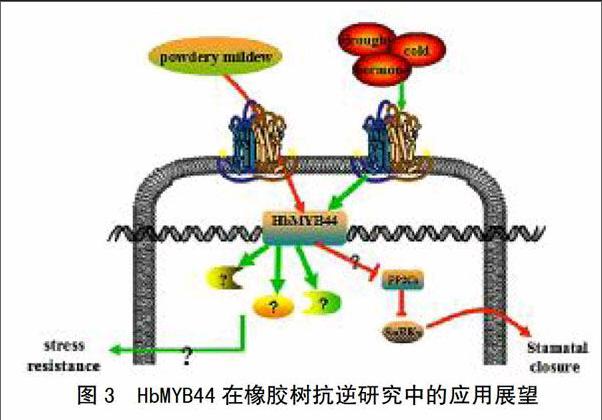
3 MYB44转录因子在橡胶树抗逆研究中的展望
尽管相关学者在多种植物中证明MYB转录因子具有多样性的功能,但在橡胶树中研究较少。研究较为深入的是与死皮相关的HbMYB1转录因子,其在橡胶树的叶片、树皮以及胶乳中表达,并且能有效地减少橡胶树死皮病的发生[13]。转基因实验证明,HbMYB1还具有抑制烟草遭遇逆境所引起的细胞死亡过程[52]。HbSM1也是与橡胶树发育有关的MYB转录因子,参与橡胶树抗逆响应过程[53]。
橡胶树在中国经常受到干旱[54]、寒害和病原菌侵染[55]等非生物与生物胁迫。最近,笔者采用分子生物学技术在橡胶树中克隆了HbMYB44转录因子基因,结合其在模式植物和作物中的研究进展,对其在橡胶树抗逆研究中的应用前景展望如图3所示。
在基因功能验证方面,将HbMYB44转入拟南芥[56]、水稻[49]、小麦[48]是最直接有效的验证方式。可将HbMYB44转入烟草、拟南芥或者橡胶草中,经测序鉴定为稳定植株后,通过采用干旱、低温、激素分别处理幼苗和白粉菌侵染叶片的方法来鉴定该基因在抗逆和抗病中的作用。
突变体技术,包括利用双突变体和四突变体研究基因功能的有效手段,例如,mybr1(MYB44)×mybr2(MYB77)双突变体比单突变体表型衰老更明显,说明MYB44和MYB77对衰老均有重要的调控作用[34]。将HbMYB44基因在拟南芥野生Col中过表达,并将其转入atmyb44突变体进行互补验证,获得稳定植株后分析转基因植株表型和经干旱、低温、激素处理后的基因差异表达规律,有助于揭示HbMYB44结构及其在植物生长发育和抗逆过程中的功能。
在转录因子鉴定方面,除了转录因子自激活活性鉴定和亚细胞定位分析,还要结合体内体外蛋白-蛋白和蛋白-DNA互作技术阐明HbMYB44调控下游基因启动子区的结合元件、互作蛋白的位点等,例如,凝胶阻滞实验证明MYB44与MBSII元件在体外具有结合活性[57],体内共转化实验证明二者不具有结合活性[41]。此外,可采用染色质免疫沉淀技术与体内共转化荧光素酶技术相结合研究HbMYB44与其互作DNA、蛋白的结合。
总之,系统地分析橡胶树HbMYB44及其互作蛋白、基因有助于阐明HbMYB44转录调控天然橡胶抗逆的分子机制,为培育橡胶树抗逆品种和研发新型抗逆栽培技术提供技术指导。
参考文献
[1] 陈 俊, 王宗阳. 植物MYB类转录因子研究进展[J]. 植物生理与分子生物学学报,2002,28(2):81-88.
[2] Ambawat S, Sharma P, Yadav N R, et al. MYB transcription factor genes as regulators for plant responses: an overview[J]. Physiol Mol Biol Plants, 2013, 19(3): 307-321.
[3] Paz-Ares J, Ghosal D, Wienand U, et al. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators [J]. Embo J, 1987, 6(12): 3 553-3 558.
[4] Chen Y H, Xiaoyuan Y, Kun H, et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family[J]. Plant Mol Biol, 2006, 60(1): 107-124.
[5] Cedroni M L, Cronn R C, Adams K L, et al. Evolution and expression of MYB genes in diploid and polyploid cotton[J]. Plant Mol Biol, 2003, 51(3): 313-325.
[6] Rabinowicz P D, Braun E L, Wolfe A D, et al. Maize R2R3 Myb genes: Sequence analysis reveals amplification in the higher plants [J]. Genetics, 1999, 153(1): 427-444.
[7] Wilkins O, Nahal H, Foong J, et al. Expansion and diversification of the Populus R2R3-MYB family of transcription factors [J]. Plant Physiol, 2009, 149(2): 981-993.
[8] Rosinski J A, Atchley W R. Molecular evolution of the Myb family of transcription factors: evidence for polyphyletic origin[J]. J Mol Evoluti, 1998, 46(1): 74-83.
[9] Ogata K, Kanei-Ishii C, Sasaki M, et al. The cavity in the hydrophobic core of Myb DNA-binding domain is reserved for DNA recognition and trans-activation [J]. Nat Struct Biol, 1996, 3(2): 178-187.
[10] Ogata K, Morikawa S, Nakamura H, et al. Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices[J]. Cell, 1994, 79(4): 639-648.
[11] 陳 清,汤浩茹,董晓莉,等. 植物Myb转录因子的研究进展 [J]. 基因组学与应用生物学,2009,28(2):365-372.
[12] Uimari A, Strommer J. Myb26: a MYB-like protein of pea flowers with affinity for promoters of phenylpropanoid genes[J]. Plant J, 1997, 12(6): 1 273-1 284.
[13] Chen S, Peng S, Huang G, et al. Association of decreased expression of a Myb transcription factor with the TPD(tapping panel dryness)syndrome in Hevea brasiliensis[J]. Plant Mol Biol, 2003, 51(1): 51-58.
[14] Lee M M, Schiefelbein J. Cell pattern in the Arabidopsis root epidermis determined by lateral inhibition with feedback[J]. The Plant Cell, 2002, 14(3): 611-618.
[15] Matsui K, Hiratsu K, Koyama T, et al. A chimeric AtMYB23 repressor induces hairy roots, elongation of leaves and stems, and inhibition of the deposition of mucilage on seed coats in Arabidopsis [J]. Plant & Cell Physiol, 2005, 46(1): 147-155.
[16] Abe H, Urao T, Ito T, et al. Arabidopsis AtMYC2(bHLH)and AtMYB2(MYB)function as transcriptional activators in abscisic acid signaling[J]. The Plant Cell, 2003, 15(1): 63-78.
[17] Suo J, Liang X, Pu L, et al. Identification of GhMYB109 encoding a R2R3 MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton(Gossypium hirsutum L.)[J]. Biochim Biophys Acta, 2003, 1630(1): 25-34.
[18] Zhou C, Li C. A Novel R2R3-MYB transcription factor BpMYB106 of birch(Betula platyphylla)confers increased photosynthesis and growth rate through up-regulating photosynthetic gene expression [J]. Front Plant Sci, 2016, 7: 315.
[19] Peng X, Liu H, Wang D, et al. Genome-wide identification of the Jatropha curcas MYB family and functional analysis of the abiotic stress responsive gene JcMYB2[J]. BMC Genomics, 2016, 17: 251.
[20] Jin H, Cominelli E, Bailey P, et al. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis[J]. EMBO J, 2000, 19(22): 6 150-6 161.
[21] Tamagnone L, Merida A, Parr A, et al. The AmMYB308 and AmMYB330 transcription factors from antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco[J]. The Plant Cell, 1998, 10(2): 135-154.
[22] Preston J, Wheeler J, Heazlewood J, et al. AtMYB32 is required for normal pollen development in Arabidopsis thaliana[J]. Plant J, 2004, 40(6): 979-995.
[23] Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana[J]. Curr Opin Plant Biol, 2001, 4(5): 447-456.
[24] Kirik V, Kolle K, Misera S, et al. Two novel MYB homologues with changed expression in late embryogenesis-defective Arabidopsis mutants[J]. Plant Mol Biol, 1998, 37(5): 819-827.
[25] 樊錦涛. 拟南芥AtMYB73响应干旱机制初探[D]. 保定:河北农业大学,2015.
[26] Zhao Y, Xing L, Wang X, et al. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes[J]. Sci Signal, 2014, 7(328): ra53.
[27] Kranz HD, Denekamp M, Greco R, et al. Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana[J]. Plant J, 1998, 16(2): 263-276.
[28] Romero I, Fuertes A, Benito M J, et al. More than 80R2R3-MYB regulatory genes in the genome of Arabidopsis thaliana[J]. Plant J, 1998, 14(3): 273-284.
[29] Creelman R A, Mullet J E. Biosynthesis and action of jasmonates in plants[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1997, 48:355-381.
[30] Reymond P, Farmer E E. Jasmonate and salicylate as global signals for defense gene expression [J]. Curr Opin Plant Biol, 1998, 1(5): 404-411.
[31] Jung C, Lyou S H, Yeu S, et al. Microarray-based screening of jasmonate-responsive genes in Arabidopsis thaliana[J]. Plant Cell Rep, 2007, 26(7): 1 053-1 063.
[32] Jung C, Shim J S, Seo J S, et al. Non-specific phytohormonal induction of AtMYB44 and suppression of jasmonate-responsive gene activation in Arabidopsis thaliana[J]. Molecules and Cells, 2010, 29(1): 71-76.
[33] Jung C, Seo J S, Han S W, et al. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis [J]. Plant Physiol, 2008, 146(2): 623-635.
[34] Jaradat M R, Feurtado J A, Huang D, et al. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence[J]. BMC Plant Biol, 2013, 13: 192.
[35] Shim J S, Jung C, Lee S, et al. AtMYB44 regulates WRKY70 expression and modulates antagonistic interaction between salicylic acid and jasmonic acid signaling[J]. Plant J, 2013, 73(3): 483-495.
[36] Li D, Li Y, Zhang L, et al. Arabidopsis ABA receptor RCAR1/PYL9 interacts with an R2R3-type MYB transcription factor, AtMYB44 [J]. Int J Mol Sci, 2014, 15(5): 8 473-8 490.
[37] Lu B B, Li X J, Sun W W, et al. AtMYB44 regulates resistance to the green peach aphid and diamondback moth by activating EIN2-affected defences in Arabidopsis[J]. Plant Biol (Stuttg), 2013, 15(5): 841-850.
[38] 吕贝贝. MYB44对HrpN_(Ea)诱导拟南芥抗虫防卫信号传导的调控作用[D]. 江苏:南京农业大学,2012.
[39] Shim J S, Choi Y D. Direct regulation of WRKY70 by AtMYB44 in plant defense responses[J]. Plant Signaling & Behavior, 2013, 8(6): e20 783.
[40] Nguyen X C, Hoang M H, Kim H S, et al. Phosphorylation of the transcriptional regulator MYB44 by mitogen activated protein kinase regulates Arabidopsis seed germinatio [J]. Biochem Biophys Res Commun, 2012, 423(4): 703-708.
[41] Persak H, Pitzschke A. Dominant repression by Arabidopsis transcription factor MYB44 causes oxidative damage and hypersensitivity to abiotic stress[J]. Int J Mol Sci, 2014, 15(2): 2 517-2 537.
[42] Hieno A, Naznin H A, Hyakumachi M, et al. Possible Involvement of MYB44-Mediated Stomatal Regulation in Systemic Resistance Induced by Penicillium simplicissimum GP17-2 in Arabidopsis[J]. Microbes and environments/JSME, 2016, 31(2): 154-159.
[43] 王艷红,肖 莹,郑舒扬,等. 白粉菌诱导的小麦品种Brock的差异表达基因解析[J]. 天津师范大学学报(自然科学版),2015,35(2):71-76.
[44] 邹保红. 染色质修饰基因HUB1和CHR5及转录因子MYB44在拟南芥防卫反应中的功能研究[D]. 南京:南京农业大学,2013.
[45] Liu R, Lu B, Wang X, et al. Thirty-seven transcription factor genes differentially respond to a harpin protein and affect resistance to the green peach aphid in Arabidopsis[J]. Journal of biosciences, 2010, 35(3): 435-450.
[46] Lü B, Sun W, Zhang S, et al. HrpNEa-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana[J]. Journal of Biosciences, 2011, 36(1): 123-137.
[47] Park J-B, Sendon P M, Kwon S H, et al. Overexpression of stress-related genes, BrERF4 and AtMYB44, in Arabidopsis thaliana alters cell expansion but not cell proliferation during leaf growth[J]. Journal of Plant Biology, 2012, 55(5): 406-412.
[48] 柳金伟,焦 娇,张洪滨,等. 转AtMYB44基因小麦的获得和检测[J]. 鲁东大学学报(自然科学版),2012,28(2):150-154.
[49] 李 敏. 拟南芥AtMYB44和LWT1基因在水稻中的遗传转化及功能验证[D]. 合肥: 安徽农业大学, 2012.
[50] Li C, Chang P P, Ghebremariam K M, et al. Overexpression of tomato SpMPK3 gene in Arabidopsis enhances the osmotic tolerance[J]. Biochem Biophys Res Commun, 2014, 443(2): 357-362.
[51] Seo J S, Sohn H B, Noh K, et al. Expression of the Arabidopsis AtMYB44 gene confers drought/salt-stress tolerance in transgenic soybean[J]. Molecular Breeding, 2011, 29(3): 601-608.
[52] Peng S Q, Wu K X, Huang G X, et al. HbMyb1, a Myb transcription factor from Hevea brasiliensis, suppresses stress induced cell death in transgenic tobacco[J]. Plant Physiol Biochem, 2011, 49(12): 1 429-1 435.
[53] Qin B, Zhang Y, Wang M. Molecular cloning and expression of a novel MYB transcription factor gene in rubber tree[J]. Mol Biol Rep, 2014, 41(12): 8 169-8 176.
[54] Wang L F. Physiological and molecular responses to drought stress in rubber tree(Hevea brasiliensis Muell. Arg.)[J]. Plant Physiology and Biochemistry, 2014, 83: 243-249.
[55] Wang L F, Wang M, Zhang Y. Effects of powdery mildew infection on chloroplast and mitochondrial functions in rubber tree[J]. Tropical Plant Pathology, 2014, 39(3): 242-250.
[56] 孫伟伟. 拟南芥转录因子MYB44与葡聚糖合酶GSL5对抗桃蚜防卫反应的调控作用[D]. 南京:南京农业大学,2012.
[57] ung C, Kim Y K, Oh N I, et al. Quadruple 9-mer-based protein binding microarray analysis confirms AACnG as the consensus nucleotide sequence sufficient for the specific binding of AtMYB44[J]. Molecules and Cells, 2012, 34(6): 531-537.
