龟头包皮炎患者淋病奈瑟菌分离与鉴定及其耐药机制研究
2017-06-09葛玉梅胡庆丰朱永泽周永列吕火烊
葛玉梅,胡庆丰,朱永泽,周永列,吕火烊
龟头包皮炎患者淋病奈瑟菌分离与鉴定及其耐药机制研究
葛玉梅,胡庆丰,朱永泽,周永列,吕火烊
目的 分离鉴定龟头化脓性包皮炎病原菌,检测分离菌株耐药性并了解其耐药机制。方法 采取尿道分泌物及龟头脓包液标本,革兰染色镜检并采用Mycoplasma IST 2试剂盒检测支原体。上述标本接种哥伦比亚血平板、淋病奈瑟菌选择平板、酵母菌鉴定平板进行细菌分离培养,获得的菌落用VITEK 2-compact全自动细菌检测分析系统进行鉴定,另采用PCR检测上述标本及菌落的淋病奈瑟菌16S rRNA基因。采用K-B法检测分离菌株对5种常用抗生素的敏感性,采用β-内酰胺酶和超广谱β-内酰胺酶确证试验了解该菌株产酶情况,PCR检测该菌株耐药相关tetM、TEM、mefA和ermF基因。 结果 各标本支原体检测结果均为阴性。尿道分泌物标本分离培养结果均为阴性,龟头脓包液血平板和选择平板培养结果为阳性。VITEK 2-compact系统和16S rRNA-PCR检测结果显示分离菌株为淋病奈瑟菌。该菌株产β内酰胺酶且对青霉素G、环丙沙星、四环素耐药,其基因组携带tetM、TEM、mefA和ermF基因。结论 淋病奈瑟菌可引起龟头化脓性包皮炎,该淋病奈瑟菌菌株多重耐药并与其携带的耐药基因密切相关。
龟头包皮炎;淋病奈瑟菌;分离鉴定;耐药性;耐药基因
淋病奈瑟菌(Neisseriagonorrhoeae)俗称淋球菌,是人类常见性传播疾病淋病(gonorrhea)的病原体[1]。淋病是《中华人民共和国传染病防治法》中重点防治的乙类传染病,近年来其发病率一直占据我国性传播疾病第2位[2]。早已肯定,淋病奈瑟菌引起人泌尿生殖道急性或慢性化脓性感染,新生儿经产道感染后引起淋球菌性结膜炎[3]。然而,我们从1例龟头包皮炎患者龟头脓包液中分离出淋病奈瑟菌,该菌株同时携带四环素耐药相关tetM基因、β-内酰胺类抗生素耐药相关TEM基因、红霉素耐药相关外排基因mefA和甲基化酶基因ermF基因,表现为对多种抗生素很强的耐药性。
1 材料和方法
1.1 菌株来源及分离培养 龟头包皮化脓性感染患者男性,33岁,无尿道刺激症状,2016年2月27日就诊杭州某三甲医院泌尿外科。无菌棉棒采取尿道分泌物及龟头包皮脓包液标本,革兰染色镜检并采用Mycoplasma IST 2试剂盒(梅里埃诊断产品有限公司)检测支原体,然后将标本分别涂布淋病奈瑟菌巧克力选择平板、哥伦比亚血平板、酵母菌鉴定平板,35 ℃、5% CO2培养24 h,观察其生长情况及菌落形态。
1.2 菌株鉴定 挑取菌落革兰染色镜检后用VITEK 2-compact型法国梅里埃VITEK 2-compact全自动细菌检测分析系统及其配套的API-NH细菌鉴定卡进行鉴定[4]。此外,采用细菌基因组DNA提取试剂盒(Axygen)提取尿道口分泌物或龟头脓包液、淋病奈瑟菌分离株DNA,紫外分光光度法检测其纯度和浓度。采用淋病奈瑟菌16S rRNA基因通用引物[5]、淋球菌核酸荧光PCR试剂盒(上海复星长征医学科学有限公司)检测上述DNA标本中淋病奈瑟菌16S rRNA基因片段。引物由上海Invitrogen公司合成,上游引物序列:5′-GCT ACG CAT ACC CGC GTT GC-3′, 下游引物序列:5′-CGA AGA CCT TCG AGC AGA CA-3′。PCR参数:94 ℃ 5 min;94 ℃ 30 s、55 ℃ 30 s、72 ℃ 1 min,35个循环;72 ℃ 10 min。采用溴乙锭预染色1.5%琼脂糖凝胶电泳及Bio-Rad成像系统观察260 bp目的扩增片段。
1.3 药物敏感试验 参照国内临床通用的美国临床实验室标准化协会(CLSI)介绍的方法及判断标准[5],采用K-B法检测分离菌株对6种常见抗生素的敏感性。实验中采用淋病奈瑟菌ATCC49981株为质控菌株。
1.4 β-内酰胺酶和超广谱β-内酰胺酶确证试验 分别采用显色头孢菌素法和双纸片法进行β-内酰胺酶和超广谱β-内酰胺酶(ESBLs)确证试验[6-7]。淋病奈瑟菌分离株涂布于纸片头孢硝噻吩纸片(BioMérieux)上,即刻观察纸片颜色变化,若出现红色表明该菌产β-内酰胺酶。淋病奈瑟菌分离株涂布于巧克力琼脂(Oxoid)平板上,然后贴上头孢他啶、头孢他啶/克拉维酸、头孢噻肟、头孢噻肟/克拉维酸药物纸片(Oxoid),37 ℃孵育24 h后观察结果。若头孢他啶/克拉维酸或头孢噻肟/克拉维酸纸片抑菌环直径较头孢他啶或头孢噻肟纸片抑菌环直径≥5 cm,表明该菌株产ESBLs。实验中以不产ESBLs大肠埃希菌ATCC25922株为阴性对照、产ESBLs肺炎克雷伯菌ATCC700603株为阳性对照。
1.5 耐药基因检测 按上法提取淋病奈瑟菌分离株DNA,紫外分光光度法检测其纯度和浓度[4]。采用文献报道的引物以及Ex-Taq PCR试剂盒(TaKaRa)检测上述DNA标本中TEM、tetM、ermF、mefA耐药基因片段,反应参数同上[8-10]。引物并由上海Invitrogen公司合成,其序列(见表1)。
表1 淋病奈瑟菌耐药基因PCR引物
Tab.1 Primers for amplification of drug resistance genes ofN.gonorrhoeae

耐药基因drugresistancegenes引物序列(5'-3')primersequences产物长度/bplengthtetMF:GTGGACGAACTTTAC-CGAA501R:GCTTTGTATCTCCAA-GAACACTEM内显F:AGGAAGAGTATGAT-TCAACA535R:CTCGTCGTTTGGTAT-GGCmefAF:ACTATCATTAATCAC-TAGTGC346R:TTCTTCTGGTACTAA-AAGTGGermFF:GGATACGGTTTAGA-TATTGGG295R:TTGAAGGACAATGG-AACCTCC
F and R: upstream and downstream primers respectively
2 结 果
2.1 临床标本检查结果 龟头脓包液中可见革兰阴性双球菌,部分双球菌位于多形核白细胞内(图1A),尿道分泌物未见细菌,但两种标本支原体检测结果均为阴性。尿道分泌物各种平板培养结果均为阴性,龟头脓包液酵母菌鉴定平板培养结果阴性,但哥伦比亚血平板和淋病奈瑟菌选择平板培养结果阳性,其菌落细小、凸起、光滑、圆形、灰白色,革兰染色镜检为革兰阴性双球菌(图1B)。淋病奈瑟菌16S rRNA基因PCR结果显示,仅龟头脓包液以及淋病奈瑟菌选择平板上菌落检出淋病奈瑟菌16S rRNA基因片段(图2)。
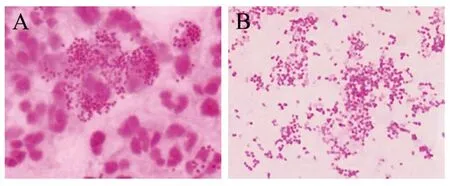
图1 龟头脓包液(A)及选择平板上菌落(B)革兰染色镜检结果(100×)Fig.1 Microscopic examination results of glans pustule (A) and colonies (B) on selective plate after Gram staining (100×)
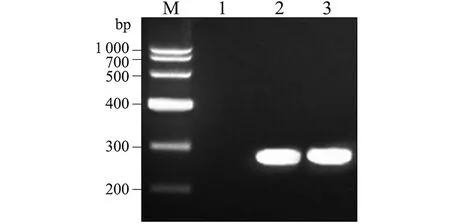
M: DNA marker(TaKaRa); 1-3: PCR results for detecting urethral secretions,balanus pustule liquids and selective plates of N. gonorrhoeae 16S rRNA genes图2 不同标本淋病奈瑟菌16S核糖体rRNA基因PCR检测结果Fig.2 PCR results for detecting different samples of N. gonorrhoeae 16S rRNA gene
2.2 分离菌株生化反应鉴定结果 分离菌株经VITEK 2-compact全自动细菌检测分析系统及其API-NH细菌鉴定卡鉴定为淋病奈瑟菌,鉴定值=99%,各生化反应中营养琼脂35 ℃培养、硝酸盐还原试验NO3(red)、NO2→N2以及麦芽糖、乳糖、蔗糖、果糖发酵试验结果均为阴性,但葡萄糖发酵试验结果阳性。
2.3 药敏试验结果 淋病奈瑟菌分离株对青霉素G、环丙沙星、四环素、红霉素耐药,但对头孢西丁、头孢噻肟敏感(表2)。
表2 淋病奈瑟菌分离株药敏试验结果
Fig.2 Results of drug sensitive test of theN.gonorrhoeaeisolate

抗生素antibioticsKB值KBvalues折点范围breakpoints结果判定results青霉素G(penicillinG)626-47耐药drug-resistant环丙沙星(ciprofloxacin)627-41耐药drug-resistant四环素(tetracycline)2430-38耐药drug-resistant头孢噻肟(cefotaxime)45>=31敏感drug-sensitive头孢西丁(cefoxitin)3323-28敏感drug-sensitive
2.4 β-内酰胺酶及ESBLs确证试验结果 淋病奈瑟菌分离株涂布的头孢硝噻吩纸片由黄色转变为红色,表明该菌株产β-内酰胺酶。头孢他啶对淋病奈瑟菌分离株抑菌环直径18 mm、头孢他啶/克拉维酸17 mm、头孢噻肟32 mm、头孢噻肟/克拉维酸35 mm。由于头孢他啶/克拉维酸或头孢噻肟/克拉维酸抑菌环直径与头孢他啶或头孢噻肟抑菌环直径之差小于5 mm,表明该菌株不产ESBLs。
2.5tetM、TEM、mefA、ermF基因PCR检测结果 淋病奈瑟菌分离株四环素耐药相关tetM基因、β-内酰胺酶TEM基因、红霉素耐药相关mefA和ermF基因PCR结果均为阳性(图3)。

M: DNA marker(TaKaRa); 1: blank control; 2-5: PCR results of tetM,TEM,mefA and ermF genes of the N. gonorrhoeae isolate图3 淋病奈瑟菌分离株tetM、TEM、mefA、ermF基因PCR检测结果Fig.3 PCR results of tetM,TEM,mefA and ermF genes of the N. gonorrhoeae isolate
3 讨 论
淋病是全球流行的人类性传播疾病,临床上主要表现为泌尿道化脓性炎症,该菌虽可上行感染引起男性附睾炎、前列腺炎或引起女性阴道炎、子宫炎[11-13],但未有引起龟头包皮炎的报道。因此,临床上一般采集尿道分泌物检测淋病奈瑟菌。本文中患者既无泌尿道化脓性炎症的症状与体征,其尿道分泌物中也未分离出淋病奈瑟菌或检出该菌16S rDNA基因片段,但在龟头包皮脓包液中不仅分离出淋病奈瑟菌,也检出了该菌16S rDNA基因,表明该患者为淋球菌龟头包皮炎。
近年来淋病奈瑟菌临床分离株对抗生素、尤其是对环丙沙星、青霉素、四环素等临床一线抗菌药物的耐药性日趋增强[14]。了解淋病奈瑟菌临床菌株的耐药谱、耐药基因型及其相互关系,不仅可指导临床合理用药,同时也有助于进一步揭示耐药表型与基因型关系并为开发新的抗菌药物提供依据。
青霉素类抗生素能与本质为细菌细胞壁肽聚糖合成相关内肽酶和羧肽酶的青霉素结合蛋白(penicilin-binding proteins,PBPs)结合,导致酶分子变构失活,细菌因细胞壁缺陷而裂解[2]。TEM基因产物β-内酰胺酶可水解青霉素类抗生素,故携带TEM基因细菌表现为对青霉素耐药[15]。环丙沙星是大环内酯类抗生素。mef基因产物为细菌排出环丙沙星等大环内酯类抗生素的外排蛋白,erm基因产物为rRNA甲基化酶,该酶可使大环内酯类抗生素作用靶位核糖体50S亚基发生改变,降低大环内酯类药物结合能力而导致耐药[16]。四环素类抗生素主要通过与细菌核糖体30S亚基结合,抑制氨基酰-tRNA与核糖体结合而阻断细菌蛋白合成[17]。tetM基因产物为核糖体保护蛋白,可对抗四环素类抗生素阻断氨基酰-tRNA与核糖体结合的作用,从而使细菌产生对四环素类抗生素的耐药性[18]。我们的药物敏感试验结果显示,淋病奈瑟菌分离株对青霉素G、环丙沙星、四环素耐药,但对头孢噻肟和头孢西丁敏感;β-内酰胺酶和ESBLs确证试验结果显示,淋病奈瑟菌分离株产β-内酰胺酶,但不产ESBLs。我们的PCR检测结果显示,淋病奈瑟菌分离株具有TEM、tetM、mef和erm基因,与该菌株耐药谱及其表型相符。
[1] Sunita G,Sarika A,Shreekant V,et al. Rice and Sanjay Ram Properdin is critical for antibody-dependent bactericidal activity againstNeisseriagonorrhoeaethat recruit C4b-binding protein[J]. J Immunol,2012,188(7): 3416-3425. DOI: 10.4049/jimmunol. 1102746
[2] Gong XD,Yue XL,Jiang N,et al. Epidemiological characteristics and trends of gonorrhea in China from 2000 to 2014[J]. Chin J Dermatol,2015,48(5): 301-306. DOI: 10.3760/cma.j.
issn.0412-4030.2015.05.002
[3] Yan J. Medical microbiology[M]. 3rd Edition,Beijing: The High Education Publication House,China,2016: 25-26,82-84. (in Chinese)
[4] Dong HL,Guo YY,Mao JF,et al. Analysis on the results of main sexually transmitted diseases tested in 6 682 male cases suspected with genitourinary infection[J]. Chin J Zoonoses,2014,30(1): 102-105. DOI: 10.3969/cjz.j.issn.1002-2694.2014.01.022
[5] David J,Farrel L. Evaluation of amplicorNeisseriagonorrhoeaePCR using cppB nested PCR and 16S rRNA PCR[J]. J Clin Microbiol,1999,37(2): 386-390. DOI: 0095-1137/99 /04.0010
[6] Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing[S]. Twenty-fourth Informational Supplement. CLSI document M100-S24. Wayne: Clinical and Laboratory Standards Institute. 2014.
[7] Laura M,Valerie C,Zaelle D,et al. FirstNeisseriagonorrhoeaegenotyping analysis in France: identification of a strain cluster with reduced susceptibility to ceftriaxone[J]. J Clin Microbiol,2009,47(11): 3540-3545. DOI: 10.1128/JCM.01236-09
[8] Joseph RD,Lalitagauri MD,Douglas JB,et al. Evolution and dissemination of extended spectrum β-lactamase-producingKlebsiellapneumoniae: Epidemiology and molecular report from the SENTRY Antimicrobial Surveillance Program (1997-2003)[J]. Diagn Microbiol Infect Dis,2005,51(1): 1-7. DOI: 10.1016/j.diagmicrobio.2004.08.001
[9] Chunxin W,Peiquan C,Dong C,et al. APseudomonasaeruginosaisolate producing the GES-5 extended-spectrum β-lactamase[J]. J Antimicrob Chemoth,2006,57(6): 1261-1262. DOI: 10.1093/jac/dkl116
[10] Li GM,Chen Qun,Chen JJ,et al. Resistance genes of multi-drug resistance strains ofNeisseriagonorrhoeae[J].Chin J Nosocomiol Vol,2005,15(8): 852-854. DOI: 10.3321/j.issn:1005-4529.2005.08.004
[11] Ros CTD,Schmitt CDS. Global epidemiology of sexually transmitted diseases[J]. Lancet,2008,10(1): 110-114. DOI: 10.1111/j.1745-7262.2008.00367
[12] Johnson MB,Criss AK. Resistance ofNeisseriagonorrhoeaeto neutrophils[J]. Front Microbiol,2011,2(1): 77-77. DOI: 10.3389/fmicb.2011.00077
[13] Yin F,Feng Z,Li X. Spatial analysis of county-based gonorrhoea incidence in mainland China from 2004 to 2009[J]. Sex Health,2012,9(3): 227-232. DOI: 10.1071/SH11052
[14] Unemo M,Nicholas RA. Emergence of multi-drug resistant,extensively drug-resistant and untreatable gonorrhea[J]. Future Microbiol,2012,7(12): 1401-1422. DOI: 10.2217/ fmb.12.117
[15] Unemo M,Shafer WM. Antimicrobial resistance inNeisseriagonorrhoeaein the 21st century: past,evolution and future[J]. Clin Microbiol Rev,2014,27(3): 587-613. DOI: 10.1128/CMR.00010-14[16] Luna VA,Cousin S,Whittington WLH,et al. Identification of the conjugative mef gene in clinicalAcinetobacterjuniiandNeisseriagonorrhoeaeisolates[J]. Antimicrob Agents CH,2000,44(9): 2503-2506. DOI: 10.1128/AAC.44.9.2503-2506.2000
[17] Flrez AB,Ammor MS,Martín PL,et al. Molecular analysis of tet(W) gene-mediated tetracycline resistance in dominant intestinal bifidobacterium species from healthy humans[J]. Appl Environ Microb,2006,72(11): 7377-7379. DOI: 10.1128/AEM.00486-06
[18] Li YW,Shi ZQ,Zhao PL,et al. The research on plasmid profiles of TEM-1 encoding gene in penicillinase-producingNeisseriagonorrhoeaeandtetMgene in high level tetracycline-resistantNeisseriagonorrhoeaein Fosha[J]. Chin J Antibiot,2014,39(3): 236-239. DOI: 10.3969/j.issn.1001-8689.2014.03.016
Isolation and identification ofNeisseriagonorrhoeaestrain from a balanoposthitis patient and drug resistance mechanism of the isolate
GE Yu-mei,HU Qing-feng,ZHU Yong-ze,ZHOU Yong-lie,LYU Huo-yang
(ZhejiangProvincialPeople’sHospital,Hangzhou310014,China)
We isolated and identified the bacterial pathogen in a pyogenic balanoposthitis patient and investigated the drug resistance and its mechanism of the isolate. Urethral secretions and balanus pustule liquids were collected for microscopic examination after Gram-staining and detection of mycoplasma usingMycoplasmaIST 2 kit. The two samples were inoculated on Columbia blood plate,N.gonorrhoeaeselective plate and chromID Candida plate for isolation. The obtained colonies were identified by VITEK 2-compact automatic bacterial detection and analysis system. Moreover,PCR was performed to detect 16S rRNA gene ofN.gonorrhoeaein the samples and colonies. KB method was applied for detecting susceptibility of five common antibiotics against the isolate. The β-lactamase and extended spectrum β-lactamase confirmatory tests were used to investigate the enzyme production of the isolate as well as drug resistance-associated tetM,TEM,mefA and ermF genes in the isolate were detected by PCR. Results showed that all the clinic samples showed negative for mycoplasma. All the isolating cultivation results of urethral secretions were negative while the balanus pustule liquids provided positive isolating cultivation in the blood and selective plates. The VITEK 2-compact system and 16S rRNA-PCR revealed that the isolated strain belongs toN.gonorrhoeae. The isolate can produce β-lactamases and resist to penicillin G,ciprofloxacin and tetracycline. The tetM,TEM,mefA and ermF genes could be found in the isolate’s genome. The patient’s balanoposthitis is caused by infection ofN.gonorrhoeae. The multidrug resistance ofNeisseriagonorrhoeaeisolate is closely associated with its carried resistant genes.
balanoposthitis;Neisseriagonorrhoeae; isolation and identification; drug resistance; resistance gene
Lyu Huo-yang,Email: geyumei1990@hotmail.com
10.3969/j.issn.1002-2694.2017.05.009
浙江省医药卫生科技计划项目(2017KY004)资助
吕火烊,geyumei1990@hotmail.com
浙江省人民医院检验中心,杭州 310014
R378.1
A
1002-2694(2017)05-0432-04
2016-11-14 编辑:刘岱伟
Supported by the Medical Scientific Research Foundation of Zhejiang Province (No. 2017KY004)
猜你喜欢
杂志排行
中国人兽共患病学报的其它文章
- 中国西北四省(区)结核分枝杆菌分离株一线药物耐药状况及其影响因素分析
- 美国《Emerging Infectious Diseases》2017年第3期有关人兽共患病论文摘译
- MicroRNA通用探针法的建立及布鲁氏菌病患者血浆microRNA-146a的检测
- miR-16在金黄色葡萄球菌脓毒症中的表达及意义探讨
- Identification of non-tuberculosis mycobacteria speciesof clinical isolates from patients clinically diagnosed with tuberculosis in Fujian Province,China
- 四对结核分枝杆菌毒素-抗毒素系统基因功能的初步研究
