苯并(a)芘致癌的表观遗传学作用机制研究进展
2017-06-05周超文胡建安
周超文,胡建安
(中南大学公共卫生学院劳动卫生与环境卫生学系,湖南长沙 410078)
·前沿论坛·
苯并(a)芘致癌的表观遗传学作用机制研究进展
周超文,胡建安
(中南大学公共卫生学院劳动卫生与环境卫生学系,湖南长沙 410078)
胡建安,博士,中南大学湘雅公共卫生学院二级教授,博士生导师。长期从事职业卫生与职业医学及毒理学教学和科研工作。1996-1999年在美国南佛罗里达大学从事毒理学研究工作。主持国家自然科学基金项目4项及教育部博士点基金等省部级项目13项。以第一或通讯作者发表学术论文100余篇。参编国家规划教材《职业卫生与职业医学》和研究生教材《分子毒理学》等8本。培养博士和硕士研究生51名。任中国毒理学会理事和湖南省劳动卫生学会副主任委员等职。

近年研究发现,环境致癌物苯并(a)芘的致癌机制除遗传毒性外,可引起全基因组甲基化减低、抑癌基因甲基化升高及原癌基因甲基化降低,亦可引起微RNA表达升高或降低、长链非编码RNA表达升高、组蛋白磷酸化水平升高、组蛋白甲基化和乙酰化失衡等表观遗传学变化。这些改变既可引起基因表达异常、染色体结构异常和不稳定性增加直接致癌,又可以引起相应的遗传毒性改变,如基因突变、基因损伤修复异常、细胞凋亡和细胞周期阻滞等协同致癌,被认为是苯并(a)芘致癌可能的表观遗传学机制。上述研究为进一步揭示苯并(a)芘引起的环境相关性疾病和职业病的发生机制及防治策略提供了科学依据。
苯并(a)芘;表观遗传学;DNA甲基化;微RNA;长链非编码RNA;组蛋白修饰
苯并(a)芘〔benzo[a]pyrene,B(a)P〕是最常见的多环芳烃类环境化学污染物,主要来源于煤和石油等燃料燃烧的烟气、烟草燃烧的烟雾、油炸食品以及汽车尾气中。B(a)P的职业暴露主要发生在焦炭的生产、煤的气化和液化、沥青的铺陈、铝的生产和烟囱清扫等过程中。国际癌症研究机构2012年已经将B(a)P归为第一类致癌物,即对人类致癌,可引起肺癌、肝癌、胃癌和皮肤癌等多种癌症。B(a)P本身并不具有致癌性,只有在机体内经过氧化代谢活化生成7,8-二羟基-9,10-环氧B(a)P〔7,8-dihydroxybenzo[a]pyrene-9,10-oxide,BPDE〕才能致癌[1]。活性代谢物BPDE可以与DNA上的鸟嘌呤形成BPDE-DNA加合物,通过引起氧化应激、基因突变和染色体畸变等遗传改变来发挥其致癌作用。
然而,最近越来越多的证据表明,表观遗传学调控在B(a)P的致癌过程中也具有非常关键的作用,B(a)P致癌的表观遗传学机制已经成为当前毒理学研究的热点。表观遗传学是在基因的核苷酸序列不发生改变的情况下,基因表达发生的可遗传的变化,包括DNA甲基化、非编码RNA、组蛋白修饰、核小体的定位与表达等多种改变{2]。本文主要对B(a)P所致几个方面的表观遗传学改变进行总结,为阐明其致癌的表观遗传学机制提供依据。
1 苯并(a)芘与DNA甲基化
DNA甲基化是在DNA甲基转移酶的催化下,甲基从供体S-腺苷甲硫氨酸甲基添加到DNA胞嘧啶残基上的过程。DNA甲基化主要发生在基因的CpG双核苷酸位点,能够抑制基因的表达。B(a)P对DNA甲基化的作用包括对全基因甲基化的作用以及对特定基因甲基化的作用。
1.1 苯并(a)芘与全基因甲基化
用B(a)P<40 μmol·L-1处理人支气管上皮细胞,发现随着浓度的增高,DNA甲基化程度呈先略增高后降低的趋势,并且能被DNA甲基转移酶抑制剂5-氮杂脱氧胞苷所抑制[3-5]。对小鼠[6]和石斑鱼幼体[7-8]的研究也显示,随着B(a)P染毒浓度的增加,全基因组甲基化的水平逐渐减低。多个人群试验表明,接触高浓度B(a)P的人群全基因组甲基化程度低于接触低浓度B(a)P的人群[9-10]。相反,B(a)P处理后的小鼠胚胎成纤维细胞全基因组DNA甲基化有明显的增高[11]。
上述多数研究表明,B(a)P染毒能降低全基因组甲基化程度,其原因是为B(a)P引起DNA加合物生成增多,从而抑制DNA甲基转移酶的活性,而细胞癌变的重要特征之一也是全基因组DNA甲基化程度降低,可以使染色体结构异常、不稳定性增加,进而出现染色体基因表达异常。少数研究发现,B(a)P染毒后全基因组甲基化程度不变甚至增加,有可能是B(a)P染毒浓度过低时出现“毒物兴奋效应”导致的,也可能因实验设计或测定方法不同引起。
1.2 苯并(a)芘与特定基因甲基化改变
特定基因甲基化的改变对癌症的发生发展有两方面的作用,即对抑癌基因,高甲基化状态会抑制其表达,促进肿瘤事件的发生;对原癌基因,低甲基化状态会解除对其的抑制,从而促进肿瘤事件的发生。研究发现,B(a)P染毒细胞后,能够诱导多种抑癌基因〔成对盒5β基因(PAX5β)、胰岛素样生长因子结合蛋白3基因(IGFBP3)、上皮性钙黏附蛋白基因、视黄酸受体β2基因(RAR-β2)和p53基因〕转变为甲基化状态;多种原癌基因(K-ras和逆转座子LINE1基因)启动子区域呈现低甲基化状态,并且基因突变的频率也随之增高[12-16]。Tao等[17]发现,B(a)P可以使雌性小鼠的原癌基因胰岛素样生长因子2基因(IGF-2)和c-myc的甲基化程度明显降低,且表达明显升高。Corrales等[8]却发现,石斑鱼受精卵染毒后2个癌基因h-ras和msh3及2个抑癌基因apc和brca1的甲基化程度明显增高;另外,2个抑癌基因p53和dazl的甲基化程度明显降低。蒋成兰等[18]通过分析云南宣威14例长期接触B(a)P的肺癌患者癌组织及癌旁组织的DNA甲基化谱发现,肺癌组织中有23 368个DNA甲基化位点显著升高。张开力等[19]在接触B(a)P胃癌患者的胃癌组织中,发现抑癌基因P16甲基化水平升高,并且随着胃癌的进展,甲基化基因种类明显增多,甲基化程度也逐渐增强。这一规律也发现于接触B(a)P的肝癌患者血细胞GSTP基因(抑癌基因)、食管癌患者RAR-β2基因(抑癌基因)、非小细胞肺癌患者抑癌基因(P16和DAPK)和焦炉工人外周血单核细胞抑癌基因(P14,P15和P16)中[20-24]。接触B(a)P的焦炉工人血液细胞中P53基因(抑癌基因)、消防员血液细胞中DUSP22基因(原癌基因)和肺癌患者肺组织中PP2A基因(抑癌基因)的甲基化水平低于正常对照组[10,25-26]。
对于特定基因的DNA甲基化,上述研究也大部分呈现出与B(a)P致癌相一致的改变,即抑癌基因甲基化程度升高,原癌基因甲基化程度降低,同时还伴随着DNA甲基转移酶相应的升高或降低。但是也有相当部分研究发现了与之相反的结果。还有研究发现,DNA甲基化改变方向与DNA甲基转移酶改变方向不一致,这些都值得进行更深入的研究。
1.3 苯并(a)芘与DNA甲基转移酶的改变
DNA甲基转移酶是催化DNA发生甲基化的酶类,包括维持性甲基化酶(DNMT1)、与DNA特定位点结合的甲基化酶(DNMT2)和从头甲基化酶(DNMT3a和DNMT3b)。研究表明,经B(a)P恶性转化的小鼠胚胎成纤维细胞和HBE细胞,DNMT1和DNMT3b的mRNA和蛋白表达均明显增高,各种DNA甲基转移酶的活性都有明显的增强[3,12,27-28]。而有研究却发现,B(a)P染毒的小鼠胚胎成纤维细胞和HeLa细胞中DNMT1和DNMT3a蛋白的表达显著降低[11,16]。还有研究发现,B(a)P终致癌物虽然不能显著的改变食管癌细胞中DNMT3a的表达水平,却能诱导DNMT3a朝向目标基因启动子区域聚集,同时显著降低DNMT3b的表达水平[22]。Tommasi等[29]发现,B(a)P染毒的雄性小鼠DNMT3a和DNMT3b表达均下降。相反,Tian等[21]发现,B(a)P暴露能够诱导肝癌患者体内DNMT1,DNMT3a和DNMT3b的表达。Leng等[30]发现,在吸烟人群中DNMT1和DNMT3a突变序列显著增加。
2 苯并(a)芘与非编码RNA
非编码RNA是指在基因转录过程中出现的不编码蛋白质的RNA。其中与表观遗传学有关的主要有小干扰RNA(small interference RNA,siRNA)、微RNA(microRNA,miRNA)以及长链非编码RNA(long noncoding RNA,lncRNA)。目前,非编码RNA主要的研究方法是先用生物芯片技术进行筛选,再用RNA印迹法和实时荧光PCR等方法进行定量验证。
2.1 苯并(a)芘与微RNA
miRNA是指一系列内源性小分子单链RNA,长约21~23个核苷酸。miRNA的功能是通过降解mRNA或者抑制mRNA翻译进行转录后调控:当miRNA与靶基因mRNA非编码区(3′-UTR)完全互补配对时,靶基因mRNA出现降解;当miRNA与靶基因mRNA非编码区不完全互补配对时,靶基因mRNA的翻译受到抑制[31]。一些研究通过生物芯片对B(a)P染毒细胞的miRNA进行筛选,选出数十种表达量有统计学差异的miRNA[32-36];另有研究针对这些筛选出来的miRNA进行实验验证,结果与生物芯片筛选结果基本一致[37-42]。还有研究进一步分析了miRNA的改变所引起的基因表达或者翻译的改变,甚至对于细胞损伤、细胞增殖和凋亡以及细胞周期的影响[43-49]。动物实验研究发现,B(a)P染毒野生型秀丽线虫后2种miRNA表达降低,6种miRNA表达增加[50]。Halappanavar等[51]用B(a)P染毒雄性小鼠,发现肺组织中有7种miRNA表达增加,6种miRNA表达降低,这些miRNA的功能主要是调控血管生成、细胞凋亡和细胞周期等。Deng等[52]通过生物芯片检测焦炉工人的血液,发现有68种miRNA存在5倍以上的表达下调。同样,Chen等[26]对比正常组织发现,接触B(a)P肺癌患者的肺癌组织中miR-34b的表达受到了明显抑制。Maccani等[53]也发现,吸烟孕妇的胎盘中miR-16,miR-21和miR-146a的表达明显降低。
上述研究中,无论是细胞实验、动物实验还是人群试验,都发现有部分的miRNA表达上调,部分的miRNA表达下调。作为一种表观遗传学调控机制,对于癌症的发生发展,miRNA调控同样具有两个方面:①B(a)P染毒后,表达上调的miRNA能够下调抑制癌症发生发展相关基因的表达;②表达下调的miRNA能够解除对促进癌症发生发展相关基因表达的抑制。这样的调控方式也在上述的部分研究中得到体现。但是,也有部分miRNA表达改变并不与癌症相关,可能是由于miRNA的功能具有多样性,调控的靶mRNA同样也具有多样性。
2.2 苯并(a)芘与长链非编码RNA
lncRNA的长度>200个核苷酸,其可以通过修饰染色质、激活或者抑制DNA转录、转录后调控mRNA表达以及作为miRNA的诱导分子来干扰基因的表达,进而在肿瘤发生发展中发挥重要的调控作用。研究显示,在B(a)P染毒的HBE细胞中,发现7种IncRNA的表达水平有明显差异,其中IncRNA AF11808的增加最为显著,其可以抑制肺癌细胞生长、迁移和侵袭[54]。同样,Hu等[55]在HBE细胞中发现,IncRNA-LOC728228的表达也明显增加,并且可以通过上调细胞周期蛋白D1的表达来促进细胞增殖。Gao等[56]在BESAS-2B细胞中也发现,IncRNA-DQ785227的表达随着B(a)P染毒时间的延长而逐渐增加,其在肺癌细胞中也有显著增多。Recio等[57]在B(a)P染毒的雌性小鼠也发现,IncRNA-p21表达明显增高。
对于lncRNA,上述所有的研究都显示出一致的趋势,即B(a)P染毒后,所研究的lncRNA表达均增加,进而促进癌症的发生发展。但是,可以明显发现,上述研究中的lncRNA仅有十余种,仅仅是总lncRNA中的极少部分。所以,针对lncRNA,还需要更多更深入的研究。
3 苯并(a)芘与组蛋白修饰
组蛋白是细胞内染色质的基本结构蛋白,被不同基团修饰的组蛋白可以通过改变基因所处的环境、染色质的结构或凝集状态以及作为信号影响下游蛋白表达的方式来调控基因的表达。组蛋白修饰有多种形式,包括甲基化、乙酰化、磷酸化、泛素化、腺苷酸化、ADP-核糖基化、生物素化和脯氨酸异构化等。
3.1 苯并(a)芘与组蛋白甲基化
组蛋白甲基化由组蛋白转移酶和组蛋白去甲基化酶催化,主要发生在H3和H4的赖氨酸和精氨酸残基上。Khanal等[58]用B(a)P染毒人乳腺癌MCF-7细胞后发现,活性组蛋白标志物二甲基化H3K4的表达明显降低。Lee等[59]在A549细胞也观察到类似结果。Ovensen等[60]也在HepG2细胞中发现三甲基化H3K4的水平明显降低。相反,Teneng等[16]在HeLa细胞中发现,L1基因启动子区域三甲基化H3K4的水平明显升高。其他研究也发现,B(a)P染毒后三甲基化H3K9和三甲基化H3K27有升高[19]。
3.2 苯并(a)芘与组蛋白磷酸化
组蛋白磷酸化发生在组蛋白N端氨基酸残基上,其可以通过磷酸基团携带的负电荷与组蛋白上的正电荷结合降低组蛋白与DNA之间的亲和力,以及通过与特异的蛋白质复合物结合来调控基因表达[61]。B(a)P染毒不同种类的细胞后均显示,细胞中磷酸化H2AX的含量随着B(a)P染毒时间的延长而逐渐增加[60,62-73],并且这种上调作用可以被氯化钴(CoCl2)增强[74],γ-生育酚甲基转移酶所抑制[75]。相反,在B(a)P染毒的人乳腺癌MCF-10A细胞研究发现,磷酸化H2AX的水平有降低的趋势[76]。另外,在细胞B(a)P染毒细胞过程中,随时间的变化观察到磷酸化H2AX水平先短暂的上调,然后缓慢降低,最后与对照组基本持平[27,77-78]。
3.3 苯并(a)芘与组蛋白乙酰化
组蛋白乙酰化是一个动态的可逆过程,乙酰化和去乙酰化的动态失衡可以影响染色质的结构和基因表达,进而影响肿瘤的发生。研究显示,B(a)P染毒HepG2细胞后,CYP1a1基因启动子区域乙酰化H3K9,H4K16和H3K14的水平均明显增加[16,78]。也有研究发现,在接触B(a)P的人乳腺癌MCF-7细胞中,乙酰化H4A的水平降低[58]。
组蛋白修饰的形式多种多样,修饰的与未修饰的组蛋白之间存在一定的平衡,并且各种修饰形式之间相互影响。所以很难通过一种组蛋白修饰的改变来衡量其对基因表达的调控效果。而且大部分的研究集中于上述的3种组蛋白修饰的改变,极少关注其他类型的组蛋白修饰,对组蛋白修饰缺乏一个完整的概括。
4 苯并(a)芘致癌的可能表观遗传学机制
根据目前的有关研究,B(a)P致癌可能的表观遗传学机制包括以下几个方面:①全基因甲基化降低,染色体结构异常、不稳定增加,进而出现染色体基因表达异常,促进癌症发生发展;②癌基因甲基化降低,癌基因激活,抑癌基因甲基化升高,解除抑癌基因的抑癌作用,通过增加基因突变频率、细胞周期阻滞、抑制癌细胞凋亡以及抑制DNA损伤修复促进癌症的发生发展;③部分miRNA表达升高,部分miRNA表达降低,通过细胞周期阻滞、抑制癌细胞凋亡以及抑制DNA损伤修复促进癌症的发生发展;④长链非编码RNA表达升高,通过促进DNA复制和修饰染色质促进癌症的发生发展;⑤组蛋白甲基化失衡和组蛋白乙酰化失衡,通过抑制DNA转录和生成异染色质促进癌症的发生发展;⑥组蛋白磷酸化升高,通过DNA双链断裂和增加DNA突变频率促进癌症的发生发展。另外,上述各种机制之间也存在相互之间的关联,共同构成一个表观遗传学调控体系(见图1)。
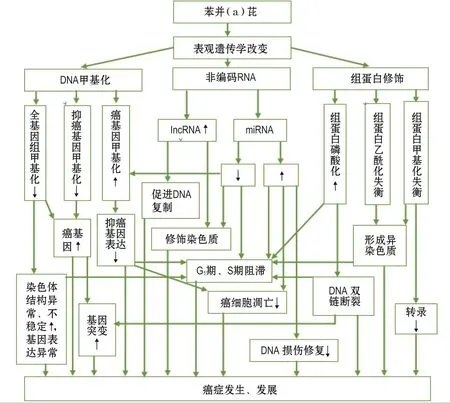
图1 苯并(a)芘致癌的可能表观遗传学机制.lncRNA:长链非编码RNA;miRNA:微RNA;↑:升高;↓:降低.
5 结语
综上所述,B(a)P可以通过表观遗传学机制引起某些基因的表达失调,进而诱导肿瘤的发生,促进肿瘤的发展(见表1)。虽然上述证据表明,B(a)P可以改变DNA甲基化水平,但是各研究结果并不完全一致,而且对于特定基因甲基化的改变大多还未见重复性实验进行验证。B(a)P暴露引起非编码RNA的改变,大部分研究还处于基因芯片筛选的阶段,具体的机制研究较少,而且缺乏一个谱系性质的描述,值得深入研究。B(a)P引起组蛋白修饰的改变,已经基本确定了组蛋白改变的区域、修饰的形式以及改变的方向(上调或者下调),但是要确定组蛋白修饰改变以后对基因表达的影响,还需要更多以及更加深入的相关研究。而且现有的研究大多数着眼于DNA甲基化、非编码RNA和组蛋白修饰,还有其他许多表观遗传学机制如核小体的定位、染色质重塑等并未涉及,因而对表观遗传学机制缺乏一个整体的评价[79]。总而言之,B(a)P的表观遗传学研究已经受到足够的重视,并且获得了相当多有价值的研究成果,这有助于丰富对B(a)P致癌机制的认识,对于进一步揭示B(a)P引起的环境相关性疾病和职业病的发生机制及相关疾病的防治都具有重要意义。

表1 B(a)P致癌的表观遗传学改变

续表1
[1] Xue W,Warshawsky D.Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage:a review[J].Toxicol Appl Pharmacol,2005,206(1):73-93.
[2] Rakyan V,Whitelaw E.Transgenerational epigen⁃etic inheritance[J].Curr Biol,2003,13(1):R6.
[3] Tao GH,Zhuang ZX,Yang LQ,Gong CM,Liu QC,Liu JJ,et al.Genomic DNA methylation in benzo(a)pyrene-induced cell malignant transformation[J].J Environ Occup Med(环境与职业医学),2014,31(5):347-351.
[4] Xi B.Joint toxicity and epigenetic alterations in 16HBE cells induced by Cr(VI)and B[a]P(Cr(VI)和B[a]P对16HBE细胞的联合毒性效应及表观遗传改变的研究)[D].Changsha:Central South Univer⁃sity(中南大学),2012.
[5] Huang H,Hu G,Cai J,Xia B,Liu J,Li X,et al. Role of poly(ADP-ribose)glycohydrolase silenc⁃ing in DNA hypomethylation induced by benzo(a)pyrene[J].BiochemBiophysResCommun,2014,452(3):708-714.
[6] Zhao L,Zhang S,An X,Tan W,Pang D,Ouyang H.Toxicological effects of benzo[a]pyrene on DNA methylation of whole genome in ICR mice[J].Cell Mol Biol(Noisy-le-grand),2015,61(5):115-119.
[7] Fang X,Thornton C,Scheffler BE,Willett KL. Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development[J].Environ Toxicol Pharmacol,2013,36(1):40-50.
[8]CorralesJ,FangX,ThorntonC,MeiW,Barbazuk WB,Duke M,et al.Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo[a]pyrene exposure[J].Comp Biochem Physiol C Toxicol Pharmacol,2014,163:37-46.
[9]Herbstman JB,Tang D,Zhu D,Qu L,Sjödin A,Li Z,et al.Prenatal exposure to polycyclic aromatic hydrocarbons,benzo[a]pyrene-DNA adducts,and genomic DNA methylation in cord blood[J].Environ Health Perspect,2012,120(5):733-738.
[10]Pavanello S,Pesatori AC,Dioni L,Hoxha M,Bollati V,Siwinska E,et al.Shorter telomere length in peripheral blood lymphocytes of workers exposed to polycyclic aromatic hydrocarbons[J].Carcinogenesis,2010,31(2):216-221.
[11]Yauk CL,Polyzos A,Rowan-Carroll A,Kortubash I,Williams A,Kovalchuk O.Tandem repeat muta⁃tion,global DNA methylation,and regulation of DNA methyltransferases in cultured mouse embry⁃onic fibroblast cells chronically exposed to chemi⁃cals with different modes of action[J].Environ Mol Mutagen,2008,49(1):26-35.
[12]Damiani LA,Yingling CM,Leng S,Romo PE,Nakamura J,Belinsky SA.Carcinogen-induced gene promoter hypermethylation is mediated by DNMT1 and causal for transformation of immortalized bron⁃chial epithelial cells[J].Cancer Res,2008,68(21):9005-9014.
[13]Song S,Lippman SM,Zou Y,Ye X,Ajani JA,Xu XC.Induction of cyclooxygenase-2 by benzo[a]pyrene diol epoxide through inhibition of retinoic acid receptor-beta 2 expression[J].Oncogene,2005,24(56):8268-8276.
[14]Glick J,Xiong W,Lin Y,Noronha AM,Wilds CJ,Vouros P.The influence of cytosine methylation on the chemoselectivity of benzo[a]pyrene diol epoxideoligonucleotide adducts determined using nanoLC/ MS/MS[J].J Mass Spectrom,2009,44(8):1241-1248.
[15]Hu W,Feng Z,Tang MS.Preferential carcinogen-DNA adduct formation at codons 12 and 14 in the human k-ras gene and their possible mechanisms[J].Biochemistry,2003,42(33):10012-10023.
[16]Teneng I,Montoya-Durango DE,Quertermous JL,Lacy ME, Ramos KS.Reactivation ofL1 retrotransposon by benzo(a)pyrene involves complex genetic and epigenetic regulation[J].Epigenetics,2011,6(3):355-367.
[17]Tao L,Li Y,Wang W,Kramer PM,Gunning WT,Lubet RA,et al.Effect of budesonide on the meth⁃ylation and mRNA expression of the insulin-like growth factor 2 and c-myc genes in mouse lung tumors[J].Mol Carcinog,2002,35(2):93-102.
[18]Jiang CL,Zhang YD,Chang H,Duan HX,He SW,Huang YC,et al.The effect of environmental carcinogens PAHs on genome-wide DNA methyla⁃tion of lung cancers(环境致癌剂多环芳烃对肺癌全基因组DNA甲基化的影响)[C]//The 16th Annual Meeting of China Association for Science and Technology.Kunming:2014:1-6.
[19]Zhang KL.A pilot study of molecular basis of gas⁃trocarcinogenesis in Dalian,a gastric cancer at risk region〔高发区(大连)胃癌发病分子基础的探讨〕[D].Dalian:Dalian Medical University(大连医科大学),2008.
[20]Jin Y,Xu P,Liu X,Zhang C,Tan C,Chen C,et al. Cigarette smoking,BPDE-DNA adducts,and aber⁃rant promoter methylations of tumor suppressor genes(TSGs)in NSCLC from Chinese population[J].Cancer Invest,2016,34(4):173-180.
[21]Tian M,Zhao B,Zhang J,Martin FL,Huang Q,Liu L,et al.Association of environmental benzo[a]pyrene exposure and DNA methylation alterations in hepatocellular carcinoma:a Chinese case-control study[J].Sci Total Environ,2016,541:1243-1252.
[22]Ye F,Xu XC.Benzo[a]pyrene diol epoxide suppresses retinoic acid receptor-beta2 expres⁃sion by recruiting DNA(cytosine-5-)-methyltrans⁃ferase 3A[J].Mol Cancer,2010,9:93.
[23] Zhang H,Li X,Ge L,Yang J,Sun J,Niu Q. Methylation of CpG island of p14(ARK),p15(INK4b)and p16(INK4a)genes in coke oven workers[J].Hum Exp Toxicol,2015,34(2):191-197.
[24]Yang P,Ma J,Zhang B,Duan H,He Z,Zeng J,et al.CpG site-specific hypermethylation of p16 INK4α in peripheral blood lymphocytes of PAH-ex⁃posed workers[J].Cancer Epidemiol Biomarkers Prev,2012,21(1):182-190.
[25]Ouyang B,Baxter CS,Lam HM,Yeramaneni S,Levin L,Haynes E,et al.Hypomethylation of dual specificityphosphatase 22 promotercorrelates with duration of service in firefighters and is induc⁃ible by low-dose benzo[a]pyrene[J].J Occup Environ Med,2012,54(7):774-780.
[26]Chen LP,Lai YD,Li DC,Zhu XN,Yang P,Li WX,et al.α4 is highly expressed in carcinogen-trans⁃formed human cells and primary human cancers[J].Oncogene,2011,30(26):2943-2953.
[27]Schnekenburger M,Peng L,Puga A.HDAC1 bound to the CYP1A1 promoter blocks histone acetylation associated with Ah receptor-mediated trans-activation[J].Biochim Biophys Acta,2007,1769(9-10):569-578.
[28]Deng WW,Yang M,Zhang ZZ,Wu M.Effects of cell malignant transformation induced by benzo[a]pyrene on DNA methylation transferases[J].J Hyg Res(卫生研究),2013,42(6):915-9,924.
[29]Tommasi S,Kim SI,Zhong X,Wu X,Pfeifer GP,Besaratinia A.Investigating the epigenetic effects of a prototype smoke-derived carcinogen in human cells[J].PLoS One,2010,5(5):e10594.
[30]Leng S,Stidley CA,Bernauer AM,Picchi MA,Sheng X,Frasco MA,et al.Haplotypes of DNMT1 and DNMT3B are associated with mutagen sensi⁃tivity induced by benzo[a]pyrene diol epoxide among smokers[J].Carcinogenesis,2008,29(7):1380-1385.
[31]Vislovukh A,Vargas TR,Polesskaya A,Groisman I. Role of 3′-untranslated region translational control in cancer development,diagnostics and treatment[J].World J Biol Chem,2014,5(1):40-57.
[32] Shen YL,Jiang YG,Greenlee AR,Zhou LL,Liu LH.MicroRNA expression profiles and miR-10a target in anti-benzo[a]pyrene-7,8-diol-9,10-epoxidetransformed human 16HBE cells[J].Biomed Envi⁃ron Sci,2009,22(1):14-21.
[33]Duan H,Jiang Y,Zhang H,Wu Y.MiR-320 and miR-494 affect cell cycles of primary murine bron⁃chial epithelial cells exposed to benzo[a]pyrene[J].Toxicol In Vitro,2010,24(3):928-935.
[34]Gulyaeva LF,Chanyshev MD,Kolmykov SK,Ushakov DS,Nechkin SS.Effect of xenobiotics on microRNA expression in rat liver[J].Biomed Khim,2016,62(2):154-159.
[35]Caiment F,Gaj S,Claessen S,Kleinjans J.Highthroughput data integration of RNA-miRNA-circRNA reveals novel insights into mechanisms of benzo[a]pyrene-induced carcinogenicity[J].Nucleic Acids Res,2015,43(5):2525-2534.
[36]Marrone AK,Tryndyak V,Beland FA,Pogribny IP. MicroRNA responses to the genotoxic carcinogens aflatoxin B1 and benzo[a]pyrene in human hepaRG cells[J].Toxicol Sci,2016,149(2):496-502.
[37]Liu LH.MiR-494 and miR-22 regulate PTEN expres⁃sion in transformed cells induced by anti-BPDE(反式二羟环氧苯并芘诱导恶变细胞中miR-494和miR-22对PTEN基因调控及其功能研究)[D].Guangzhou:Guangzhou Medical University(广州医学院),2009.
[38]Brevik A,Lindeman B,Rusnakova V,Olsen AK,Brunborg G,Duale N.Paternal benzo[a]pyrene exposure affects gene expression in the early developing mouse embryo[J].Toxicol Sci,2012,129(1):157-165.
[39]Qi GZ,Liu CX,Zhang ZB,Zhou M,Wei LD,Pang YQ.The function of microRNAs in benzo(a)pyrene-induced human airway cell transformation[J].J Youjiang Med Univ Natl(右江民族医学院学报),2013,35(6):754-757.
[40]Li D,Wang Q,Liu WC,Duan H,Zeng X,Zhang B,et al.Aberrant expression of miR-638 contributes to benzo(a)pyrene-induced human celltransforma⁃tion[J].Toxicol Sci,2012,125(2):382-391.
[41]Barkley LR,Santocanale C.MicroRNA-29a regu⁃lates the benzo[a]pyrene dihydrodiol epoxide-induced DNA damage response through Cdc7 kinase in lung cancer cells[J].Oncogenesis,2013,2:e57.
[42]Wu Y.The functions of miR-106a and miR-17-5p in malignant transformation of cells induced by anti-BPDE(miR-106a和miR-17-5p在反式二羟环氧苯并芘诱导细胞恶变中的作用)[D].Guangzhou:Guangzhou Medical university(广州医学院),2010.
[43]Han Z,Zhang Y,Xu Y,Ji J,Xu W,Zhao Y,et al. Cell cycle changes mediated by the p53/miR-34c axis are involved in the malignant transformation of human bronchial epithelial cells by benzo[a]pyrene[J].Toxicol Lett,2014,225(2):275-284.
[44]Choi YM,An S,Lee EM,Kim K,Choi SJ,Kim JS,et al.CYP1A1 is a target of miR-892a-mediated post-transcriptional repression[J].Int J Oncol,2012,41(1):331-336.
[45]Zhao Y,Liu H,Li Y,Wu J,Greenlee AR,Yang C,et al.The role of miR-506 in transformed 16HBE cells induced by anti-benzo[a]pyrene-trans-7,8-dihydrodiol-9,10-epoxide[J].Toxicol Lett,2011,205(3):320-326.
[46]Niziolek-Kierecka M,Dreij K,Lundstedt S,Stenius U. γH2AX,pChk1,and Wip1 as potential markers of persistent DNA damage derived from dibenzo[a,l]pyrene and PAH-containing extracts from contami⁃nated soils[J].Chem Res Toxicol,2012,25(4):862-872.
[47]Rieswijk L,Brauers KJ,Coonen ML,van Breda SG,Jennen DG,Kleinjans JC.Evaluating micro RNA profiles reveals discriminative responses following geno⁃toxic or non-genotoxic carcinogen exposure in pri⁃mary mouse hepatocytes[J].Mutagenesis,2015,30(6):771-784.
[48]Hecht E,Zago M,Sarill M,Rico de Souza A,Gomez A,Matthews J,et al.Aryl hydrocarbon receptor-dependent regulation of miR-196a expres⁃sion controls lung fibroblast apoptosis but not prolif⁃eration[J].Toxicol Appl Pharmacol,2014,280(3):511-525.
[49]Malik AI,Williams A,Lemieux CL,White PA,Yauk CL.Hepatic mRNA,microRNA,and miR-34atarget responses in mice after 28 days exposure to doses of benzo(a)pyrene that elicit DNA damage and mutation[J].Environ Mol Mutagen,2012,53(1):10-21.
[50]Wu H,Huang C,Taki FA,Zhang Y,Dobbins DL,Li L,et al.Benzo-α-pyrene induced oxidative stress inCaenorhabditis elegansand the potential involvementsofmicroRNA[J].Chemosphere,2015,139:496-503.
[51]Halappanavar S,Wu D,Williams A,Kuo B,Godschalk RW,Van Schooten FJ,et al.Pulmonary gene and microRNA expression changes in mice exposed to benzo(a)pyrene by oral gavage[J].Toxicology,2011,285(3):133-141.
[52]Deng Q,Huang S,Zhang X,Zhang W,Feng J,Wang T,et al.Plasma microRNA expression and micronuclei frequency in workers exposed to poly⁃cyclic aromatic hydrocarbons[J].Environ Health Perspect,2014,122(7):719-725.
[53]Maccani MA,Avissar-Whiting M,Banister CE,McGonnigal B,Padbury JF,Marsit CJ.Maternal cigarette smoking during pregnancy is associatedwith downregulation of miR-16,miR-21,and miR-146a in the placenta[J].Epigenetics,2010,5(7):583-589.
[54]Yang Q,Zhang S,Liu H,Wu J,Xu E,Peng B,et al.Oncogenic role of long noncoding RNA AF118081 in anti-benzo[a]pyrene-trans-7,8-dihy⁃drodiol-9,10-epoxide-transformed 16HBE cells[J].Toxicol Lett,2014,229(3):430-439.
[55]Hu G,Yang T,Zheng J,Dai J,Nan A,Lai Y,et al. Functionalrole and mechanism oflncRNA LOC728228 in malignant 16HBE cells transformed by anti-benzopyrene-trans-7,8-dihydrodiol-9,10-epoxide[J].Mol Carcinog,2015,54(Suppl 1):E192-E204.
[56]Gao L,Mai A,Li X,Lai Y,Zheng J,Yang Q,et al. ncRNA-DQ786227-mediated cell malignant trans⁃formation induced by benzo(a)pyrene[J].Toxicol Lett,2013,223(2):205-210.
[57]Recio L,Phillips SL,Maynor T,Waters M,Jackson AF,Yauk CL.Differential expression of long noncoding RNAs in the livers of female B6C3F1 mice exposed to the carcinogen furan[J].Toxicol Sci,2013,135(2):369-379.
[58]Khanal T,Kim D,Johnson A,Choubey D,Kim K. Deregulation of NR2E3,an orphan nuclear recep⁃tor,by benzo(a)pyrene-induced oxidative stressis associated with histone modification status change of the estrogen receptor gene promoter[J].Toxicol Lett,2015,237(3):228-236.
[59]Lee E,Jin D,Lee BB,Kim Y,Han J,Shim YM,et al.Negative effect of cyclin D1 overexpression on recurrence-free survival in stageⅡ-ⅢA lung adeno⁃carcinoma and its expression modulation by vorino⁃statin vitro[J].BMC Cancer,2015,15:982.
[60]Ovesen JL,Schnekenburger M,Puga A.Hydrocarbon receptor ligands of widely different toxic equivalency factors induce similar histone marks in target gene chromatin[J].Toxicol Sci,2011,121(1):123-131.
[61]Zhao SL,Fang JY.Advances in research on histone phosphorylation[J].Chin J Cell Biol(细胞生物学杂志)2009,31(2):178-182.
[62]Rossner P Jr,Rossnerova A,Beskid O,Tabashidze N,Libalova H,Uhlirova K,et al.Nonhomologous DNA end joining and chromosome aberrations in human embryonic lung fibroblasts treated with envi⁃ronmental pollutants[J].Mutat Res,2014,763-764:28-38.
[63]Verma N,Pink M,Rettenmeier AW,Schmitz-Spanke S.Benzo[a]pyrene-mediated toxicity in primary pig bladder epithelial cells:a proteomic approach[J].J Proteomics,2013,85:53-64.
[64] JaminEL,RiuA,DoukiT,DebrauwerL,Cravedi JP,Zalko D,et al.Combined genotoxic effects of a polycyclic aromatic hydrocarbon(B(a)P)and an heterocyclic amine(PhIP)in relation to colorectal carcinogenesis[J].PLoS One,2013,8(3):e58591.
[65] Yan C,Lu J,Zhang G,Gan T,Zeng Q,Shao Z,et al.Benzo[a]pyrene induces complex H2AX phosphorylation patterns by multiple kinases including ATM,ATR,and DNA-PK[J].Toxicol In Vitro,2011,25(1):91-99.
[66] Smart DJ,Ahmedi KP,Harvey JS,Lynch AM. Genotoxicity screening via the γH2AX by flow assay[J].Mutat Res,2011,715(1-2):25-31.
[67] Tang SC,Sheu GT,Wong RH,Huang CY,Weng MW,Lee LW,et al.Expression of glutathione S-transferase M2 in stageⅠ/Ⅱnon-small cell lung cancer and alleviation of DNA damage exposure to benzo[a]pyrene[J].Toxicol Lett,2010,192(3):316-323.
[68]Ohnuki G,Toyooka T,Ibuki Y.UVB In solar-simu⁃lated light causes formation of BaP-photoproducts capable ofgenerating phosphorylated histone H2AX[J].Mutat Res,2010,702(1):70-77.
[69] MrozRM,SchinsRP,LiH,JimenezLA,Drost EM,Holownia A,et al.Nanoparticle-driven DNA damage mimics irradiation-related carcino⁃genesis pathways[J].Eur Respir J,2008,31(2):241-251.
[70] Zhou C,Li Z,Diao H,Yu Y,Zhu W,Dai Y,et al. DNA damage evaluated by gammaH2AX foci forma⁃tion by a selective group of chemical/physical⁃stressors[J].Mutat Res,2006,604(1-2):8-18.
[71] Al-Anati L,Viluksela M,Strid A,Bergman Å,Andersson PL,Stenius U,et al.Hydroxyl metabolite of PCB 180 induces DNA damage signaling and enhances the DNA damaging effect of benzo[a]pyrene[J].Chem Biol Interact,2015,239:164-173.
[72] Quesnot N,Rondel K,Audebert M,Martinais S,Glaise D,Morel F,et al.Evaluation of genotoxicity using automated detection of γH2AX in metabolically competent HepaRG cells[J].Mutagenesis,2016,31(1):43-50.
[73] Liang J,Zhu H,Li C,Ding Y,Zhou Z,Wu Q. Neonatal exposure to benzo[a]pyrene decreases the levels of serum testosterone and histone H3K14 acetylation of the StAR promoter in the testes of SD rats[J].Toxicology,2012,302(2-3):285-291.
[74]Schults MA,Timmermans L,Godschalk RW,Theys J,Wouters BG,van Schooten FJ,et al. Diminished carcinogen detoxification is a novel mechanism for hypoxia-inducible factor 1-mediated genetic instability[J].J Biol Chem,2010,285(19):14558-14564.
[75]Lu G,Xiao H,Li GX,Picinich SC,Chen YK,Liu A,et al.A gamma-tocopherol-rich mixture of tocopherols inhibits chemically induced lung tumorigenesis in A/J mice and xenograft tumor growth[J].Carcinogenesis,2010,31(4):687-694.
[76]Rathore K,Wang HC.Tea catechin extract in inter⁃vention of chronic breast cell carcinogenesis induced by environmental carcinogens[J].Mol Carcinog,2012,51(3):280-289.
[77]Mattsson A,Jernström B,Cotgreave IA,Bajak E. H2AX phosphorylation in A549 cells induced by the bulky and stable DNA adducts of benzo[a]py⁃rene and dibenzo[a,l]pyrene diol epoxides[J].Chem Biol Interact,2009,177(1):40-47.
[78]Bradley C,van der Meer R,Roodi N,Yan H,Chandrasekharan MB,Sun ZW,et al.Carcinogeninduced histone alteration in normal human mam⁃mary epithelial cells[J].Carcinogenesis,2007,28(10):2184-2192.
[79]Chappell G,Pogribny IP,Guyton KZ,Rusyn I. Epigenetic alterations induced by genotoxic occu⁃pational and environmental human chemical car⁃cinogens:A systematic literature review[J].Mutat Res Rev Mutat Res,2016,768:27-45.
Research progress in cancer epigenetics mechanisms of benzo(a)pyrene
ZHOU Chao-wen,HU Jian-an
(Department of Occupational and Environmental Health,School of Public Health,Central South University,Changsha 410078,China)
In recent years,researches on cells,animals,and human beings have found that the carcinogenic mechanism of environmental carcinogen benzo(a)pyrene〔B(a)P〕can reduce methyla⁃tion of the whole genes,increase the tumor suppressor gene methylation and reduce the gene methyla⁃tion of proto-oncogene,in addition to the genetic toxicity.It can also cause changes in small RNA expression,the increase of long-chain non-coded RNA expression and imbalance in histone phosphor⁃ylation expressions.These changes can cause abnormalities in gene expression and chromosome structure and instability,directly leading to cancer.These changes can also cause the corresponding changes of genetic toxicity,such as gene mutation,abnormal genetic damage repair,increas of cell apoptosis and cell cycle arrest.All these are considered to be potential epigenetic mechanisms of B(a)P. Existing researches have provided the scientific basis for the mechanism of and prevention counter⁃measures for environment-related diseases and vocational diseases caused by B(a)P.
benzo(a)pyrene;epigenetics;DNA methylation;microRNA;long ioncocling RNA;histone modification
The project supported by National Natural Science Foundation of China(81372966)
HU Jian-an,E-mail:jiananhu@csu.edu.cn,Tel:(0731)84805460,Fax:(0731)84803006
R99
:A
:1000-3002-(2017)05-0375-10
10.3867/j.issn.1000-3002.2017.05.001
2017-01-13 接受日期:2017-05-10)
(本文编辑:齐春会)
国家自然科学基金(81372966)
胡建安,E-mail:jiananhu@csu.edu.cn,Tel:(0731)84805460,Fax:(0731)84803006
