P2X7 receptor antagonism in amyotrophic lateral sclerosis
2017-06-05RonaldSluyter,RachaelBartlett,DianeLy等
P2X7 receptor antagonism in amyotrophic lateral sclerosis
In our study (Bartlett et al., 2017), intraperitoneal (i.p.) injection of BBG (45.5 mg/kg) into SOD1G93Amice 3 times per week from 62 days of age (late pre-onset) delayed weight loss and prolonged survival of female, but not male, mice. BBG treatment also delayed weight loss in both genders combined. BBG treatment had no effect on clinical score or motor coordination in either gender, nor did it prevent motor neuron loss, microgliosis, or affect the amount of lumber SOD1 or P2X7 protein at end-stage. Therefore, results from this study demonstrate that P2X7 antagonism with BBG has some therapeutic benefit in ALS, at least in female SOD1G93Amice.
During the course of our pre-clinical trial (Bartlett et al., 2017), two similar studies were published (Cervetto et al., 2013; Apolloni et al., 2014). These studies also reported some therapeutic benefit with BBG treatment in SOD1G93Amice, but with notable differences to our study (Figure 1). In the first of these studies (Cervetto et al., 2013), injection of BBG (45.5 mg/kg i.p.) every 2 days from day 90 (onset) improved motor coordination in mice of either gender, with greater improvement in male than female mice. BBG treatment also delayed weight loss in male and both genders combined, but not in female mice. BBG treatment did not alter survival. In the second of these studies (Apolloni et al., 2014), injection of BBG (50 mg/kg i.p.) 3 times per week from day 100 (late pre-onset) improved motor coordination, but did not alter disease onset or survival, with no differences observed between genders. Injection of BBG (50 mg/kg i.p.) 3 times per week from day 135 (onset) had no effect on motor coordination, disease onset or survival (Apolloni et al., 2014). SOD1G93Amice were also injected with a higher dose of BBG (250 mg/kg i.p.) 3 times per week from either day 40 (asymptomatic), day 70 (pre-onset) or day 100 (late pre-onset) (Apolloni et al., 2014). BBG treatment from day 40 or 70 did not alter any observed disease parameter, but BBG treatment from day 100 delayed disease onset, and improved clinical score and motor coordination, but did not delay weight loss or prolong survival. BBG treatment from day 100 also increased motor neuron survival, and reduced microgliosis and pro-inflammatory markers (nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and interleukin-1β) at end-stage. As with treatment using BBG at 50 mg/kg, no differences were observed between genders with this compound at 250 mg/kg.
The effect of BBG treatment on the survival of female SOD-1G93Amice in our study (Bartlett et al., 2017) paralleled the prolonged survival observed in female, but not male, heterozygous and homozygous P2X7 knockout (P2X7KO)/SOD1G93Amice compared to P2X7 wild-type (P2X7WT)/SOD1G93Amice (Apolloni et al., 2013). In contrast, genetic ablation of P2X7 anticipated clinical onset, worsened disease progression and increased motor neuron loss, microgliosis, astrogliosis and pro-inflammatory markers (NADPH oxidase and inducible nitric oxide synthase) towards or at end-stage (Apolloni et al., 2013). Consistent with this, BBG treatment in our study caused an increase in pro-inflammatory marker serum monocyte chemoattractant protein-1 in end-stage SOD1G93Amice (Bartlett et al., 2017), although group sizes were insufficiently powered to detect a significant difference. Together these two studies demonstrate a dual role for P2X7 activation in this model of ALS (at least in female SOD1G93Amice) by promoting disease progression but limiting aspects of neuroinflammation. Moreover, it may be of value to compare the effects of BBG treatment in P2X7WT/SOD1G93Aand P2X7KO/SOD1G93Amice (which to the best of our knowledge has not been reported) to confirm P2X7 antagonism by BBG in SOD1G93Amice expressing functional P2X7.
Collectively the four studies discussed above (Apolloni et al., 2013, 2014; Cervetto et al., 2013; Bartlett et al., 2017) reveal a complex role for P2X7 in the SOD1G93Amurine model of ALS and by extension ALS in humans. The various and contrasting gender differences observed with P2X7 antagonism or genetic ablation in these studies remain unexplained, but it is well known that disease progression in this model of ALS is gender dependent (Heiman-Patterson et al., 2005). It is noteworthy, that disease progression accelerates in ovariectomized SOD1G93Amice and that this effect can be reversed by administration of 17β-oestradiol (Choi et al., 2008), a compound which can also inhibit P2X7 activation (Cario-Toumaniantz et al., 1998). Whilst, theseobservations do not completely reconcile the gender differences observed between the pre-clinical trials with BBG in SOD1G93Amice (Cervetto et al., 2013; Apolloni et al., 2014; Bartlett et al., 2017), they do suggest possible interactions between P2X7 and 17β-oestradiol, which may influence treatment design and disease outcomes in ALS.
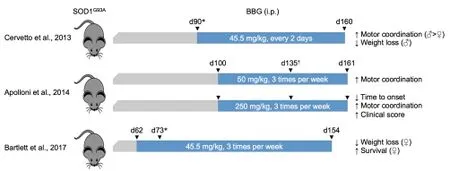
Figure 1 P2X7 antagonism with Brilliant Blue G (BBG) can reduce amyotrophic lateral sclerosis (ALS) progression in mice.
Gender differences aside, consideration of the pre-clinical trials with BBG (Cervetto et al., 2013; Apolloni et al., 2014; Bartlett et al., 2017) along with the P2X7 ablation study (Apolloni et al., 2013) indicate that P2X7 antagonism may be of most therapeutic benefit when treatment commences during late pre-onset or early onset. However in the absence of suitable biomarkers to predict disease onset and given that the average time to diagnosis is 10–12 months in humans (Mathis et al., 2017), the potential application of P2X7 antagonism in ALS is currently limited. Thus, given the current knowledge about ALS in humans, future pre-clinical studies of P2X7 antagonism in the SOD1G93Amurine model of ALS would be best served with drug treatments commencing at disease onset to translate any therapeutic benefits observed in mice to people with ALS. Moreover, pre-clinical studies of P2X7 antagonism are required in other transgenic mouse models of ALS, such as TAR DNA-binding protein 43 kDa (TDP-43) or C9Orf72 (Mathis et al., 2017), to determine if this approach has therapeutic benefits in ALS associated with molecular events other than mutant SOD1.
A limitation of pre-clinical trials with BBG in SOD1G93Amice (and other murine models of disease) is that this compound is not specific for P2X7. BBG can impair murine P2X7 with a half maximal inhibitory concentration of 2 µM (Bartlett et al., 2013), but this compound can also inhibit other channels including murine neuronal voltage-gated sodium channels at similar concentrations to murine P2X7 (Jo and Bean, 2011). Despite this caveat, BBG has been a well-recognized P2X7 antagonist since the year 2000, and has been the most widely used P2X7 antagonistin vivoto date since its first use almost a decade ago in rodents models of multiple sclerosis, Alzheimer’s disease, Huntington’s disease or spinal cord injury (Bhattacharya and Biber, 2016). Nonetheless, future studies exploring P2X7 antagonism in ALS should consider using specific P2X7 antagonists, many of which are inhibitory at concentrations lower than that of BBG (Bhattacharya and Biber, 2016).
An emerging limitation of pre-clinical trials with BBG in SOD1G93Amice is whether this compound can cross an intact blood-brain barrier. In the absence of blood-brain barrier damage, BBG demonstrates no significant occupancy of rat brain P2X7, whilst the brain to plasma ratio of BBG may be as low as 0.0065, which is well below a target ratio of 0.2 or more (Bhattacharya and Biber, 2016). Consistent with these findings, in SOD1G93Amice treated with BBG 3 times per week for up to 90 days (Bartlett et al., 2017), we observed no blue coloration of the brains or spinal cords either macroscopically or microscopically, despite extensive blue coloration of the skin macroscopically, and of the livers and spleens microscopically (Bartlett R, Sluyter R, Yerbury JJ, unpublished). Thus, pre-clinical studies with P2X7 antagonists that can cross the blood-brain barrier (Bhattacharya and Biber, 2016) may be required to determine if P2X7 antagonism is of therapeutic benefit in ALS.
在使用传感器前,需要在系统中执行注册阶段。首先,证书中心生成传感器的公钥K1,并发送给相应的传感器。其次,传感器使用公钥K1加密其身份信息idn和随机数R,并将加密结果E1返回给证书中心。最后,证书中心收到加密结果E1后,用其私钥K′1来解密,并对其中的信息进行审核。如果审核通过,则向传感器发送其使用的公钥和私钥(Ks,K′s)。
Our recent study (Bartlett et al., 2017) combined with studies by others (Cervetto et al., 2013; Apolloni et al., 2014) show that P2X7 antagonism has therapeutic benefits in SOD1G93Amice, but the observed gender differences between these studies remain unexplained. Nevertheless these studies suggest that P2X7 may play a role in ALS progression, which is supported by other observations in rodents and humans with ALS (Volonte et al., 2016). However, much work is required to ascertain a potentially complex role of this receptor in this multifaceted, currently incurable neurodegenerative disorder, and to determine if P2X7 represents a realistic therapeutic target in people with ALS.
The laboratory of Ronald Sluyter is currently supported by the MND Research Institute of Australia and the Stanford Family MND Research Grant. The laboratory of Justin J. Yerbury is currently supported by the National Health and Medical Research Council of Australia, and a US Department of Defense Therapeutic Ideas Grant.
Ronald Sluyter*, Rachael Bartlett, Diane Ly, Justin J. Yerbury
School of Biological Sciences and Center for Medical and Molecular Bioscience, University of Wollongong, Wollongong, NSW, Australia; Illawarra Health and Medical Research Institute, NSW, Wollongong, Australia
*Correspondence to:Ronald Sluyter, Ph.D., rsluyter@uow.edu.au.
Accepted:2017-05-04
orcid:0000-0003-4909-686X (Ronald Sluyter)
How to cite this article:Sluyter R, Bartlett R, Ly D, Yerbury JJ (2017) P2X7 receptor antagonism in amyotrophic lateral sclerosis. Neural Regen Res 12(5):749-750.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Open peer reviewers:Kentaro Hatano, Luis Gandia, Antonio G. García.
Additional file:Open peer review reports 1, 2, and 3.
Apolloni S, Amadio S, Montilli C, Volonte C, D’Ambrosi N (2013) Ablation of P2X7 receptor exacerbates gliosis and motoneuron death in the SOD1-G93A mouse model of amyotrophic lateral sclerosis. Hum Mol Genet 22:4102-4116.
Apolloni S, Amadio S, Parisi C, Matteucci A, Potenza RL, Armida M, Popoli P, D’Ambrosi N, Volonte C (2014) Spinal cord pathology is ameliorated by P2X7 antagonism in a SOD1-mutant mouse model of amyotrophic lateral sclerosis. Dis Model Mech 7:1101-1109.
Bartlett R, Sluyter V, Watson D, Sluyter R, Yerbury JJ (2017) P2X7 antagonism using Brilliant Blue G reduces body weight loss and prolongs survival in female SOD1G93A amyotrophic lateral sclerosis mice. PeerJ 5:e3064.
Bartlett R, Yerbury JJ, Sluyter R (2013) P2X7 receptor activation induces reactive oxygen species formation and cell death in murine EOC13 microglia. Mediators Inflamm 2013:271813.
Bhattacharya A, Biber K (2016) The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 64:1772-1787.
Cario-Toumaniantz C, Loirand G, Ferrier L, Pacaud P (1998) Non-genomic inhibition of human P2X7 purinoceptor by 17beta-oestradiol. J Physiol 508:659-666.
Cervetto C, Frattaroli D, Maura G, Marcoli M (2013) Motor neuron dysfunction in a mouse model of ALS: Gender-dependent effect of P2X7 antagonism. Toxicology 311:69-77.
Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H (2008) Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci 268:40-47.
Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM (2005) Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci 236:1-7.
Jo S, Bean BP (2011) Inhibition of neuronal voltage-gated sodium channels by Brilliant Blue G. Mol Pharmacol 80:247-257.
Mathis S, Couratier P, Julian A, Corcia P, Le Masson G (2017) Current view and perspectives in amyotrophic lateral sclerosis. Neural Regen Res 12:181-184.
Volonte C, Apolloni S, Parisi C, Amadio S (2016) Purinergic contribution to amyotrophic lateral sclerosis. Neuropharmacology 104:180-193.
10.4103/1673-5374.206643
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Efficacy of intraorbital electroacupuncture for diabetic abducens nerve palsy: study protocol for a prospective single-center randomized controlled trial
- Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety
- The brain activation pattern of the medial temporal lobe during chewing gum: a functional MRI study
- Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine
- Correlation between photoreceptor injuryregeneration and behavior in a zebrafish model