Correlation between photoreceptor injuryregeneration and behavior in a zebrafish model
2017-06-05YajieWangShijiaoCaiJianlinCuiYangChenXinTangYuhaoLi
Ya-jie Wang, Shi-jiao Cai, Jian-lin Cui Yang Chen Xin Tang, Yu-hao Li
1 Key Laboratory of Tumor Microenviroment and Neurovascular Regulation, Nankai University School of Medicine, Tianjin, China
2 Cataract Center, Tianjin Eye Hospital, Tianjin, China
Correlation between photoreceptor injuryregeneration and behavior in a zebrafish model
Ya-jie Wang1,2,#, Shi-jiao Cai1,#, Jian-lin Cui1, Yang Chen1, Xin Tang2,*, Yu-hao Li1,*
1 Key Laboratory of Tumor Microenviroment and Neurovascular Regulation, Nankai University School of Medicine, Tianjin, China
2 Cataract Center, Tianjin Eye Hospital, Tianjin, China
How to cite this article:Wang YJ, Cai SJ, Cui JL, Chen Y, Tang X, Li YH (2017) Correlation between photoreceptor injury-regeneration and behavior in a zebrafish model. Neural Regen Res 12(5):795-803.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This work was supported by the National Natural Science Foundation of China, No. 81301080, 81671179; the Fundamental Research Funds for the Central Universities in China, No. 63161215; the Natural Science Foundation of Tianjin of China, No. 15JCYBJC24400, 15JCQNJC10900.
Graphical Abstract
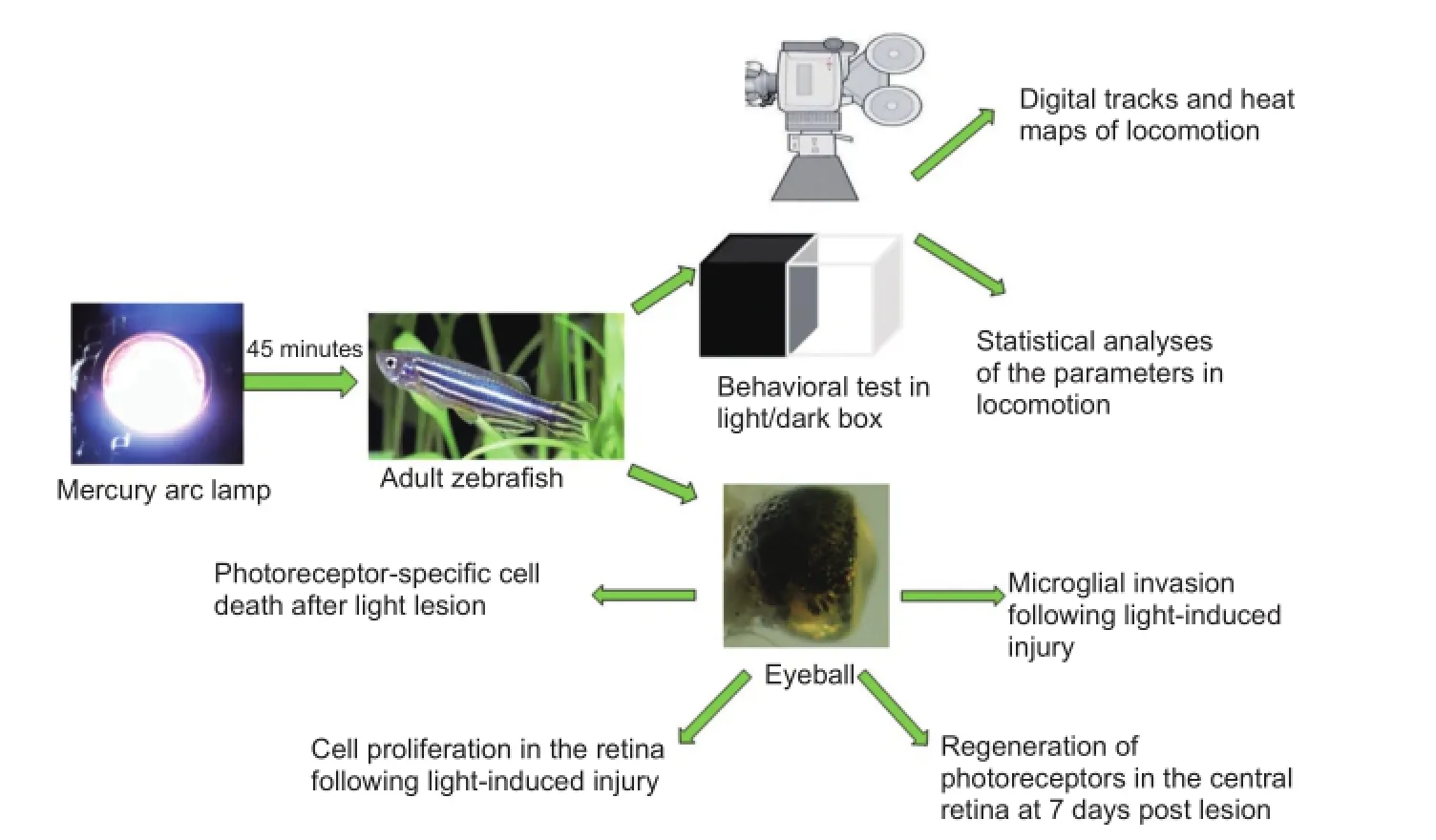
The process of injury and regeneration of photoreceptor cells in zebrafish retina
Direct exposure to intensive visible light can lead to solar retinopathy, including macular injury. The signs and symptoms include central scotoma, metamorphopsia, and decreased vision. However, there have been few studies examining retinal injury due to intensive light stimulation at the cellular level. Neural network arrangements and gene expression patterns in zebrafish photoreceptors are similar to those observed in humans, and photoreceptor injury in zebrafish can induce stem cell-based cellular regeneration. Therefore, the zebrafish retina is considered a useful model for studying photoreceptor injury in humans. In the current study, the central retinal photoreceptors of zebrafish were selectively ablated by stimulation with high-intensity light. Retinal injury, cell proliferation and regeneration of cones and rods were assessed at 1, 3 and 7 days post lesion with immunohistochemistry andin situhybridization. Additionally, a light/dark box test was used to assess zebrafish behavior. The results revealed that photoreceptors were regenerated by 7 days after the light-induced injury. However, the regenerated cells showed a disrupted arrangement at the lesion site. During the injury-regeneration process, the zebrafish exhibited reduced locomotor capacity, weakened phototaxis and increased movement angular velocity. These behaviors matched the morphological changes of retinal injury and regeneration in a number of ways. This study demonstrates that the zebrafish retina has a robust capacity for regeneration. Visual impairment and stress responses following high-intensity light stimulation appear to contribute to the alteration of behaviors.
nerve regeneration; optic nerve injury; light injury; photoreceptor cell; cell proliferation; retinal regeneration; light/dark test; behavior; zebrafish; phototaxis; immunohistochemistry; in situ hybridization; neural regeneration
Introduction
In the zebrafish, as in all vertebrates, the retina is an extension of the encephalon; it develops from a neuroepithelial sheet of embryonic stem cells, differentiates in a scheduled spatiotemporal pattern and grows into the neurosensory retina (Randlett et al., 2011). Although both the structure and function of the zebrafish retina are remarkably similar to those of the human retina, there is a marked difference in neuronal regeneration between the two species. Whereas human retinal neurons exhibit poor self-repair capacity after injury, retinal neurogenesis takes place throughout the zebrafish life cycle (Gramage et al., 2015). Any insult that significantly depletes retinal neurons in zebrafish stimulates robust neuronal regeneration, during which Müller glia serve as stem cells (Craig et al., 2010; Gemberling et al., 2013). Therefore, the zebrafish retina is considered a powerful model for studying neuronal regenerationin vivo(Nelson et al., 2013).
The light/dark test has traditionally been used in mice to measure anxiety-like behaviors and investigate the mechanisms of drug-induced neurobehavioral alterations (Ibironke and Modupe, 2015; Li et al., 2016). This test is based on findings suggesting that rodents innately exhibit scototaxis (preference for dark) and spontaneous exploratory behavior, causing them to avoid unfamiliar and bright environments (Lahouel et al., 2016; Mlyniec et al., 2016). Recently, the light/dark test has been applied in behavioral neuroscience studies of zebrafish involving high-throughput neurophenotyping and screening of genetic mutations and psychotropic drugs (Cachat et al., 2010; Nunes et al., 2016; Song et al., 2016). Unlike rodents, zebrafish exhibit phototaxis (Blaser and Penalosa, 2011), and zebrafish larvae have been shown to be attracted to light and to avoid darkness when illumination was manipulated (Chen and Engert, 2014). However, the behavioral changes that occur in zebrafish during the retinal injury-regeneration process are not well understood.
In the present study, adult zebrafish was used as an animal model for investigating the regeneration of photoreceptors, and to examine associated behavioral changes during retinal injury and regeneration. We examined the following parameters: (1) the morphological characteristics of a retinal photoreceptor lesion induced by high-intensity light treatment; (2) the morphological changes to the lesion site following cell proliferation and regeneration of photoreceptors; and (3) the behaviors of adult zebrafish following retinal injury and regeneration. The current results demonstrated that the light/ dark test can be applied in the evaluation of retinal status following high-intensity light-induced injury in zebrafish.
Materials and Methods
Experimental animals and light treatment
Normally pigmented wild-type (AB strain) adult zebrafish (6–12 months of age) were used in this study. The animals were maintained in a fish facility at 28.5°C with a 10/14-hour dark/light cycle (Westerfield, 2007). To induce selective photoreceptor death, animals were exposed to high-intensity light from a mercury arc lamp (> 180,000 lx) for 45 minutes. The study protocol was approved by the Experimental Animal Ethics Committee of Nankai University of China. The experiment follows the national guidelines for the Care and Use of Laboratory Animals, and the “Consensus author guidelines on animal ethics and welfare” by the International Association for Veterinary Editors. The article was prepared in accordance with the “Animal Research: Reporting ofIn VivoExperiments Guidelines”.
Seventy adult fish were randomly divided into seven groups, as follows: 0 days post lesion (0 dpl, control), 1 day post lesion (1 dpl), 2 days post lesion (2 dpl), 3 days post lesion (3 dpl), 4 days post lesion (4 dpl), 5 days post lesion (5 dpl), and 7 days post lesion (7 dpl) for morphological assay. In addition, eight fish were examined in the behavioral test.
Immunohistochemistry
Untreated and light-treated fish were euthanized with 0.1% 3-aminobenzoic acid ethyl ester methanesulfonate (Sigma, St. Louis, MO, USA) after light onset, and eyecups were harvested. The excised eyes were fixed in 4% paraformaldehyde, dehydrated in 20% sucrose in 0.1 M phosphate buffered saline (pH 7.4), frozen in Optimal Cutting Temperature Compound (Sakura Finetek, Torrance, CA, USA), cryosectioned at 10 μm with a cryostat (Leica CM1850, Wetzlar, Germany) and mounted on glass slides. Immunohistochemistry was performed using standard procedures (Wang et al., 2014). Four primary antibodies were used in this study: anti-proliferating cell nuclear antigen (PCNA; 1:1,000; clone PC-10, Sigma), Zpr1 (1:200; Zebrafish International Resource Center, Eugene, OR, USA), Zpr3 (1:200, Zebrafish International Resource Center), and 4C4 (1:200, provided by Dr. Hitchcock) for labeling of proliferating cells, cones, rods and microglia, respectively. For PCNA immunolabeling, slides were first incubated for 20 minutes at 95–98°C in 0.01 M sodium citrate buffer (pH 6.0) and 0.05% Tween-20 prior to immunostaining (Nelson et al., 2013). The secondary antibody was a fluorescent-labeled Cy3 (1:500; Millipore, Billerica, MA, USA). The sections were counterstained with a 1:1,000 dilution of 4′,6-diamidino-2-phenylindole (DAPI; Sigma) to label the nuclei. The time points of immunohistochemistry were: Zpr1 and Zpr3 staining, 0 (control), 1, 3 and 7 dpl; 4C4 staining, 0, 1, 2 and 3 dpl; PCNA staining, 2, 3, 4 and 5 dpl. Ten fish were examined at each time point.
In situhybridization
To identify the regenerated cones and rods,in situhybridization was performed on cryosections at 7 days using a standard protocol (Luo et al., 2012). Two digoxigenin-labeled probes were used in this study. For photoreceptors, cones and rods were specifically labeled using mRNA probes forphosphodiesterase 6c(pde6c, GenBankNM_200871) andrhodopsin(GenBank NM_131084), respectively. The cDNA encoding pde6c was linearized withSalI, and the riboprobes were synthesized with T7 polymerase. The cDNA encodingrhodopsinwas linearized with Apa I, and the riboprobes were synthesized with SP6 polymerase. The hybrid concentration was 200ng pde6c orrhodopsinprobe in 100 μl hybridization buffer. Riboprobes encoding the sense strand of the respective cDNAs were used as negative controls. On the second day, digoxigenin was immunolabeled using an alkaline phosphatase-conjugated antibody (Roche Diagnostics, Indianapolis, IN, USA). On the third day, 4-nitrobluetetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche) was applied as the enzymatic substrate. Ten fish were processed at 7 dpl.
Behavioral test
Experiments were carried out at 0 dpl (control), 1 dpl, 3 dpl, and 7 dpl. All behavioral tests were performed between 14:00 and 17:00 to avoid the influence of circadian rhythms on visual sensitivity and locomotor activity (Li et al., 2012; Kopp et al., 2016). A light/dark box (half-white/half-black tank, 20.0 cm × 9.6 cm × 10.0 cm) was used for the behavioral tests. The light/dark box was filled with 4 cm system water (3.6 g/L sea salt; NaHCO3is used to adjust pH to 7.2) at room temperature. Two lamps were used to control illumination and to ensure that the lighting conditions of all areas of the apparatus were homogeneous. Zebrafish were placed into the light/dark box and allowed to acclimatize for 15 minutes before monitoring began (Zhao et al., 2012; Fang et al., 2015). Next, the fish were allowed to freely explore the light/dark box for 10 minutes. Fish movement was tracked using a camera positioned above the light/dark box. All digital tracks were analyzed using Ethovision XT software (Noldus Information Technology, Wageningen, the Netherlands). To further examine the movement, three swimming speeds were defined: slow (< 1 cm/s), medium (≥ 1 cm/s, ≤20 cm/s) and fast (> 20 cm/s). Twelve parameters were analyzed: total movement time, fast movement time, medium movement time, slow movement time, total movement distance, distance in the white region, white distance ratio (white distance/total distance), transition times to white, time spent in the white region, white time ratio (time spent in the white region/total movement time), velocity and angular velocity.
Photography and image analysis
Images of immunohistochemistry-stained sections were captured with an FV 1000 confocal microscope (Olympus, Japan). Images ofin situhybridization were photographed with a DP71 digital camera mounted on a BX51 microscope (Olympus). Image J software (1.49X, NIH, http://rsb.info. nih.gov/ij/) was used to convert the fluorescent images of the PCNA staining to 8-bit greyscale images prior to thresholding and determining the positive staining of each image.
Statistical analysis
Data were expressed as the mean ± SD. Statistical analysis was performed with GraphPad Software (version 5.0, Graph-Pad Software, La Jolla, USA). A normal distribution of the data was confirmed using a one-sample Kolmogorov-Smirnov test. For each parameter, groups at different time points were analyzed with one-way analysis of variance and the least significant differencettest. AP-value of 0.05 was set as the threshold value for statistical significance.
Results
High-intensity light treatment induces photoreceptor injury in the central retina
In zebrafish, sufficient light stimulation results in the dispersion of melanin in skin melanocytes (Taylor et al., 2012). This camouflage reaction is a response to environmental changes and is under neuroendocrine control (Bernardos et al., 2007; Taylor et al., 2012). In the present study, the zebrafish became darkly pigmented after 45 minutes of exposure to light (data not shown). Intense, short-term light treatment has been shown to selectively kill photoreceptors (including cones and rods) in a narrow horizontally oriented band across the nasal-temporal axis of the retina (Craig et al., 2008; Taylor et al., 2012). Photoreceptor injury can be detected using Zpr1 and Zpr3 antibodies to specifically label cones and rods, respectively, with immunohistochemistry (Huang et al., 2012; Wang et al., 2014). In the present study, the Zpr1- and Zpr3-positive cells in the control retina were arranged in a regular pattern (Figure 1A, E). At 1 dpl, the cones and rods exfoliated from the outer nuclear layer (ONL) to the outer segment layer (OSL) in the central retina (Figure 1B, F; white brackets). At 2 dpl, the ONLs were thinner than in the control group, and Zpr1-positive cells were absent in the central retina (Figure 1C; white bracket), whereas the Zpr3-positive cells were disorganized (Figure 1G; white bracket). The arrangement of cells in the ONL was completely interrupted at 3 dpl, and Zpr1- and Zpr3-positive cells were absent in the OSL (Figure 1D, H, white brackets). In other regions of the retina, the ONL exhibited normal thickness and a normal quantity of Zpr1- and Zpr3-positive cells in the OSL.
To further characterize photoreceptor damage, we examined the distribution of microglia using immunohistochemistry with the 4C4 antibody using a previously reported method (Wang et al., 2014). In the present study, only a few 4C4-expressing cells were detected in the control retina, mainly in the plexiform layers or the retinal pigment epithelium (RPE, Figure 2A). More 4C4-expressing cells were detected at the injury site in the OSL of the retina at 1 dpl (Figure 2B). Numerous microglia adopted an amoeboid phenotype with enlarged cell bodies and irregular shapes; these microglia aggregated around cellular debris in the OSL and the pigment epithelial layer of the central retina at 2 and 3 dpl (Figure 2C, D). Together, the Zpr1, Zpr3 and 4C4 expression results indicate that high-intensity light treatment selectively induces central retinal photoreceptor death.
Cellular proliferation was triggered at lesion site to regenerate photoreceptors
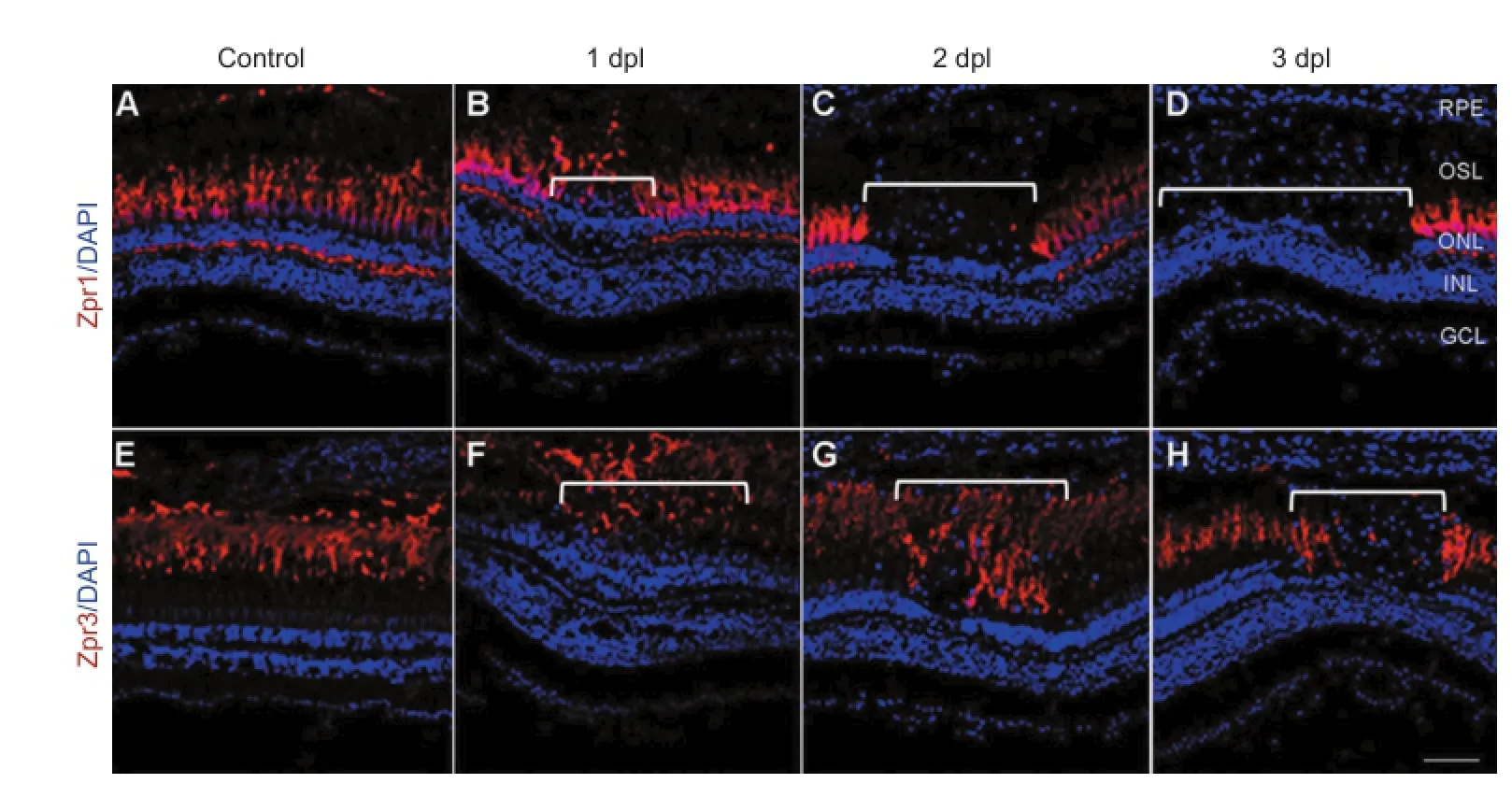
Figure 1 Photoreceptor-specific cell death after light treatment (confocal microscope).

Figure 2 Microglial invasion following light-induced injury (confocal microscope).
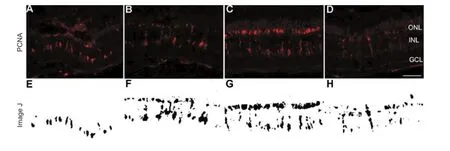
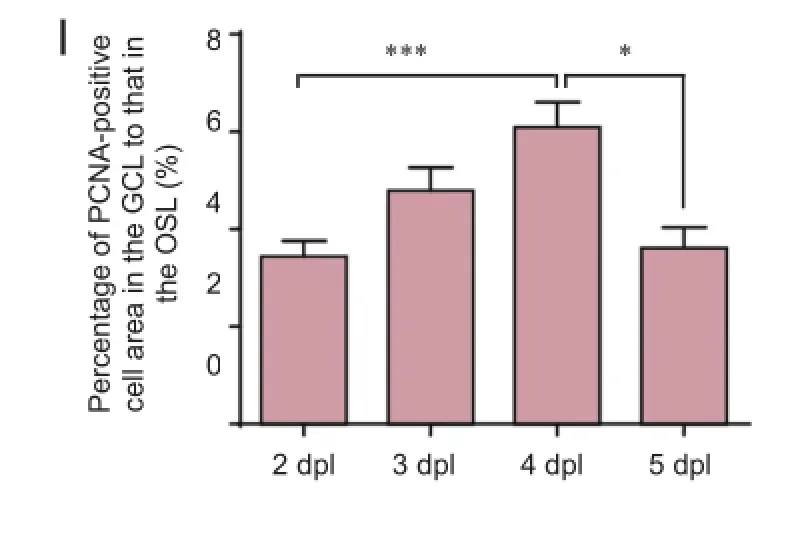
Figure 3 Cell proliferation in the retina following light-induced injury.
Once retinal cells complete the differentiation process, they lose the ability to proliferate, and no longer express PCNA (Nelson et al., 2013). Therefore, the anti-PCNA antibody can be used to identify precursor cells and evaluate cell proliferation (Bailey et al., 2010; Craig et al., 2010). At 2 dpl, scattered PCNA-positive cells gathered in clusters at the top of the inner nuclear layer (INL, Figure 3A, E). More PCNA-positive cells were detected extending from the INL to the ONL at 3 dpl (Figure 3B, F). At 4 dpl, the proliferating cells had almost finished their migration to the ONL and had reached their peak number (Figure 3C, G). During this process, PCNA-positive cells underwent morphological changes. When the proliferating cell clusters were located in the INL, they exhibited short spindle shapes, then transformed into long spindle shapes (Figure 3A, B). However, clusters in the ONL were round and compact (see Vihtelic and Hyde, 2000) (Figure 3C). The percentage of PCNA-positive cells was scored to quantify the findings. Cellular proliferation increased un-til 4 dpl (Figure 3I;P< 0.05), then decreased significantly at 5 dpl (Figure 3D, H, I;P< 0.05).
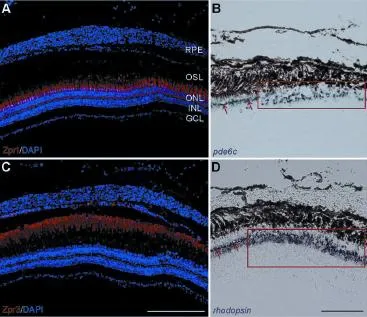
Figure 4 Regeneration of photoreceptors in the central retina at 7
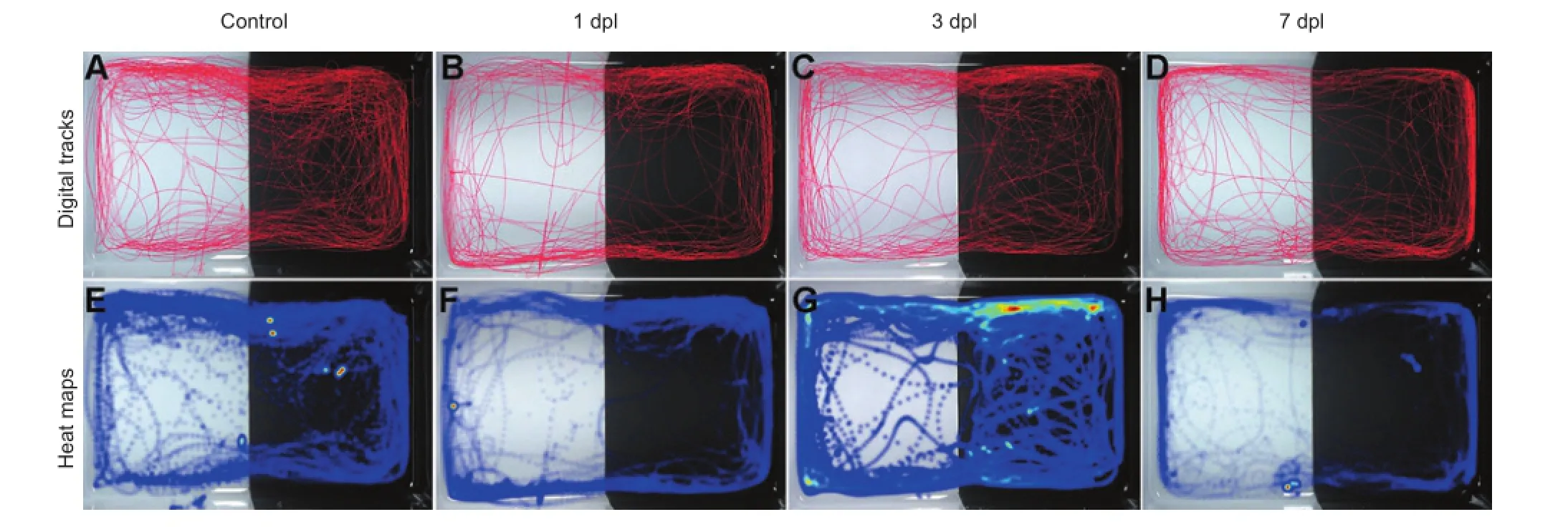
Figure 5 Digital tracks and heat maps of locomotion in adult zebrafish following light-induced injury.
Two approaches were used to visualize the regeneration of retinal photoreceptors at 7 dpl. First, the neuronal differentiation of cones and rods was detected using immunochemistry with cell-type-specific antibodies. DAPI staining did not show any alteration in the retinas, and the ONLs exhibited a normal thickness at 7 dpl. Zpr1 and Zpr3 were strongly and continuously expressed in the OSL of the retina (Figure 4A, C). Therefore, the results from Zpr1 and Zpr3 staining showed that cones and rods were well differentiated and filled in the lesion site. Second, cell-type-specific mRNA probes, pde6c andrhodopsin, were used to label the cones and rods, respectively,viain situhybridization (Lewis et al., 2010). Positive pde6c andrhodopsinsignals were detected at the lesion site of the ONL. However, the regenerated cells were densely arranged with thicker cell layers (Qin et al., 2009; Figure 4B, D; frames). This result suggests that the regenerated cones and rods had lost their regular patterns (Figure 4B, D; arrows). The results from immunostaining andin situhybridization suggest that the progenitors were triggered to proliferate and differentiate to cones or rods, resulting in regeneration of the photoreceptors with a disordered arrangement after 7 days.
Locomotor capacity and phototaxis were reduced after light-induced injury
To examine the functional changes associated with photoreceptor injury and regeneration, behavioral tests were performed in adult zebrafish. There were four time points in the trial: 0 dpl (control), 1 dpl, 3 dpl and 7 dpl. The digital tracks and heat maps are shown in Figure 5. The heat map shows that fish from the 3 dpl group appeared more frequently in the dark region than fish from the control group. This result was in line with the severity of retinal injury. The digital tracks were then analyzed according to 12 parameters. The total movement time (Figure 6A) and medium movement time (Figure 6C) showed a V-curve tendency, which changed from descending to ascending during retinal injury and regeneration. The total movement time and medium movement time decreased markedly at 1 dpl and increased slightly and gradually at 3 dpl and 7 dpl, although the movement times were still lower than in the control group at 7 dpl (Figure 6A, C;P< 0.05). In contrast, no significant differences in fast movement time were found among the four groups (Figure 6B). However, slow movement time (Figure 6D) showed a gradual increase following light-induced injury, and the movement time was significantly longer in the 3 dpl and 7 dpl groups compared with the control group (Figure 6D;P< 0.05). The total movement distance also showed a similar V-curve tendency, and the reduction was significant in the 1 dpl and 3 dpl groups compared with the control group (Figure 6E;P< 0.05). The parameters of phototaxis, including the distance in the white region (Figure 6F), white distance ratio (Figure 6G), time spent in the white region (Figure 6I) and white time ratio (Figure 6J) also showed a V-curve tendency with a significant reduction at 1 dpl (P< 0.05). For the transition times to white, the fish swam from the black area to the white area an average of 34 times before the injury; however, when the fish received the light-induced injury, the transitions decreased dramatically to an average of 19 times at 1 dpl, then increased slightly at 3 dpl and 7 dpl(Figure 6H;P< 0.05). The velocity of fish in the 1 dpl, 3 dpl and 7 dpl groups was lower than in the control group. However, these differences were not statistically significant (Figure 6K). Interestingly, the change in angular velocity over time resembled an inverted V-curve. After the light-induced injury, the angular velocity gradually increased. At 3 dpl, fish with the most severe retinal injury showed a markedly increased angular velocity compared with the control group (Figure 6L;P< 0.01). The angular velocity dropped at 7 dpl, but was still higher than in the control group (Figure 6L;P< 0.05). Taken together, these data suggest that the locomotor capacity and phototaxis of adult zebrafish are reduced after retinal injury, and recover to some extent during the self-repair process.
Discussion
The zebrafish retina is an important model for neuronal regeneration (Meyers et al., 2012; Sun et al., 2016). In response to injury, intrinsic stem cells become activated at the lesion site and enter the mitotic cycle to directly regenerate photoreceptors, and mature cells then integrate into existing neural circuits (Thummel et al., 2010). The current study not only evaluated retinal status following exposure to high-intensity light, but also introduced a novel approach for evaluating zebrafish vision and adaptation based on behavioral assessment.
In the present study, high-intensity light was used to induce selective death of photoreceptors in the central retina. Following light-induced injury, photoreceptors became disorganized and began exfoliating from the ONL to the OSL, which was inconsistent with the results of a previous study (Qin et al., 2009). Interestingly, the present results revealed that the rods underwent slower apoptosis and that fewer rods underwent apoptosis compared with cones at a particular stage of the light lesion. Various light treatments are known to cause different types of photoreceptor loss in adult zebrafish. Constant bright light has been shown to cause more damage to rods than cones (Thummel et al., 2010), whereas short exposure to high-intensity light causes more marked damage to cones (Thomas et al., 2012). This discrepancy may be related to the light adaptation of rod photoreceptors (Weber et al., 2013). Following intense light irradiation, pigment granules in the RPE migrate to the zone between the rod outer segments and the cone outer segments. The RPE protects rods by scattering light back to cones (Hodel et al., 2006), resulting in differential susceptibility in response to light-induced lesions between rods and cones. In addition, under high-intensity light exposure, cones and rods produce large amounts of reactive oxygen species, surpassing the absorption capacity of the RPE (Tarboush et al., 2014) and eventually causing damage to the photoreceptor cells. In the present study, the photoreceptors were hardly detectable and the ONL was interrupted in the central retina at 3 dpl. Dying photoreceptors release a variety of cytokines that attract phagocytes (Fernando et al., 2016). Thus, in the present study, microglia were continuously recruited to the injury area, where they performed phagocytosis and cleanup (Casano et al., 2016). The invasion of microglia reached a peak at 3 dpl, when the apoptosis of cones and rods was also at a maximum.
In zebrafish retina, neuronal injury can rapidly stimulate the proliferation of stem cells (progenitors) (Gramage et al., 2015). Following the selective death of photoreceptors, two stem cell populations, the Müller glia and the ONL rod precursors, have been shown to re-enter the cell cycle, with the former differentiating into cones and rods and the latter differentiating only into rods (Thummel et al., 2010). In the current study, PCNA-positive cells gathered in clusters and migrated to the top of the INL, starting at 2 dpl. Cell proliferation peaked at 4 dpl. After 7 days of recovery, the cones and rods were well differentiated and filled the lesion site. However, the regenerated cells did not exhibit their original pattern within the regenerated lesion site, and the regenerated cones showed a more irregular arrangement than the rods. This finding supports the notion that the establishment of cone patterns is more sensitive to change, and is more readily compromised by changes in the retinal milieu (Stenkamp and Cameron, 2002; Jimeno and Santos, 2016; Smiley et al., 2016).
The present findings raise several important questions, including what functional changes take place during this process, and how the effects of these changes on vision can be evaluated. In the current study, a light/dark box test was used as a direct and non-invasive approach for investigating behavioral changes during retinal injury and repair in zebrafish. Surprisingly, the behavioral changes correlated with morphological changes in the retina in three ways. First, the locomotor capacity of the zebrafish decreased following high-intensity light-induced injury to the retina. At 1 dpl or 3 dpl, the fish exhibited a shorter total movement time, a shorter medium movement time, a shorter total movement distance and a longer slow movement time. Second, the injured fish exhibited weakened phototaxis, exhibited as decreases in the distance in the white region, the white distance ratio, the time spent in the white region, the white time ratio and the transition times to white. These findings are in accord with the notion that fish with injuries to the central retina exhibited a reduced tendency to approach light. Morphologically, in the central retina, the cones and rods began to undergo apoptosis at 1 dpl, exhibited the most severe retinal coloboma at 3 dpl and had regenerated by 7 dpl. The ONL was thinnest at 3 dpl and recovered to almost normal at 7 dpl. It has been reported that modest declines in photoreceptor number with slight thinning of the ONL do not elicit a significant alteration in retinal architecture and visual response, but when the loss of photoreceptors is severe and the thickness of the ONL drops by more than 50%, the light-evoked responses disappear (Saade et al., 2013). Some behavioral parameters revealed a slight difference between 1 dpl and 3 dpl in the present study, which may have been caused by adaptation to blindness, and anxiety (Huang et al., 2013). In addition to low vision, anxiety related to high-intensity light may have also been an important parameter for altering the locomotor activity in zebrafish, introducingmore complexity in the behavioral results (Peng et al., 2016). Finally, the light stimulus used in the current study had a substantial impact on angular velocity, which is a useful indicator of stress (Lima et al., 2016; Nema et al., 2016). The pattern of angular velocity resembled an inverted “V”, which is consistent with the loss of photoreceptors. Increased angular velocity has been reported to be an early indicator of retinal damage (Fernandes et al., 2016). Both the light stimulus and impaired vision can induce stress in the zebrafish, which brought about an alteration in angular velocity. The current findings demonstrate that the loss and recovery of photoreceptors in the central retina result in an alteration of locomotor capacity, phototaxis and angular velocity.
Overall, the data indicate that photoreceptors can regenerate at 7 days after a high-intensity light-induced injury to the retina, and that the regenerated cells exhibit a disrupted arrangement at the lesion site. During the injury-regeneration process, the behaviors of adult zebrafish changed in concert with the morphological changes observed in the retina. Therefore, the light/dark test can provide a useful method for evaluating visual function in adult zebrafish. Reduced locomotor capacity, weakened phototaxis and increased angular velocity can indicate the presence of visual impairment, and the behavioral changes observed during the light/dark test are an accurate indicator of photoreceptor degeneration and regeneration in zebrafish following a high-intensity light-induced retinal injury.
In the current study, a light/dark test was used as a new method for studying retinal degeneration and regeneration in zebrafish after a high-intensity light-induced retinal injury. A light stimulus and self-repair activity can induce changes in photoreceptors, influence vision, and evoke anxiety, consequently altering the locomotor capacity and phototaxis of fish. The current behavioral results were consistent with the observed morphological changes. The present findings therefore suggest that the light/dark test can be used as a novel method for evaluating vision in zebrafish, and to identify new treatments for retinal diseases. While it is not clear about the molecular mechanisms between light injury and locomotor capacity, more research on the mechanisms of the behavioral alterations will be conducted in the future.
Author contributions:YHL conceived and designed the study. YHL and XT supervised the work. YJW, SJC, and YC performed the experiments. YJW and JLC analyzed the data. YHL and YJW wrote the paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR (2010) The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res 91:601-612.
Bernardos RL, Barthel LK, Meyers JR, Raymond PA (2007) Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci 27:7028-7040.
Blaser RE, Penalosa YM (2011) Stimuli affecting zebrafish (Danio rerio) behavior in the light/dark preference test. Physiol Behav 104:831-837.
Cachat J et al. (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5:1786-1799.
Casano AM, Albert M, Peri F (2016) Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Rep 16:897-906.
Chen X, Engert F (2014) Navigational strategies underlying phototaxis in larval zebrafish. Front Syst Neurosci 8:39.
Craig SE, Calinescu AA, Hitchcock PF (2008) Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J Ocul Biol Dis Infor 1:73-84.
Craig SE, Thummel R, Ahmed H, Vasta GR, Hyde DR, Hitchcock PF (2010) The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci 51:3244-3252.
Fang YW, Lei XD, Li X, Chen YN, Xu F, Feng XZ, Wei SH, Li YH (2015) A novel model of demyelination and remyelination in a GFP-transgenic zebrafish. Biol Open 4:62-68.
Fernandes M, Amorim J, Vasconcelos V, Teles LO (2016) Resilience assessment of a biological early warning system based on the locomotor behavior of zebrafish (Danio rerio). Environ Sci Pollut Res Int 23:18858-18868.
Fernando N, Natoli R, Valter K, Provis J, Rutar M (2016) The broadspectrum chemokine inhibitor NR58-3.14.3 modulates macrophage-mediated inflammation in the diseased retina. J Neuroinflammation 13:47.
Gemberling M, Bailey TJ, Hyde DR, Poss KD (2013) The zebrafish as a model for complex tissue regeneration. Trends Genet 29:611-620.
Gramage E, D’Cruz T, Taylor S, Thummel R, Hitchcock PF (2015) Midkine-a protein localization in the developing and adult retina of the zebrafish and its function during photoreceptor regeneration. PLoS One 10:e0121789.
Hodel C, Neuhauss SC, Biehlmaier O (2006) Time course and development of light adaptation processes in the outer zebrafish retina. Anat Rec A Discov Mol Cell Evol Biol 288:653-662.
Huang KH, Ahrens MB, Dunn TW, Engert F (2013) Spinal projection neurons control turning behaviors in zebrafish. Curr Biol 23:1566-1573.
Huang T, Cui J, Li L, Hitchcock PF, Li Y (2012) The role of microglia in the neurogenesis of zebrafish retina. Biochem Biophys Res Commun 421:214-220.
Ibironke GF, Modupe OG (2015) Non cholinergic dependent mechanism of Ocimum gratissimum induced neurobehavioural alterations in mice. Afr J Med Med Sci 44:213-220.
Jimeno D, Santos E (2016) A new functional role uncovered for RASGRF2 in control of nuclear migration in cone photoreceptors during postnatal retinal development. Small GTPases 8:26-30.
Kopp R, Legler J, Legradi J (2016) Alterations in locomotor activity of feeding zebrafish larvae as a consequence of exposure to different environmental factors. Environ Sci Pollut Res Int doi:10.1007/s11356-016-6704-3.
Lahouel A, Kebieche M, Lakroun Z, Rouabhi R, Fetoui H, Chtourou Y, Djamila Z, Soulimani R (2016) Neurobehavioral deficits and brain oxidative stress induced by chronic low dose exposure of persistent organic pollutants mixture in adult female rat. Environ Sci Pollut Res Int 23:19030-19040.
Lewis A, Williams P, Lawrence O, Wong RO, Brockerhoff SE (2010) Wild-type cone photoreceptors persist despite neighboring mutant cone degeneration. J Neurosci 30:382-389.
Li J, Liu QT, Chen Y, Liu J, Shi JL, Liu Y, Guo JY (2016) Involvement of 5-HT1A receptors in the anxiolytic-like effects of quercitrin and evidence of the involvement of the monoaminergic system. Evid Based Complement Alternat Med 2016:6530364.
Li X, Montgomery J, Cheng W, Noh JH, Hyde DR, Li L (2012) Pineal photoreceptor cells are required for maintaining the circadian rhythms of behavioral visual sensitivity in zebrafish. PLoS One 7:e40508.
Lima MG, Silva RX, Silva Sde N, Rodrigues Ldo S, Oliveira KR, Batista Ede J, Maximino C, Herculano AM (2016) Time-dependent sensitization of stress responses in zebrafish: a putative model for post-traumatic stress disorder. Behav Processes 128:70-82.
Luo J, Uribe RA, Hayton S, Calinescu AA, Gross JM, Hitchcock PF (2012) Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Dev 7:33.
Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA (2012) beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev 7:30.
Mlyniec K, Starowicz G, Gawel M, Frackiewicz E, Nowak G (2016) Potential antidepressant-like properties of the TC G-1008, a GPR39 (zinc receptor) agonist. J Affect Disord 201:179-184.
Nelson CM, Ackerman KM, O’Hayer P, Bailey TJ, Gorsuch RA, Hyde DR (2013) Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci 33:6524-6539.
Nema S, Hasan W, Bhargava A, Bhargava Y (2016) A novel method for automated tracking and quantification of adult zebrafish behaviour during anxiety. J Neurosci Methods 271:65-75.
Nunes ME, Muller TE, Braga MM, Fontana BD, Quadros VA, Marins A, Rodrigues C, Menezes C, Rosemberg DB, Loro VL (2016) Chronic treatment with paraquat induces brain injury, changes in antioxidant defenses system, and modulates behavioral functions in zebrafish. Mol Neurobiol doi:10.1007/s12035-016-9919-x.
Peng X, Lin J, Zhu Y, Liu X, Zhang Y, Ji Y, Yang X, Zhang Y, Guo N, Li Q (2016) Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions. Pharmacol Biochem Behav 145:55-65.
Qin Z, Barthel LK, Raymond PA (2009) Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci U S A 106:9310-9315.
Randlett O, Norden C, Harris WA (2011) The vertebrate retina: a model for neuronal polarization in vivo. Dev Neurobiol 71:567-583.
Saade CJ, Alvarez-Delfin K, Fadool JM (2013) Rod photoreceptors protect from cone degeneration-induced retinal remodeling and restore visual responses in zebrafish. J Neurosci 33:1804-1814.
Smiley S, Nickerson PE, Comanita L, Daftarian N, El-Sehemy A, Tsai EL, Matan-Lithwick S, Yan K, Thurig S, Touahri Y, Dixit R, Aavani T, De Repentingy Y, Baker A, Tsilfidis C, Biernaskie J, Sauvé Y, Schuurmans C, Kothary R, Mears AJ (2016) Establishment of a cone photoreceptor transplantation platform based on a novel cone-GFP reporter mouse line. Sci Rep 6:22867.
Song C, Yang L, Wang J, Chen P, Li S, Liu Y, Nguyen M, Kaluyeva A, Kyzar EJ, Gaikwad S, Kalueff AV (2016) Building neurophenomics in zebrafish: effects of prior testing stress and test batteries. Behav Brain Res 311:24-30.
Stenkamp DL, Cameron DA (2002) Cellular pattern formation in the retina: retinal regeneration as a model system. Mol Vis 8:280-293.
Sun Z, Zhang M, Liu W, Tian J, Xu G (2016) Photoreceptor IRBP prevents light induced injury. Front Biosci (Landmark Ed) 21:958-972.
Tarboush R, Novales Flamarique I, Chapman GB, Connaughton VP (2014) Variability in mitochondria of zebrafish photoreceptor ellipsoids. Vis Neurosci 31:11-23.
Taylor S, Chen J, Luo J, Hitchcock P (2012) Light-induced photoreceptor degeneration in the retina of the zebrafish. Methods Mol Biol 884:247-254.
Thomas JL, Nelson CM, Luo X, Hyde DR, Thummel R (2012) Characterization of multiple light damage paradigms reveals regional differences in photoreceptor loss. Exp Eye Res 97:105-116.
Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR (2010) Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res 90:572-582.
Vihtelic TS, Hyde DR (2000) Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol 44:289-307.
Wang YJ, He ZZ, Fang YW, Xu Y, Chen YN, Wang GQ, Yang YQ, Yang Z, Li YH (2014) Effect of titanium dioxide nanoparticles on zebrafish embryos and developing retina. Int J Ophthalmol 7:917-923.
Weber A, Hochmann S, Cimalla P, Gartner M, Kuscha V, Hans S, Geffarth M, Kaslin J, Koch E, Brand M (2013) Characterization of light lesion paradigms and optical coherence tomography as tools to study adult retina regeneration in zebrafish. PLoS One 8:e80483.
Westerfield M (2007) The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press.
Zhao T, Zondervan-van der Linde H, Severijnen LA, Oostra BA, Willemsen R, Bonifati V (2012) Dopaminergic neuronal loss and dopamine-dependent locomotor defects in Fbxo7-deficient zebrafish. PLoS One 7:e48911.
Copyedited by Knight B, Maxwell R, Wang J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Yu-hao Li, M.D. or Xin Tang, M.D.,
Yu-hao Li, M.D. or Xin Tang, M.D.,
liyuhao@nankai.edu.cn or tangprofessor@aliyun.com.
#These authors contributed equally to this study.
orcid: 0000-0003-2022-9526 (Yu-hao Li) 0000-0002-7501-2837 (Xin Tang)
10.4103/1673-5374.206651
Accepted: 2017-04-02
杂志排行
中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Efficacy of intraorbital electroacupuncture for diabetic abducens nerve palsy: study protocol for a prospective single-center randomized controlled trial
- Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety
- The brain activation pattern of the medial temporal lobe during chewing gum: a functional MRI study
- Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine
- Correlation between white matter damage and gray matter lesions in multiple sclerosis patients