The brain activation pattern of the medial temporal lobe during chewing gum: a functional MRI study
2017-06-05Youn-HeeChoi,WooHyukJang,Sang-UkIm等
The brain activation pattern of the medial temporal lobe during chewing gum: a functional MRI study
The human brain is known to be influenced by environmental stimuli (Feeney et al., 1982; Kaplan, 1988). Therefore, research on the brain activation pattern by external stimuli has been an important topic in neuroscience (Kaplan, 1988). Chewing gum has been known to have a positive effect on cognition, including alertness, attention, cognitive processing speed, and memory (Stephens and Tunney, 2004; Smith, 2009; Onyper et al., 2011; Allen and Smith, 2012; Hirano et al., 2013). Many studies have reported that chewing gum can improve memory function, although there is some controversy on this topic (Nielson and Jensen, 1994; Stephens and Tunney, 2004; Tucha et al., 2004; Miles and Johnson, 2007; Smith, 2009). Chewing gum has been shown to improve memory function by enhancing glucose delivery to the brain, increasing adrenergic arousal (Stephens and Tunney, 2004), and initiating cortical activation in areas related to memory (Sesay et al., 2000; Takada and Miyamoto, 2004). However, the possible brain activation pattern of the medial temporal lobe has not been clarified so far.
The medial temporal lobe, which is essential for memory function, comprises the hippocampus, entorhinal cortex, perirhinal cortex, and parahippocampal cortex (Poldrack and Gabrieli, 1997; Eichenbaum, 2002; Brand, 2003; Squire et al., 2004; Karnik, 2009). The hippocampal-entorhinal complex is most closely related to the encoding and consolidation of information on episodic memory among declarative memory (Eichenbaum, 2002; Brand, 2003; Squire et al., 2004). Consequently, this region involves transmission of information for neocortical longterm storage (Brand, 2003).
Functional neuroimaging techniques, especially functional MRI (fMRI), have been widely used for investigation of cortical activation by external stimuli (Wexler et al., 1997; Kim et al., 2011). Since 1997, several studies have reported on brain activation in the frontal lobe, parietal lobe, and cerebellum during chewing (Sesay et al., 2000; Onozuka et al., 2002, 2003; Takada and Miyamoto, 2004; Quintero et al., 2013). However, to the best of our knowledge, there is no study on brain activation of the medial temporal lobe, which is closely related to memory function.
In this study, we attempted to investigate the brain activation pattern of the medial temporal lobe during chewing gum using fMRI. Eight healthy right-handed subjects, consisting of four males and four females, mean age of 23 ± 2.8 (range 20–29 years) years with no history of neurological, physical, or psychiatric illness were recruited into this study. The eight subjects were the college students who participated voluntarily through the recruitment notice. All subjects understood the method of fMRI of this study and provided written consent. The study protocol was approved by Yeungnam University Hospital Institutional Review Board (YUH-14-0425-D7).
The subjects were secured in a supine position with a frame. Tasks were performed using a block paradigm (21-second control, 21-second stimulation: three cycles). Chewing gum was performed using a tasteless and odorless gum (Ivoclar Vivadent AG, Schann, Liechtenstein, South Korea) in the right molars under metronome guidance (1 Hz) and gum was held in the right cheek during resting phase. Each task was performed three times.
A 1.5T Philips Gyroscan Intera scanner (Philips, Ltd.; Best, the Netherlands) was used for blood oxygenation level dependent (BOLD) fMRI. Echo Planar Imaging BOLD images were acquired over identical 20 axial sections. BOLD-weighted Echo Planar Imaging parameters were as follows: repetition time/echo time: 2 seconds/60 ms; field of view: 210 mm; flip angle: 90°; matrix size: 64 × 64, and slice thickness: 5 mm. In addition, the following parameters were employed for T1-weighted anatomical reference images: 20 axial, 5-mm slice thickness and matrix 128 × 128 (Schaechter et al., 2006). All images were acquired parallel to the anterior and posterior commissure line.
SPM 8 software (Wellcome Department of Cognitive Neurology, London, UK) was used for analysis of fMRI data. The functional images were nonlinearly realigned by slice timing and motion correction. These data were co-registered using the T1-weighted image as a template for each participant and normalized to the MNI (Montreal Neurological Institute) template (Cherubini et al., 2007). Normalized images were then smoothed using a Gaussian kernel at a full width at half maximum of 8 mm. For detection of changes in BOLD signals, control condition data were subtracted from stimulated condition data. Random-effect group analysis was used for comparison of differences in brain activations. SPMt-maps were computed, and voxels were considered significant (uncorrected threshold ofP< 0.05, more than five voxels).
For quantitative analysis of volume data mapped to the cortical surface, we used version 5.61 of the Computerized Anatomical Reconstruction and Editing Toolkit (CARET) (Van Essen, 2002). We projected functional group results onto the left hemisphere of the Human Colin surface-based atlas mapped to the PALS-B12 surface (‘Population-Average Landmark- and Surface-Based’-atlas; derived from structural MRI volumes of 12 normal young adults) (Nakahara et al., 2001; Van Essen et al., 2001; Van Essen, 2005). Data values in voxels that intersected the cortical surface were directly mapped to the vertices of each participant-specific fiducial cortical surface using the intersections of enclosing voxels and nodes. Nodes representing an individual hemisphere were deformed to the standard PALS-B12 atlas sphere with 73,730 nodes using selective landmarks and spherical alignment (Van Essen, 2005). The results of fMRI activation for the groups were mapped on the flatmap template of the PALS-B12. Regions of interest (ROIs) were set at the medial temporal cortex (the entorhinal cortex: Broadmann area [BA] 28, perirhinal cortex: BA35, and parahippocampal cortex: BA36) (Brodmann and Gary, 2006; Augustinack et al., 2014). Voxel counts were used as measures of the amounts of cortical activation.
Group analysis of fMRI data showed activation in the left medial temporal lobe, including the hippocampus, during chewing gum (Figure 1). Aaccording to BA, we observed activations in the entorhinal cortex (BA28, voxel counts: 124) and the parahippocampal cortex (BA36, voxel counts: 106). However, activation was not observed in the perirhinal cortex (BA35).
In this study, we investigated the brain activation pattern of the medial temporal lobe during chewing gum. According to our findings, the medial temporal lobe, including the hippocampus, was activated during chewing gum. In addition, the entorhinal cortex and the parahippocampal cortex were mainly activated according to BA on the flat map template. The medial temporal lobe consisted of the hippocampus and the parahippocampal region, which comprises BA28, 35, and 36 (Poldrackand Gabrieli, 1997; Eichenbaum, 2002; Brand, 2003; Squire et al., 2004; Karnik, 2009). This lobe is critical in persistence of memory representations bridging the gap between shortterm and long-term memory (Poldrack and Gabrieli, 1997; Eichenbaum, 2002; Brand, 2003; Squire et al., 2004). The parahippocampal region is an important convergence site for neocortical input to the hippocampus by receiving input from multiple cortical association areas (Poldrack and Gabrieli, 1997; Eichenbaum, 2002; Brand, 2003; Squire et al., 2004). Therefore, our results showed that activation of the hippocampus and the parahippocampal region during chewing gum might be related to memory function through functional linkage between these two brain regions. It appears that our results are coincided with the results of previous studies which demonstrated that chewing gum is related to improvement of declarative memory (Nielson and Jensen, 1994; Wilkinson et al., 2002; Stephens and Tunney, 2004). Further studies on this topic should be encouraged.
Several functional neuroimaging studies have reported cortical activation patterns induced by chewing gum in normal subjects (Momose et al., 1997; Sesay et al., 2000; Onozuka et al., 2002, 2003; Takada and Miyamoto, 2004; Quintero et al., 2013). In 1997, using Positron Emission Tomography, Momose et al. (1997) reported that chewing gum increased regional cerebral blood flow in the primary sensorimotor cortex (SM1), supplementary motor area, insula, cerebellum, and striatum. Subsequently, using xenon-enhanced computed tomography, Sesay et al. (2000) found that regional cerebral blood flow was increased in the fronto-temporal cortex, caudate nucleus, thalamus, rolandic areas, insula, cingulate, and cerebellum. Using fMRI, Onozuka et al. (2002) reported activation of the sensorimotor cortex, supplementary motor area, insula, thalamus, and cerebellum during chewing gum. The next year, the same researchers reported similar results to those from the study reported in 2002; however, they demonstrated that the cortical activation patterns differed according to the age of subjects (Onozuka et al., 2003). Furthermore, using fMRI, Takada and Miyamoto (2004) reported that chewing gum induced activation of the primary SM1, premotor area, supplementary motor area, inferior frontal cortex, middle frontal gyrus, intraparietal cortex, and superior parietal lobe. In a recent study, using fMRI, Quintero et al. (2013) reported that the precentral gyrus, post-central gyrus, posterior cerebellar lobes, rostrum of the corpus callosum, anterior cingulated gyrus, and head of the caudate nucleus were activated during chewing gum. As a result, as far as we are aware, this is the first study to demonstrate the activation pattern of the medial temporal lobe during chewing gum.
In conclusion, this study found that chewing gum induced activation of the contralateral medial temporal lobe,i.e., the hippocampus, entorhinal cortex, and parahippocamal cortex. Our results suggest that chewing gum can effectively stimulate the medial temporal lobe, which is an important area for declarative memory. However, it is a limitation of our study that we used uncorrected threshold ofPvalue < 0.05 which is not a strict value. Whether activation of the medial temporal lobe can protect against memory loss with aging or improve memory impairment in patients with brain injury should be investigated in future studies.
This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
Youn-Hee Choi, Woo Hyuk Jang, Sang-Uk Im, Keun-Bae Song, Hee-Kyung Lee, Han Do Lee, You Sung Seo, Sung Ho Jang*
Department of Preventive Dentistry, School of Dentistry, Kyungpook National University, Daegu, Republic of Korea (Choi YH, Im SU, Song KB)
Department of Occupational Therapy, College of Health Science, Kangwon National University, Samcheok, Republic of Korea (Jang WH)
Departments of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea (Lee HK, Lee HD, Seo YS, Jang SH)
*Correspondence to:Sung Ho Jang, M.D., strokerehab@hanmail.net.
Accepted:2017-02-20
orcid:0000-0001-6383-5505 (Sung Ho Jang)
Allen AP, Smith AP (2012) Effects of chewing gum and time-on-task on alertness and attention. Nutr Neurosci 15:176-185.
Augustinack JC, Magnain C, Reuter M, van der Kouwe AJ, Boas D, Fischl B (2014) MRI parcellation of ex vivo medial temporal lobe. Neuroimage 93 (Pt 2):252-259.
Brand M, Markowitsch HJ (2003) Amnesia: Neuroanatomic and Clinical Issues. New York: McGraw-Hill.
Brodmann K, Gary LJ (2006) Brodmann’s localization in the cerebral cortex: the principles of comparative localisation in the cerebral cortex based on cytoarchitectonics. New York, NY: Springer.
Cherubini A, Luccichenti G, Peran P, Hagberg GE, Barba C, Formisano R, Sabatini U (2007) Multimodal fMRI tractography in normal subjects and in clinically recovered traumatic brain injury patients. Neuroimage 34:1331-1341.
Eichenbaum H (2002) The cognitive neuroscience of memory: an introduction. Oxford; New York: Oxford University Press.
Feeney DM, Gonzalez A, Law WA (1982) Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217:855-857.
Hirano Y, Obata T, Takahashi H, Tachibana A, Kuroiwa D, Takahashi T, Ikehira H, Onozuka M (2013) Effects of chewing on cognitive processing speed. Brain Cogn 81:376-381.
Kaplan MS (1988) Plasticity after brain lesions: contemporary concepts. Arch Phys Med Rehabil 69:984-991.
Karnik M (2009) Medial temporal lobe structure and function. Saint Louis: UMI dissertation publishing.
Kim MJ, Hong JH, Jang SH (2011) The cortical effect of clapping in the human brain: A functional MRI study. NeuroRehabilitation 28:75-79.
Miles C, Johnson AJ (2007) Chewing gum and context-dependent memory effects: a re-examination. Appetite 48:154-158.
Momose T, Nishikawa J, Watanabe T, Sasaki Y, Senda M, Kubota K, Sato Y, Funakoshi M, Minakuchi S (1997) Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with (1)(5)O-labelled water and magnetic resonance imaging. Arch Oral Biol 42:57-61.
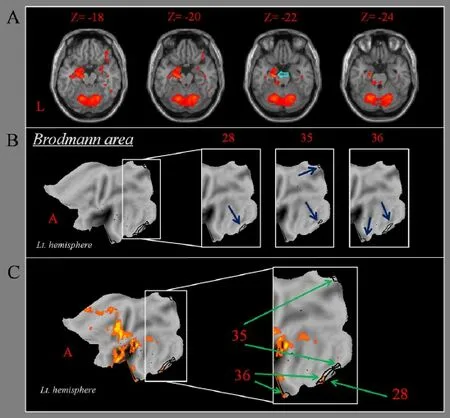
Figure 1 Brain activation and Brodmann area during chewing gum in a healthy right-handed subject.
Nakahara H, Doya K, Hikosaka O (2001) Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences - a computational approach. J Cogn Neurosci 13:626-647.
Nielson KA, Jensen RA (1994) Beta-adrenergic receptor antagonist antihypertensive medications impair arousal-induced modulation of working memory in elderly humans. Behav Neural Biol 62:190-200.
Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, Saito S (2002) Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res 81:743-746.
Onozuka M, Fujita M, Watanabe K, Hirano Y, Niwa M, Nishiyama K, Saito S (2003) Age-related changes in brain regional activity during chewing: a functional magnetic resonance imaging study. J Dent Res 82:657-660.
Onyper SV, Carr TL, Farrar JS, Floyd BR (2011) Cognitive advantages of chewing gum. Now you see them, now you don’t. Appetite 57:321-328.
Poldrack RA, Gabrieli JD (1997) Functional anatomy of long-term memory. J Clin Neurophysiol 14:294-310.
Quintero A, Ichesco E, Myers C, Schutt R, Gerstner GE (2013) Brain activity and human unilateral chewing: an fMRI study. J Dent Res 92:136-142.
Schaechter JD, Stokes C, Connell BD, Perdue K, Bonmassar G (2006) Finger motion sensors for fMRI motor studies. Neuroimage 31:1549-1559.
Sesay M, Tanaka A, Ueno Y, Lecaroz P, De Beaufort DG (2000) Assessment of regional cerebral blood flow by xenon-enhanced computed tomography during mastication in humans. Keio J Med 49 Suppl 1:A125-128.
Smith A (2009) Effects of chewing gum on mood, learning, memory and performance of an intelligence test. Nutr Neurosci 12:81-88.
Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27:279-306.
Stephens R, Tunney RJ (2004) Role of glucose in chewing gum-related facilitation of cognitive function. Appetite 43:211-213.
Takada T, Miyamoto T (2004) A fronto-parietal network for chewing of gum: a study on human subjects with functional magnetic resonance imaging. Neurosci Lett 360:137-140.
Tucha O, Mecklinger L, Maier K, Hammerl M, Lange KW (2004) Chewing gum differentially affects aspects of attention in healthy subjects. Appetite 42:327-329.
Van Essen DC (2002) Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol 12:574-579.
Van Essen DC (2005) A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage 28:635-662.
Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH (2001) An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 8:443-459.
Wexler BE, Fulbright RK, Lacadie CM, Skudlarski P, Kelz MB, Constable RT, Gore JC (1997) An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging 15:385-396.
Copyedited by Li CH, Song LP, Zhao M
10.4103/1673-5374.206656
How to cite this article:Choi YH, Jang WH, Im SU, Song KB, Lee HK, Lee HD, Seo YS, Jang SH (2017) The brain activation pattern of the medial temporal lobe during chewing gum: a functional MRI study. Neural Regen Res 12(5):812-814.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The particiants understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
杂志排行
中国神经再生研究(英文版)的其它文章
- Cerebral mechanism of puncturing at He-Mu point combination for functional dyspepsia: study protocol for a randomized controlled parallel trial
- Efficacy of intraorbital electroacupuncture for diabetic abducens nerve palsy: study protocol for a prospective single-center randomized controlled trial
- Stem cell transplantation for spinal cord injury: a meta-analysis of treatment effectiveness and safety
- Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine
- Correlation between photoreceptor injuryregeneration and behavior in a zebrafish model
- Correlation between white matter damage and gray matter lesions in multiple sclerosis patients