磁化传递成像和酰胺质子转移成像联合评价新生儿脑损伤的初步研究
2017-05-12郑阳王晓明
郑阳,王晓明
磁化传递成像和酰胺质子转移成像联合评价新生儿脑损伤的初步研究
郑阳,王晓明*

目的应用酰胺质子转移成像(amide proton transfer,APT)联合磁化传递(magnetization transfer,MT)成像对新生儿从脑内环境角度评估新生儿脑损伤。材料与方法新生儿共38名,脑损伤13例,为病例组;常规MRI检查脑内无异常25例为对照组。常规MRI检查后补充APT-MT成像扫描。测量所有新生儿双侧额叶深部白质、基底节区、枕叶深部白质的APT值以及磁化传递率(magnetization transfer ratio,MTR)值(以下用APT/MTR表示),以及病例组病灶区及其对侧的APT/MTR值。采用SPSS 19.0软件进行统计分析。结果(1)对照组中,额叶深部白质、基底节及枕叶深部白质APT/MTR值均有显著性差异(P<0.05)。(2)对照组中,APT/MTR值随孕龄增长而逐渐升高。(3)病例组病灶内APT/ MTR值低于对侧(P<0.05)。结论围产期缺氧缺血是全脑代谢变化,APT-MT成像可从内环境及分子水平评估新生儿脑损伤。
磁共振成像;新生儿;脑;酰胺质子转移成像;磁化传递成像
Key wordsMagnetic resonance imaging; Neonatal; Brain; Amide proton transfer; Magnetization transfer imaging
缺氧缺血脑损伤是围产期多种原因引起的脑组织病变,是一种全脑的缺氧缺血(hypoxic ischemic,HI)后再灌注性脑损伤。目前再灌注损伤在HIE发病中的作用日益受到重视,当脑组织由低灌注转移到再灌注时,会出现一系列病理生理改变。当脑内HI时,有氧能量代谢过程障碍[1-3],有氧代谢转为无氧代谢,无氧代谢过程产生乳酸,脑组织内乳酸增多,堆积的乳酸可以使糖代谢受到抑制,使ATP耗竭,从而使细胞内酸中毒加重[4-5]。
新生儿围产期各种原因造成的脑损伤,会引起脑组织内环境的改变。有研究表明,HI后脑组织酸中毒,哺乳动物脑组织细胞内pH值约在7.2~7.3左右,细胞外pH值约在7.3~7.4左右[6-8]。脑内pH的调节至关重要,对于脑内蛋白质的结构及酶的作用尤为明显,因此及时了解及调控细胞内pH值对脑组织改善是非常重要的。能否应用磁共振技术反映这种脑组织内环境的变化是一个新的课题,近年来一种新的磁共振内对比技术即酰胺质子转移(amide proton transfer,APT)成像[9],可以通过水的信号变化来反映蛋白质及体内酸碱度的变化。APT成像信号取决于酰胺质子和自由水质子的交换速率[10],而这种交换速率依赖于体内的酸碱度及蛋白质浓度[11-13]。APT技术正是基于上述原理利用水的信号产生内对比。目前,该技术已经在多方面进行应用研究[14-19]。
磁化传递(magnetization transfer,MT)成像是通过测量生物组织内大分子中的质子和自由水质子的相互作用来实现的[20],大分子中的质子包括与蛋白质、其他大分子、膜结合的质子。不同组织的磁化传递量是不同的,对组织的磁化传递进行定量分析,可以了解组织的特征,最常用的定量指标是磁化传递率(magnetization transfer ratio,MTR),MTR代表生物大分子完全或部分饱和所导致的信号百分比。本研究通过APT联合MT成像对新生儿脑损伤进行评估,从脑内环境角度评估新生儿脑损伤的病理生理变化。
1 材料与方法
1.1 研究对象及分组
以本院新生儿病房申请MR扫描者为研究对象,患者住院原因主要有呼吸道感染、腹泻、发热、皮肤黄染等,临床医师怀疑脑部有病变者,申请MRI检查。排除脑内占位,发育迟缓,先天畸形以及脑内代谢疾病等,其中常规MRI发现不同程度脑白质损伤和(或)脑梗死,作为病例组;与病例组孕龄相匹配(校正孕龄天数相差±2 d),且常规MRI未发现神经系统异常者,作为对照组。以上诊断均由2名及以上有经验的影像医师共同完成。以上研究设计获得本院伦理委员会批准(伦理编号:2016PS280K)。在获得患儿监护人知情同意并经过临床医生对新生儿状态评估得到允许的前提下,完成常规MRI检查后立即行APT-MT 成像扫描。所有新生儿在常规检查前 30 min给予5 %的水合氯醛(50 mg/kg)灌肠,检查时注意保暖及舒适。
1.2 常规MR扫描设备及参数
所有新生儿采用Philips 3.0 T MR仪(Achieva 3.0 T TX;Philips Healthcare Systems,Best,the Netherlands)进行扫描,笔形束,二阶匀场。体线圈发射,八通道头线圈(SENSE)接收。常规MR扫描方案:T1WI、T2WI、DWI。常规MRI序列参数如下:T1WI采用FFE序列:TR 200 ms,TE 2.3 ms,FOV 188 mm×155 mm,矩阵256×180,层厚5 mm,扫描时间36.8 s ;T2WI采用TSE序列:TR 200 ms,TE 4.6 ms,FOV 180 mm×161 mm,矩阵224×162,层厚5 mm,扫描时间42.8 s。
1.3 APT-MT序列及图像后处理
APT-MT序列:APT-MT扫描一次采集,后处理过程中分别得出APT及MT的图像及数据。所有新生儿采用横断位T1WI定位,定位于基底节层面。病例组增加显示病灶最大层面。 APT-MT采用多次偏置射频脉冲采集方法[21],连续频率饱和脉冲500 ms。APT-MT图像采集和Z谱采集相结合同时采集。在距离水的共振频率不同位置处(0,±0.25,±0.5,±0.75,±1,±1.5,±2,±2.5,±3 (2),±3.25(4),±3.5 (8),±3.75 (4),±4 (2),±4.5,±5,±6 ppm和15.6 ppm;括号里的数字代表采集的次数,没有括号标记的表示采集一次(采集多个频率饱和射频脉冲,未施加射频激励的图像作为标准化图像。这种采集方法能够校正B0场的不均匀性,得出的APT图像有较好的信噪比,并且使扫描时间能够更加接近于临床应用,单层扫描时间4 min16 s。APT-MT扫描参数如下:TR 4000 ms;TE 8.1 ms;矩阵108×71,FOV 170 mm×145 mm,层厚5 mm。将 APT-MT 原始数据导入交互式数据分析语言中的程序(IDL;Research Systems,Inc.,Boulder,CO,USA)进行分析测值并重建出伪彩图。首先,获得基于体素的Z谱(标准化的信号强度,Ssat/S0是31个偏置脉冲的函数,Ssat是施加饱和脉冲后的信号强度,S0是未施加饱和脉冲的信号强度)。通过一个12阶的多项式拟合,获得Z谱最低点的位置,得到B0场的不均匀分布,从而对Z谱进行场校正。在经过校正后的Z谱取左右对称的±3.5 ppm (APT)及15.6 ppm (MT)数据点,进行非均匀性分析[MTRasym(3.5 ppm)=Ssat(-3.5 ppm)/S0-Ssat(3.5 ppm)/ S0]、MTR=1-Ssat/S0最终获得APT及MT权重磁共振成像,APT及MTR值用百分比表示。
1.4 感兴趣区的选择及数据后处理
感兴趣区(region of interest,ROI)的选择由有经验的影像医师来完成,所有新生儿选择双侧额叶深部白质、基底节及枕叶深部白质(图1),病例组增加病灶及病灶对侧相同部位的ROI。以T1WI作为参考,小心地在APT及MT伪彩图中勾画并测量APT/MTR值。APT-MT伪彩图中信号由高到低显示为由红~蓝,相应APT/MTR值为由大到小。ROI选择注意避开颅骨、脑脊液及脑室。病例组ROI选择应在病变范围内,不超过病变边缘。
1.5 统计学分析
数据统计学处理采用软件SPSS 19.0软件处理,计量资料以均数±标准差表示。采用配对样本t检验,分析对照组各部位双侧APT/MTR值是否存在差异,若无差异,双侧APT/MTR值按部位纳入各组进行分析;采用ANOVA分析,比较对照组额叶、基底节及枕叶深部白质之间APT-MT值是否存在显著性差异。采用两样本t检验分析病例组病灶及对侧APT-MT值是否存在差异。P<0.05为差异有统计学意义。
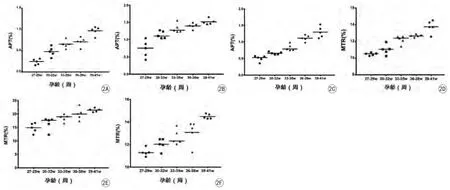
图2 对照组新生儿脑内APT/MTR值随孕龄(周)的变化。A~C:为对照组脑内额叶深部白质、基底节及枕叶深部白质APT值随孕龄变化趋势;D~F:为对照组脑内额叶深部白质、基底节及枕叶深部白质MTR值随孕龄变化趋势。脑内不同部位APT/MTR值随孕龄增长而升高Fig. 2 Changes between gestational age (weeks) and APT/MTR values of control group. Changes between gestational age (weeks) and APT values in the deep white matter of the frontal lobe (A), basal ganglia (B), and deep white matter of the occipital lobe (C). Changes between gestational age (weeks) and MTR values in the deep white matter of the frontal lobe (D), basal ganglia (E), and deep white matter of the occipital lobe (F).
2 结果
2.1 研究对象
常规MRI发现脑损伤者(新生儿脑白质不同程度损伤9例,左脑半球新发大面积脑梗死1例,局灶脑梗死3例)共13例,作为病例组。孕龄27~41周,中位年龄为34周+5 d。其中早产儿8例,中位年龄32周+1 d;足月儿5例,中位年龄37周+4 d。
与病例组孕龄匹配(校正孕龄相差≤2 d),且常规MRI脑内无异常表现新生儿25名(不包括出生窒息,先天畸形,发育落后等神经系统疾病)作为对照组,孕龄27~41周,中位年龄为36周+1 d。其中包括早产儿13例,中位年龄为32周+3 d;足月儿12例,中位年龄为37周+5 d。
2.2 对照组新生儿APT/MTR值统计及分析
对照组中,各部位(额叶深部白质、基底节及枕叶深部白质) APT/MTR值双侧均无显著性差异(P>0.05)。因此,各部位双侧APT/MTR值分别纳入各部位统计。额叶深部白质、基底节及枕叶深部白质之间APT/MTR值存在显著性差异(P<0.05),由大到小依次为:基底节,枕叶深部白质,额叶深部白质。对照组新生儿各部位(双侧额叶深部白质、基底节及枕叶深部白质) APT/MTR值随孕龄(周)增加呈升高趋势(图2,3)。
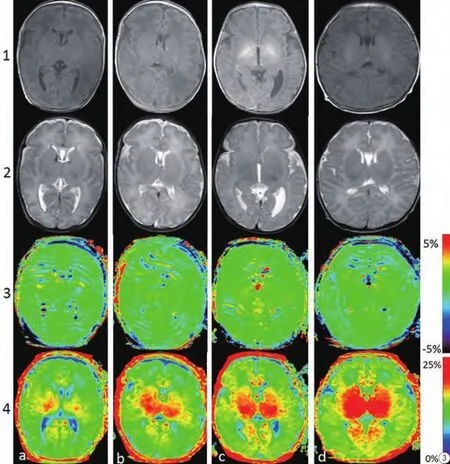
图3 对照组不同修正月龄的新生儿脑MR轴面图像。a~c列为早产儿脑(修正孕龄28周+1 d、30周+2 d、35周+5),d列为足月儿脑(40周+3 d)。第一排为T1WI,第二排为相应的T2WI,第三排为APT图像,第四排为MT图像。由图3可见,随着修正孕龄增长与髓鞘化形成,APT信号逐渐升高(受限于对比度),MT成像信号也增高(基底节显示最为明显)Fig. 3 Axial MRI Images of neonatal brain at different corrected gestational ages. Columns a—d represent images from neonates with corrected gestational ages of 28 w+1 d、30 w+2 d、35 w+ 5, and 40 w+3 d, respectively. Images from the 4 rows are as follows: row 1=T1WI; row 2=T2WI images; row 3=APT images; and row 4 = MTR images. From Figure 3, we can conclude that with increased growth associated with age, the APT signal appears to gradually increase (signal is somewhat limited by image contrast). The MT signal is increased with gestational ages.
2.3 病例组APT/MTR值统计及比较
病例组13名脑损伤新生儿,病灶侧APT/ MTR值与对侧相对正常区域进行比较,结果显示,病灶侧APT/ MTR值与对侧存在显著差异(P<0.05):病灶APT=0.45%±0.15%,病灶对侧APT=0.95%±病灶MTR=12.68%± 2.03%,病灶对MTR= 17.52%±2.12%(图4)。病变区APT/MTR信号较对侧减低(图5)。
4 讨论
新生儿脑正常状态下内环境的成分及理化性质在一定范围内保持稳态,稳态的维持对于脑细胞的生存及脑发育十分重要[6]。若发育过程中某种原因导致代谢障碍或供能不足,脑组织缺氧导致局部乳酸等代谢物堆积,脑内环境也将改变[22]。脑内MTR值的高低主要与组织中的大分子(脑内主要为髓鞘内的胆固醇、脂类)的含量有关[23],通过 MTR可以间接得到组织的内部组成,提供组织特征。早产儿与足月儿脑发育程度不同,脑内蛋白含量及髓鞘化程度不同。APT值与蛋白质浓度及pH值均呈正相关,APT-MT值越大表示蛋白质含量及半固态大分子含量的增加。APT是建立在MT技术基础之上的,基于蛋白质及多肽中的酰胺质子与水质子之间的交换,反映游离的蛋白质氨基质子浓度的变化。由于APT技术对组织内酸碱度敏感[24],在急性HI时,假定酰胺质子浓度和温度保持在恒定,APT值的变化主要反映pH的变化。
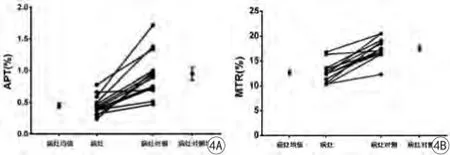
图4 病灶侧与病灶对侧APT/MTR值分布及比较。病灶内APT/MTR值明显低于病灶对侧Fig. 4 Distribution and comparison of lesion and contralateral region APT/MTR values. The APT/MTR values within lesion are lower than that of contralateral region.
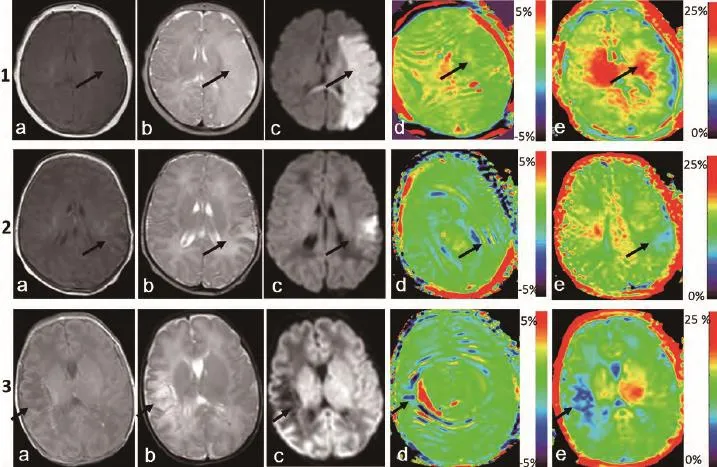
图5 病例组新生儿脑MR轴面图像。a、b、c分别是T1WI、T2WI及DWI序列,d、e分别为APT及MT伪彩图。第1行:女,38周新生儿。左脑半球新发大面积脑梗死;左脑半球呈大片长T1、长T2信号;DWI呈高信号,累及左颞、枕部及胼胝体压部;图d、e显示位于左脑半球的梗死病灶较右侧显示为低信号(黑色箭头所示,由于APT图像分辨率有限,病灶显示不如MT图像明显)。病灶及病灶对侧APT/MTR值分别为:APT:0.48%,1.50%;MTR:12.86%,18.43%。第2行:女,足月。左颞叶近期新发梗死;左颞叶DWI序列呈高信号,APT及MTR伪彩图中显示病灶(黑色箭头所示)信号减低。病灶及病灶对侧APT/MTR值分别为:APT:0.44%,0.93%;MTR:13.43%,16.76%。第3行:男,36周+5 d。右颞叶软化灶形成;右颞叶病灶DWI序列显示为低信号,d、e显示病灶(黑色箭所示)信号减低(由于软化灶形成,脑组织部分蛋白水解,病灶处水含量增多,APT图像中病灶中出现较多伪影)。病灶及病灶对侧APT/MTR值分别为:APT:0.29%,0.91%;MTR:10.25%,16.31%Fig. 5 Axial MRI Images of neonatal brain of case group. a, b and c respectively represent T1WI, T2WI and DWI sequence, figure d, e respectively represent APT and MT images. Row 1: Female, newborn, 38 weeks. Left hemisphere emerging massive cerebral infarction. Left brain hemisphere displayed as long T1 and long T2. DWI showed hyperintensity, involving left temporal, and occipital lobes and the corpus callosum. APT-MT pseudo color, with left brain hemisphere of infarction lesions (black arrow, APT=0.48%, MTR=12.86%) showing a lower signal than the right hemisphere (APT=1.50%, MTR=18.43%). Row 2: Female, full-term infant. Left temporal new cerebral infarction. Left brain hemisphere displayed as DWI hyperintensity. APT-MT pseudo color, with left temporal of infarction lesions (black arrow, APT=0.44%, MTR=13.43%) showing a lower signal than the right hemisphere (APT=0.93%, MTR=16.76%). Row 3: Male, 36 w+5 d, right temporal infarction. Right temporal displayed as DWI hyperintensity. APT-MT pseudo color, with right temporal of infarction lesions (black arrow, APT=0.29%, MTR=10.25%) showing a lower signal than the right hemisphere (APT=0.91%, MTR=16.31%). Due to the formation of softening foci, partial proteolysis of brain tissue, water content in lesions increased, some artifacts appear in APT images in the lesions.
本研究结果显示,对照组中早产儿与足月儿脑内各部位APT/MTR值不同,这是由于在不同孕龄时期,脑内发育的程度不同,脑内蛋白质浓度不同所致,而APT/MTR值技术对于蛋白质浓度敏感,能够探测到早产儿与足月儿之间的这种改变。而各部位的APT/MTR值不同,结果显示APT/ MTR值由大到小依次为:基底节区、枕叶深部白质、额叶深部白质,这与髓鞘化顺序从下至上,先中央后周围,由背侧向腹侧相一致[25-26],这正符合脑发育的顺序。当由于某种原因,如早产造成血管发育不良或脑细胞缺氧缺血时,有氧代谢过程受到障碍,能量来源转为依靠糖无氧酵解,无氧酵解产生乳酸,使细胞发生酸中毒,此时酸中毒进一步导致糖代谢受到抑制,代谢率及ATP产生率下降,从而使细胞内酸中毒恶性循环加重[27]。并且,由于无氧代谢能量不足,细胞膜无法维持正常的离子泵功能,Na+-H+、K+-H+离子交换紊乱,导致H+潴留在细胞内,造成脑组织内乳酸堆积[1,3,28],导致代谢性酸中毒,病变组织的pH值较其他正常组织低,同时,缺氧也会导致少突胶质细胞损伤[29-30],会导致发育障碍,蛋白质合成受到影响,也会导致APT/MTR值的降低。
对于缺氧缺血带来的内环境中酸碱度发生的改变,MRI常规序列是无法通过水的信号探测到的,而APT-MT技术正是一种通过组织内蛋白质浓度和酸碱度产生内对比的新技术[31]。APT成像中,酰胺质子与水的交换速率是关键因素[32-33],而这种交换速率取决于蛋白质浓度及pH值。Zhou等[10]的小鼠实验结果显示,交换速率对于体内酸碱度非常敏感,pH值每改变0.5个单位,交换速率就会发生50%~70%的改变,这种敏感性使APT技术能够应用于新生儿脑损伤的评估。
图4患儿1~3病灶处DWI序列分别为高信号、低信号,表明患儿1、2为近期新发梗死,患儿3为梗死慢性期或软化灶形成期。患儿1、2 APT/MTR值减低不如患儿3明显,即DWI序列高信号的病灶中,APT/MTR值较DWI低信号病灶高,分析其主要原因可能是新发病灶处于细胞毒性水肿期[34],脑组织蛋白质变化不如梗死慢性期或软化灶明显。梗死慢性期或软化灶内脑组织蛋白质水解,蛋白浓度减低,APT/MTR值进一步降低,故本研究观察到陈旧梗死灶APT/MTR值较新发梗死值更低。因此笔者推测,APT-MT成像能够反映病灶损伤的时期,为梗死发生的时间提供信息。具体反映梗死急性期及慢性期的APT/MTR临界值需要进一步研究。
同时,病例组结果可以得出,针对组织内pH值敏感的APT技术能够从内环境水平向发病原因更进一步进行探测及无创评估脑损伤,这在将来应用于体内无创探测组织内生化环境有很大前景。值得注意的是,APT技术是在一定条件下应用的:在急性缺氧缺血时[35],假定酰胺质子浓度基本不变,HI导致的脑组织酸中毒是APT值变化的主要原因。同时,小范围的温度波动对于交换速率的影响是忽略不计的。APT技术在临床应用之前,需要解决的问题有很多[35-37],如直接水饱和效应、磁化传递及提高信噪比,如何提供更好的图像,以及合适的扫描时间、偏置射频脉冲的能量及翻转角度等需要研究。总之,APT技术利用内源性蛋白质及酸碱度无创地成像,可以对新生儿脑损伤进行评估,对深入理解脑发育过程及理解脑损伤机制有帮助。
新生儿围产期缺氧缺血存在着内环境酸碱度的变化,APT-MT技术可以通过内环境中蛋白质浓度和酸碱度的变化来评估脑损伤,为深入理解新生儿脑损伤提供了新的思路。
[References]
[1] Liu Y, Jissendi-Tchofo P, Metens T. MR imaging, diffusion imaging, and proton MR spectroscopy at 3T in full-term neonates with hypoxic-ischemic encephalopathy. Chin J Magn Reson Imaging, 2011, 2(1): 13-18.
[2] Malik GK, Pandey M, Kumar R, et al. MR imaging and in vivo proton spectroscopy of the brain in neonates with hypoxic ischemic encephalopathy. Eur J Radiol, 2002, 43(1): 6-13.
[3] Khong PL, Tse C, Wong IY, et al. Diffusion-weighted imaging and proton magnetic resonance spectroscopy in perinatal hypoxicischemic encephalopathy: association with neuromotor outcome at 18 months of age. J Child Neurol, 2004, 19(11): 872-881.
[4] Wang HW, Wang XM, Guo QY. The correlation between DTI parameters and levels of AQP-4 in the early phases of cerebral edema after hypoxic-ischemic/reperfusion injury in piglets. Pediatr Radiol, 2012, 42(8): 992-999.
[5] Distefano G, Praticò AD. Actualities on molecular pathogenesis and repairing processes of cerebral damage in perinatal hypoxic-ischemic encephalopathy. Ital J Pediatr, 2010, 36: 63.
[6] Uria-Avellanal C, Robertson NJ. Na+/H+exchangers and intracellular pH in perinatal brain injury. Transl Stroke Res, 2014, 5(1): 79-98.
[7] Hamakawa H, Murashita J, Yamada N, et al. Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci, 2004, 58(1): 82-88.
[8] Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol, 2010, 11(1): 50-61.
[9] Schmidt H, Schwenzer NF, Gatidis S, et al. Systematic Evaluation of Amide Proton Chemical Exchange Saturation Transfer at 3 T: Effects of Protein Concentration, pH, and Acquisition Parameters. Invest Radiol, 2016, 51(10): 635-646.
[10] Zhou J, Payen J, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med, 2003, 9(8): 1085-1090.
[11] Yan K, Fu Z, Yang C, et al. Assessing amide proton transfer (APT) MRI contrast origins in 9 L gliosarcoma in the rat brain using proteomic analysis. Mol Imaging Biol, 2015, 17(4): 479-487.
[12] Zhou J, Wilson DA, Sun PZ, et al. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med, 2004, 51(5): 945-952.
[13] Jokivarsi KT, Grohn HI, Grohn OH, et al. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magn Reson Med, 2007, 57(4): 647-653.
[14] Togao O, Kessinger CW, Huang G, et al. Characterization of lung cancer by amide proton transfer (APT) imaging: an in-vivo study in an orthotopic mouse model. PLoS One, 2013, 8(10): e77019.
[15] Dula Adrienne N, Arlinghaus Lori R, Dortch Richard D, et al. Amide proton transfer imaging of the breast at 3 T: establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med, 2013, 70(1): 216-224.
[16] Klomp Dennis WJ, Dula Adrienne N, Arlinghaus Lori R, et al. Amide proton transfer imaging of the human breast at 7 T: development and reproducibility. NMR Biomed, 2013, 26(10):1271-1277.
[17] Jia G, Abaza R, Williams JD. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging, 2011, 33(3): 647-654.
[18] Gerigk L, Schmitt B, Stieltjes B. 7 Tesla imaging of cerebral radiation necrosis after arteriovenous malformations treatment using amide proton transfer (APT) imaging. J Magn Reson Imaging, 2012, 35(5): 1207-1209.
[19] Sun PZ, Zhou J, Sun W, et al. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab, 2007, 27(6): 1129-1136.
[20] Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed, 2001, 14(2): 57-64.
[21] Wen Z, Hu S, Huang F, et al. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage, 2010, 51(2): 616-622.
[22] Robertson NJ, Cowan FM, Cox IJ, et al. Brain alkaline intracellular pH after neonatal encephalopathy. Ann Neurol, 2002, 52(6): 732-742.
[23] Kucharczyk W, Macdonald PM, Stanisz GJ, et al. Relaxation and magnetization transfer of white matter lipidsat MR imaging: importance of cerebtosides and pH. Radiology, 1994, 192(2): 521-529.
[24] Zhou J. Amide proton transfer imaging of the human brain. Methods Mol Biol, 2011, 711: 227-237.
[25] Barkovich AJ, Kjos BO, Jackson DE Jr, et al. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology, 1988, 166(1 pt 1): 173-180.
[26] Oishi K, Mori S, Donohue PK, et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage, 2011, 56(1): 8-20.
[27] Wang HW, Wang XM, Guo QY. The correlation between DTI parameters and levels of AQP-4 in the early phases of cerebral edema after hypoxic-ischemic reperfusion injury in piglets. Pediatric Radiology, 2012, 42(8): 992-999.
[28] Malik GK, Pandey M, Kumar R, et al. MR imaging and in vivo proton spectroscopy of the brain in neonates with hypoxic ischemic encephalopathy. Eur J Radiol, 2002, 43(1): 6-13.
[29] Fern R, Möller T. Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci, 2000, 20(1): 34-42.
[30] Matute C, Alberdi E, Domercq M. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci, 2001, 24(4): 224-230.
[31] Zhou J, Lal B, Wilson DA, et al. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med, 2003, 50(6): 1120-1126.
[32] Sun PZ, Zhou J, Huang Judy, at el. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia. Magn Reson Med, 2007, 57(2): 405-410.
[33] Sun PZ, Wang E, Cheung JS. Imaging acute ischemic tissue acidosis with pH-sensitive endogenous amide proton transfer (APT) MRI: correction of tissue relaxation and concomitant RF irradiation effects toward mapping quantitative cerebral tissue PH. Neuroimage, 2012, 60(1): 1-6.
[34] Yilmaz U. Diffusion-weighted imaging in acute stroke. Radiologe, 2015, 5(9): 771-774.
[35] Liu D, Zhou J, Xue R, et al. Quantitative characterization of nuclear overhauser enhancement and amide proton transfer effects in the human brain at 7 tesla. Magn Reson Med, 2013, 70(4): 1070-1081
[36] Zhou J, Payen J, van Zijl PC. The interaction between magnetization transfer and blood-oxygen level-dependent effects. Magn Reson Med, 2005, 53(2): 356-366.
[37] Scheidegger R, Vinogradov E, Alsop DC. Amide proton transfer imaging with improved robustness to magnetic fi eld inhomogeneity and magnetization transfer asymmetry using saturation with frequency alternating RF irradiation. Magn Reson Med, 2011, 66(5): 1275-1285.
Evaluation of brain injury in neonates by magnetization transfer imaging combined amide proton transfer imaging: a preliminary study
ZHENG Yang, WANG Xiao-ming*
Department of Radiology, Shengjing Hospital of China Medical University, Shenyang 110004, China
*Correspondence to: Wang XM, E-mail: wangxm024@163.com
Objective:To evaluate neonatal brain injury at the internal environmental level with the application of amide proton transfer (APT) imaging and magnetization transfer (MT) imaging by measuring the APT and MTR values of the brain.Materials and Methods:A total of 38 neonatal patients who underwent MRI examination were enrolled in the study. Among them, there were 25 newborns with no abnormalities and 13 cases with brain injury who underwent conventional MRI (T1WI, T2WI, DWI) examination. After obtaining informed consent and permission of clinicians, routine MRI was followed by additional APT-MT scan. APT-MT imaging is single slice scanning, performed at the basal ganglia level in all neonates, and in the case group, with increased localization at the level of lesion, and with the contralateral relatively normal area as self-control. The APT/MTR values of bilateral frontal subcortical white matter, basal ganglia and occipital subcortical white matter were measured for all neonates, as well as the APT/MTR values of the lesion and contralateral areas. Several statistical methods were used for statistical analysis.Results:In the control group, bilateral frontal subcortical white matter, basal ganglia and occipital subcortical white matter had no signif i cant difference in APT/MTR values (P>0.05). Between the different parts of the brain, the differences among the APT/ MTR of the frontal lobes, basal ganglia, and occipital lobes were signif i cant,P<0.05. In addition, the APT/MTR values of the above brain regions were found to have a positive correlation with gestational age. In the case group, there were significant differences in APT values between the lesion side and contralateral area, being significantly lower in lesion side than the contralateral side (P<0.05).Conclusions:From changes in the pH level in the neonatal brain, APT-MT imaging can help to understand neonatal brain injury.
国家自然科学基金(编号:30570541、30770632、81271631)
中国医科大学附属盛京医院放射科,沈阳 110004
王晓明,E-mail:wangxm024@163. com
2016-12-19
接受日期:2017-01-10
R445.2;R737.9
A
10.12015/issn.1674-8034.2017.03.006
郑阳, 王晓明. 磁化传递成像和酰胺质子转移成像联合评价新生儿脑损伤的初步研究. 磁共振成像, 2017, 8(3): 189-195.
Received 19 Dec 2016, Accepted 10 Jan 2017
ACKNOWLEDGMENTSThis study was supported by National Natural Science Foundation of China (NO. 30570541, 30770632, 81271631).
