Aerobic exercise combined with huwentoxin-I mitigates chronic cerebral ischemia injury
2017-05-03haifengMaoJunXieJiaqinChenChangfaTangweiChenBocunZhouruiChenhonglinQuChuzuwu
hai-feng Mao, Jun Xie, Jia-qin Chen, Chang-fa Tang wei Chen Bo-cun Zhou rui Chen hong-lin Qu, Chu-zu wu
1 Key Laboratory of Physical Fitness and Exercise Rehabilitation of Hunan Province, Hunan Normal University, Changsha, Hunan Province, China
2 College of Physical Education, Yichun University, Yichun, Jiangxi Province, China
Aerobic exercise combined with huwentoxin-I mitigates chronic cerebral ischemia injury
hai-feng Mao1,2, Jun Xie2, Jia-qin Chen1,*, Chang-fa Tang1, wei Chen1, Bo-cun Zhou1, rui Chen1, hong-lin Qu1,2, Chu-zu wu2
1 Key Laboratory of Physical Fitness and Exercise Rehabilitation of Hunan Province, Hunan Normal University, Changsha, Hunan Province, China
2 College of Physical Education, Yichun University, Yichun, Jiangxi Province, China
How to cite this article:Mao HF, Xie J, Chen JQ, Tang CF, Chen W, Zhou BC, Chen R, Qu HL, Wu CZ (2017) Aerobic exercise combined with huwentoxin-I mitigates chronic cerebral ischemia injury. Neural Regen Res 12(4):596-602.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Funding:This work was supported by a grant from the Science and Technology Plans of Jiangxi Province Education Department of China, No. GJJ14705; a grant from the Science and Technology Plans of Health and Family Planning Commission of Jiangxi Province of China, No. 20175563.
Graphical Abstract
Neuroprotective ef f ect of huwentoxin-I (HWTX-I) in combination with exercise on mouse models of chronic cerebral ischemia
Ca2+channel blockers have been shown to protect neurons from ischemia, and aerobic exercise has signif i cant protective ef f ects on a variety of chronic diseases.e present study injected huwentoxin-I (HWTX-I), a spider peptide toxin that blocks Ca2+channels, into the caudal vein of a chronic cerebral ischemia mouse model, once every 2 days, for a total of 15 injections. During this time, a subgroup of mice was subjected to treadmill exercise for 5 weeks. Results showed amelioration of cortical injury and improved neurological function in mice with chronic cerebral ischemia in the HWTX-I + aerobic exercise group.e combined ef f ects of HWTX-I and exercise were superior to HWTX-I or aerobic exercise alone. HWTX-I ef f ectively activated the Notch signal transduction pathway in brain tissue. Aerobic exercise up-regulated synaptophysin mRNA expression.ese results demonstrated that aerobic exercise, in combination with HWTX-I, ef f ectively relieved neuronal injury induced by chronic cerebral ischemiaviathe Notch signaling pathway and promoting synaptic regeneration.
nerve regeneration; chronic cerebral ischemia; aerobic exercise; huwentoxin-I; Notch signaling pathway; calcium overload; neural regeneration
Introduction
Chronic cerebral ischemia, a sustained modest reduction in cerebral blood fl ow, is associated with neuronal damage and cognitive decline (Cechetti et al., 2012). Previous studies have focused on the mechanisms involved in chronic cerebral ischemia (Antipenko et al., 2016; Edrissi et al., 2016). In recent years, the ischemic mechanism of calcium overload in brain injury has become a hot research topic (Kostic et al., 2015; Zhang et al., 2016; Skovsted et al., 2017; Wang et al., 2017a).e Notch signaling pathway plays an important role in the regulation of cell differentiation, proliferation, and apoptosis, as well as a series of physiological and pathological processes (Huang et al., 2016; Demitrack et al., 2017; Li et al., 2017; You et al., 2017). Previous studies have shown that the Notch signaling pathway af f ects neuronal regeneration in cerebral ischemia animal models (Wang et al., 2009; Liu et al., 2011; Tao et al., 2014). Intracellular calcium overload is a main factor in apoptosis with cerebral ischemic injury (Li et al., 2015). Calcium channel blockers also provide protective ef f ects on ischemic brain injury in animal models (Maniskas et al., 2016).
In the present study, the chronic cerebral ischemia model was established in mice to investigate the ef f ects of aerobic exercise and HWTX-I on chronic cerebral ischemia.
Materials and Methods
Animals
Forty healthy, male, Kunming mice, 5–7 weeks of age and weighing 28–32 g, were provided by Hunan Slack Jingda Experimental Animals Co., Ltd., China (license No. SCXK (Xiang) 2011-0003). The study protocol was approved by the Hunan Normal University Medical Ethics Committee (Approval number: 2015(17)).e experimental design followed the national guidelines for the Care and Use of Laboratory Animals, and“Consensus author guidelines on animal ethics and welfare”by the International Association for Veterinary Editors. The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines.”
Establishment of chronic cerebral ischemia models
The mice were placed in a supine position and a 1.5-cm long skin incision was made in the upper part of the neck.e subcutaneous fat, fascia, and muscle were separated to the trachea.e right common carotid artery was separated and permanently ligated using 3-0 surgical line. The color of the right eye changed from bright-red to grayish-white following ligation of the right common carotid artery. Aer regaining consciousness, the right eye remained closed and became dark red, although it was determined that a low-f l ow blood supply was obtainedviathe Willis circle after right brain ischemia (Figure 1).ese symptoms suggested successful establishment of the chronic cerebral ischemia model (Yoshizaki et al., 2008; Zhao et al., 2014).
Drug administration
HWTX-I (200 μg/ampoule, batch number: 010620, purity: 99.5%; license number: 260085434; Sinobioway Biomedicine Co., Ltd., Xiamen, Fujian Province, China) was provided by Institute of Protein Chemistry and Molecular Biology Laboratory of Hunan Normal University, China. Four days aer group assignment, mice in the HWTX-I and HWTX-I + aerobic exercise groups were administered 0.15 mL toxin solution to the tail vein at a dose of 0.05 μg/g. Mice in the model and aerobic exercise groups were administered the same volume of physiological saline, once every 2 days, for a total of 15 times over 30 days.
Exercise training
Following model establishment, mice in the aerobic exercise and HWTX-I + aerobic exercise groups were subjected to treadmill exercise by gradually increasing the running speed and time (Figure 2) (Sheng et al., 2015) for 5 weeks as follows: a) on days 1–3 of week 1, the speed was 7 m/min for 30 minutes, with a slope of 0 degrees; b) on days 4–6 of week 1, the training time was increased by 10 minutes, and the running speed increased by 1 m/min for each training; c) on day 6, the training time was 60 minutes, and the running speed was 10 m/min; d) on day 7, the mice rested; 3) weeks 2–5, the speed was 10 m/min for 60 minutes, with a slope of 0 degrees, six times a week until the end of week 5.
Neurological function assessment
A 28-point neurological def i cit score fi rst developed by Clark et al. (1997) was used to assess neurological functions in the mice, which included focal and general functional impairment scores. Each mouse group was scored according to the Clark neurological function scale at immediately aer group assignment and at the fih weekend. A higher score represented greater damage.
Tissue extraction
Nissl staining
Tissues were embedded in paraffin wax. Tissue sections approximately 5 μm thick were prepared using a rotary microtome (Jinhua YIDI Medical Appliance Co., Ltd., Jinhua, Zhejiang Province, China).e sections were then subjected to Nissl stained using toluidine blue (ShangHai EKEAR Bio@Tech Co., Ltd., Shanghai, China).e staining was observed and photographed using a light microscope (Olympus, Tokyo, Japan).
Real-time polymerase chain reaction (RT-PCR)

Figure 1 Establishment of chronic cerebral ischemia models.

Table 1 Primers used for real time-polymerase chain reaction

Figure 2 Aerobic exercise.
Brain tissue and blood total RNA was extracted using Trizol reagent (TakaRa Biotechnology (Dalian) Co., Ltd., Dalian, Liaoning Province, China) and the Magnetic Total RNA Kit (code No. E31004; Shanghai GenePharma Co., Ltd., Shanghai, China).e absorbance value of the extracted total RNA was determined using ultraviolet spectrophotometry, with anA260/A280ratio > 1.7. PCR primers were designed using mouse cDNA sequences from Gene Bank and Primer 5.0 soware, and were synthesized by Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China (Table 1).
Genomic DNA was removed as follows: 2.0 μL 5 × g DNA Eraser Buf f er was mixed with 1.0 μL gDNA Eraser and 7.0 μL total RNA. The samples were incubated at 42°C for 2 minutes, and the reaction was terminated at 4°C. Reverse transcription: 10.0-μL reaction solution contained 1.0 μL PrimeScript RT Enzyme Mix I, 1.0 μL RT Primer Mix, and 4.0 μL 5× PrimeScript Buf f er 2. Rnase-free dH2O was added to a fi nal volume of 20 μL.e samples were incubated at 37°C for 15 minutes and 85°C for 5 seconds to obtain fi rst-strand cDNA.e reagents used in the reverse-transcription reaction were PrimeScriptTMRT reagent Kit with gDNA Eraser (TaKaRa Biotechnology (Dalian) Co., Ltd).
A SYBR®Premix Ex TaqTMII (Tli RNaseH Plus) kit (Ta-KaRa Biotechnology (Dalian) Co., Ltd.) was used for RTPCR. The total reaction volume was 20 μL and contained the following: 2 μL cDNA template, 10 μL SYBR®Premix Ex Taq II (Tli RNaseH Plus) (2×), 0.8 μL forward primer, 0.8 μL reverse primer, 0.4 μL ROX-Reference Dye II (50×), 6 μL nuclease-free water. Reactions were prepared on ice. PCR was performed using an ABI 7500 Fast Real-time PCR System (Foster, CA, USA) at 95°C for 30 seconds, 95°C for 3 seconds, and 60°C for 30 seconds for 40 cycles. Data were processed using an ABI 7500 Fast.
Statistical analysis
All data, expressed as the mean ± SD, were analyzed with SPSS 22.0 software (IBM, Armonk, NY, USA). The sample means were compared using one-way analysis of variance.P< 0.05 represented a signif i cant difference.
Results
Ef f ects of aerobic exercise and HWTX-I on neurological function in chronic cerebral ischemia mice
As shown in Figure 3, there was no signif i cant difference in neurological def i cits between the groups prior to any interventions. At the fifth weekend, compared with the model group, focal and general neurological deficit scores significantly decreased in the intervention groups (P< 0.01). Compared with the HWTX-I group, general neurological def i cit scores signif i cantly decreased in the HWTX-I + aerobic exercise group (P< 0.05).
Ef f ects of aerobic exercise and HWTX-I on morphological changes in the cerebral cortex of chronic cerebral ischemia mice

Figure 3 Ef f ects of aerobic exercise and HWTX-I on neurological function in mice with chronic cerebral ischemia.

Figure 4 Ef f ects of aerobic exercise and HWTX-I on morphological changes in the cerebral cortex of chronic cerebral ischemia mice (Nissl staining, × 200).
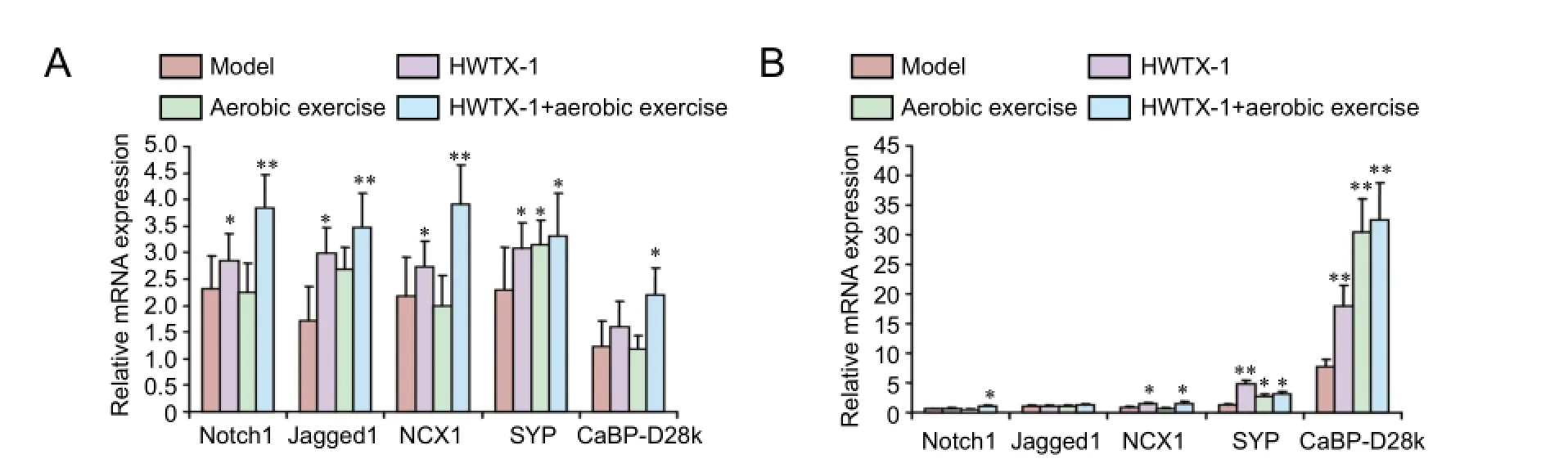
Figure 5 Ef f ects of aerobic exercise and HWTX-I on Notch1, Jagged1, NCX1, SYP, and CaBP-D28k mRNA expressions in the brain (A) and blood (B) of chronic cerebral ischemia mice (real-time polymerase chain reaction).
Ischemia results in cellular morphological changes (Kitabatake et al., 2015; Arteaga et al., 2016; Zhang et al., 2017). To determine the neuroprotective ef f ects of aerobic exercise and HWTX-1 on these morphological changes, we analyzed Nissl staining in the cerebral cortex. In the model group, neurons in the cerebral cortex were deeply stained, with the presence of pyknosis, obvious vacuoles, nuclear necrosis, and distinct neuronal loss. In the HWTX-I group, vacuoles were not obvious, part of the nuclei and Nissl bodies were visible in the cerebral cortex, but the neurons were darkly stained and exhibited pyknosis. In the aerobic exercise group, the cerebral cortex exhibited a distinct loss of neurons and vacuoles; the neurons were darkly stained, and some neurons exhibited pyknosis, although morphology was better than in the model group. In the HWTX-I + aerobic exercise group, there were less vacuoles in the cortical neurons; the nuclei were lightly stained and exhibited distinct nucleoli; although pyknosis was still detectible, there was less neuronal loss; the Nissl bodies were clear and gathered to the periphery in the cytoplasm (Figure 4).
Ef f ects of aerobic exercise and HWTX-I on Notch1, Jagged1, NCX1, synaptophysin (SYP), and CaBP-D28k mRNA expression in the blood and brains of chronic cerebral ischemia mice
As displayed in Figure 5A, mRNA expressions of Notch1, Jagged1, NCX1, SYP, and CaBP-D28k were highest in the HWTX-I + aerobic exercise group compared with the model, HWTX-1, and aerobic exercise groups. Compared withthe model group, mRNA expressions of Notch1, Jagged1, NCX1, and SYP were signif i cantly decreased in brain tissue of mice in the HWTX-I group (P< 0.05). Compared with the model group, SYP mRNA expression was significantly increased in brain tissue of mice in the aerobic exercise group (P< 0.05). Compared with the model group, mRNA expressions of Notch1, Jagged1, NCX1, SYP, and CaBP-D28k were significantly increased in brain tissues of mice in the HWTX-I + aerobic exercise group (P< 0.01 orP< 0.05).
As shown in Figure 5B, mRNA expressions of Notch1, Jagged1, NCX1, and CaBP-D28k in the HWTX-I + aerobic exercise group, and SYP in the HWTX-I group, were highest. Compared with the model group, mRNA expressions of NCX1, SYP, and CaBP-D28k were signif i cantly increased in the blood of mice in the HWTX-I group (P< 0.01 orP<0.05). Compared with the model group, mRNA expressions of CaBP-D28k and SYP were significantly increased in the aerobic exercise group (P< 0.01 orP< 0.05). Compared with the model group, mRNA expressions of Notch1, NCX1, SYP, and CaBP-D28k were signif i cantly increased in the HWTX-I + aerobic exercise group (P< 0.01 orP< 0.05).
Discussion
Shamsaei et al. (2015) confirmed a sparse distribution of hippocampal CA1 neurons in rats with cerebral ischemia, with a signif i cantly decreased number of normal cells. Naderi et al. (2017) found that minocycline pretreatment signif i cantly attenuated ischemia-induced pyramidal cell death and microglial activation in the CA1 region, and provided neuroprotective ef f ects on cerebral ischemia-induced memory def i cits. Shamsaei et al. (2015) used Nissl staining to show that pre-ischemic exercise significantly reduced necrotic cell death in the hippocampal CA1 region and prevented memory def i cits aer cerebral ischemia. Wang et al. (2017b) described a series of 11 studies that showed the signif i cant effects of astragaloside IV on ameliorating neurological function scores following ischemic stroke.ese studies suggested that different interventions exert neuroprotective effects following cerebral ischemia/reperfusion injury through antioxidant, anti-inf l ammatory, or anti-apoptotic properties.
SYP is a 38-kDa integral membrane protein closely related to synaptic structure and function; it has been identified in almost all synaptic terminals of the central and peripheral nervous systems. Pinheiro et al. (2014) showed that SYP protein expression was strongly associated with synaptic formation and function, and SYP protein expression decreased aer ischemia. Hou et al. (2011) suggested that willed movement therapy promotes recovery of neurological functions in rats with cerebral ischemia, which could be related to increased SYP expression. He et al. (2017) showed that bone marrow stromal cell treatment signif i cantly improved neurobehavioral performance following ischemic brain injury by SYP and growth-associated protein 43 expression. Fonteles et al. (2016) showed that increased synaptogenic activity and anti-inflammatory action exert protective effects on memory in a permanent middle cerebral artery occlusion mouse model. Results from the present study suggested that the aerobic exercise-induced increase in SYP mRNA expression following cerebral ischemic injury is responsible for recovery of neurological function.
The calcium-binding protein CaBP-D28k is expressed in neurons of most brain regions, and CaBP-D28k-positive neurons have a direct relationship with ischemic brain injury (Yenari et al., 2001). Ca2+overload plays a role in ischemia-reperfusion injury in neurons. Neuronal injury can be delayed or avoided using voltage-gated calcium channel inhibitors to prevent Ca2+inf l ux. CaBP-D28k selectively binds with Ca2+and down-regulates intracellular-free calcium levels. Ouh et al. (2013) determined that ischemic damage reduces expression of calcium-binding proteins, thereby leading to cell death. Results from Lee et al. (2013) showed that longer maintenance of calcium-binding proteins may contribute to less and more delayed neuronal death/damage in young animals. Yenari et al. (2001) concluded that Calbindin D28K overexpression leads to neuroprotection in an animal model of central nervous system injury. Sodium-calcium exchangers (NCX) are ion transporters that are widely expressed in animal cell membranes. Ca2+concentration stability in cells is maintained by pumping Ca2+out from the cytoplasm using reverse transport. It is believed that NCX1 plays a protective role in neuronal injury (Boscia et al., 2012; Vinciguerra et al., 2014; Secondo et al., 2015). NCX1 and the calcium-binding protein Calretinin cooperate within the striatum to confer tolerance against cerebral ischemia (Boscia et al., 2016). Results from the Formisano et al. (2013) study demonstrate that the RE1-silencing transcription factor may represent a potential drug target for treating brain ischemia by regulating NCX1 expression.is resulted in delayed or reduced neuronal damage, andthe ef f ect was increased through aerobic exercise. Although the Ca2+overload took place during the time window prior to intervention, HWTX-I increased NCX1 expression and promoted Ca2+outf l ow, thereby reducing the Ca2+concentration and delaying neuronal damage. Circulating nucleic acid includes circulating DNA and RNA. Recently, the detection of nucleic acid molecules in peripheral blood circulation has become a hot research topic (Guo et al., 2017; Huang et al., 2017). Although circulating nucleic acid can serve as a sensitive and ef f ective tumor biomarker (Roth et al., 2011; Perkins et al., 2012; Greenberg et al., 2014), very little is known about the application of circulating RNA in cerebral ischemic injury. Our results showed that Notch1 and NCX1 mRNA expression in peripheral blood coincided with a low degree of injury, suggesting that these molecules could serve as peripheral blood markers for cerebral ischemia detection.
Results from this study suggested that HWTX-I and moderate-intensity treadmill exercise for 5 weeks alone and used as a combined intervention in a mouse model of chronic cerebral ischemia effectively reduced brain tissue damage. Aerobic exercise aer cerebral ischemia resulted in increased SYP mRNA expression, suggesting a role for SYP in the recovery of neurological function. Although both HWTX-1 and exercise reduced injury, the mechanisms of action were different. HWTX-I combined with aerobic exercise was superior to HWTX-I or aerobic exercise alone.
Acknowledgments:We are grateful to Dr. Zhong-hua Liu at the Department of Biochemistry, College of Life Sciences , Hunan Normal University of China for his useful suggestions in this study.
Author contributions:JQC and CFT conceived and designed the study. HFM, JX, WC, BCZ, HLQ, and CZW performed the experiments. HFM and RC wrote the paper. All authors approved the f i nal version of the paper.
Conf l icts of interest:None declared.
Plagiarism check:This paper was screened twice using CrossCheck to verify originality before publication.
Peer review:This paper was double-blinded and stringently reviewed by international expert reviewers.
Antipenko EA, Deryugina AV, Gustov AV (2016) The system stress-limiting action of mexidol in chronic cerebral ischemia. Zh Nevrol Psikhiatr Im S S Korsakova 116:28-31.
Arteaga O, Revuelta M, Urigüen L, Martínez-Millán L, Hilario E, Álvarez A (2016) Docosahexaenoic acid reduces cerebral damage and ameliorates long-term cognitive impairments caused by neonatal hypoxia–ischemia in rats. Mol Neurobiol doi: 10.1007/s12035-016-0221-8:1-19.
Boscia F, D’Avanzo C, Pannaccione A, Secondo A, Casamassa A, Formisano L, Guida N, Sokolow S, Herchuelz A, Annunziato L (2012) Silencing or knocking out the Na(+)/Ca(2+) exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ 19:562-572.
Boscia F, Casamassa A, Secondo A, Esposito A, Pannaccione A, Sirabella R, Pignataro G, Cuomo O, Vinciguerra A, de Rosa V, Annunziato L (2016) NCX1 exchanger cooperates with calretinin to confer preconditioning-induced tolerance against cerebral ischemia in the striatum. Mol Neurobiol 53:1365-1376.
Cechetti F, Worm PV, Elsner VR, Bertoldi K, Sanches E, Ben J, Siqueira IR, Netto CA (2012) Forced treadmill exercise prevents oxidative stress and memory def i cits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem 97:90-96.
Chen JQ, Zhang YQ, Dai J, Luo ZM, Liang SP (2005) Antinociceptive ef f ects of intrathecally administered huwentoxin-I, a selective N-type calcium channel blocker, in the formalin test in conscious rats. Toxicon 45:15-20.
Clark WM, Lessov NS, Dixon MP, Eckenstein F (1997) Monof i lament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res 19:641-648.
Colbourne F, Li H, Buchan AM, Clemens JA (1999) Continuing postischemic neuronal death in CA1: inf l uence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke 30:662-668.
Demitrack ES, Gif f ord GB, Keeley TM, Horita N, Todisco A, Turgeon DK, Siebel CW, Samuelson LC (2017) NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol 312:G133-144.
Edrissi H, Schock SC, Hakim AM,ompson CS (2016) Microparticles generated during chronic cerebral ischemia increase the permeability of microvascular endothelial barriers in vitro. Brain Res 1634:83-93.
Fonteles AA, de Souza CM, de Sousa Neves JC, Menezes AP, Santos do Carmo MR, Fernandes FD, de Araujo PR, de Andrade GM (2016) Rosmarinic acid prevents against memory def i cits in ischemic mice. Behav Brain Res 297:91-103.
Formisano L, Guida N, Valsecchi V, Pignataro G, Vinciguerra A, Pannaccione A, Secondo A, Boscia F, Molinaro P, Sisalli MJ, Sirabella R, Casamassa A, Canzoniero LM, Di Renzo G, Annunziato L (2013) NCX1 is a new rest target gene: role in cerebral ischemia. Neurobiol Dis 50:76-85.
Geng X, Sun T, Li JH, Zhao N, Wang Y, Yu HL (2015) Electroacupuncture in the repair of spinal cord injury: inhibiting the Notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res 10:394-403.
Greenberg ES, Chong KK, Huynh KT, Tanaka R, Hoon DS (2014) Epigenetic biomarkers in skin cancer. Cancer Lett 342:170-177.
Guo Y, Vickers K, Xiong Y, Zhao S, Sheng Q, Zhang P, Zhou W, Flynn CR (2017) Comprehensive evaluation of extracellular small RNA isolation methods from serum in high throughput sequencing. BMC Genomics 18:50.
Ha YP, Wang ZL, Lei H, Ding RR, Jiang XF, Wang KK, Shen ZH, Jie W (2016) Ef f ects of over-expression of Notch1 intracellular domain on the differentiation of c-Kit+ bone marrow mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu 20:785-792.
He X, Jiang L, Dan QQ, Lv Q, Hu Y, Liu J, Wang SF, Wang TH (2017) Bone marrow stromal cells promote neuroplasticity of cerebral ischemic rats via a phosphorylated CRMP2-mediated mechanism. Behav Brain Res 320:494-503.
Hou DR, Shawuti S, Liu JF, Zhu HX, Deng JF, Hu ZY, Zhou J, Liu YX (2011) Ef f ect of willed movement therapy on GFAP and SYP expression in rats with cerebral ischemia-reperfusion. Nan Fang Yi Ke Da Xue Xue Bao 31:1543-1546.
Huang T, Zhou Y, Cheng AS, Yu J, To KF, Kang W (2016) NOTCH receptors in gastric and other gastrointestinal cancers: oncogenes or tumor suppressors? Mol Cancer 15:80.
Huang X, O’Connor R, Kwizera EA (2017) Gold nanoparticle based platforms for circulating cancer marker detection. Nanotheranostics 1:80-102.
Kitabatake TT, Marini Lde C, Goncalves RB, Bertolino G, de Souza HC, de Araujo JE (2015) Behavioral ef f ects and neural changes induced by continuous and not continuous treadmill training, post bilateral cerebral ischemia in gerbils. Behav Brain Res 291:20-25.
Kostic M, Ludtmann MH, Bading H, Hershf i nkel M, Steer E, Chu CT, Abramov AY, Sekler I (2015) PKA Phosphorylation of NCLX reverses mitochondrial calcium overload and depolarization, promoting survival of PINK1-def i cient dopaminergic neurons. Cell Rep 13:376-386.
Lee YJ, Yan BC, Park JH, Ahn JH, Kim IH, Lee JC, Lee HY, Kim YM, Won MH, Cho JH (2013) Differences of calcium binding proteins immunoreactivities in the young hippocampal CA1 region from the adult following transient ischemic damage. J Neurol Sci 326:40-47.
Li GJ, Yang Y, Yang GK, Wan J, Cui DL, Ma ZH, Du LJ, Zhang GM (2017) Slit2 suppresses endothelial cell proliferation and migration by inhibiting the VEGF-Notch signaling pathway. Mol Med Rep 15:1981-1988.
Li LH, Tian XR, Hu ZP (2015)e key target of neuroprotection aer the onset of ischemic stroke: secretory pathway Ca(2+)-ATPase 1. Neural Regen Res 10:1271-1278.
Lin L, Cai WM, Qin CJ, Miao LC, Yun LT, Hua Y, Weilin L (2012) Intervention of TLR4 signal pathway cytokines in severe liver injury with obstructive jaundice in rats. Int J Sports Med 33:572-579.
Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG (2011) MicroRNA prof i ling in subventricular zone aer stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6:e23461.
Maniskas ME, Roberts JM, Aron I, Fraser JF, Bix GJ (2016) Stroke neuroprotection revisited: Intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J Cereb Blood Flow Metab 36:721-730.
Naderi Y, Sabetkasaei M, Parvardeh S, Moini Zanjani T (2017) Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol 28:214-222.
Ouh IO, Kim YM, Gim SA, Koh PO (2013) Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells. Lab Anim Res 29:156-161.
Perez-Pinzon MA, Yenari MA, Sun GH, Kunis DM, Steinberg GK (1997) SNX-111, a novel, presynaptic N-type calcium channel antagonist, is neuroprotective against focal cerebral ischemia in rabbits. J Neurol Sci 153:25-31.
Perkins G, Yap TA, Pope L, Cassidy AM, Dukes JP, Riisnaes R, Massard C, Cassier PA, Miranda S, Clark J, Denholm KA,way K, Gonzalez De Castro D, Attard G, Molife LR, Kaye SB, Banerji U, de Bono JS (2012) Multi-purpose utility of circulating plasma DNA testing in patients with advanced cancers. PLoS One 7:e47020.
Pinheiro Fernandes FD, Fontenele Menezes AP, de Sousa Neves JC, Fonteles AA, da Silva AT, de Araujo Rodrigues P, Santos do Carmo MR, de Souza CM, de Andrade GM (2014) Caffeic acid protects mice from memory def i cits induced by focal cerebral ischemia. Behav Pharmacol 25:637-647.
Roth C, Pantel K, Müller V, Rack B, Kasimir-Bauer S, Janni W, Schwarzenbach H (2011) Apoptosis-related deregulation of proteolytic activities and high serum levels of circulating nucleosomes and DNA in blood correlate with breast cancer progression. BMC Cancer 11:4.
Secondo A, Pignataro G, Ambrosino P, Pannaccione A, Molinaro P, Boscia F, Cantile M, Cuomo O, Esposito A, Sisalli MJ, Scorziello A, Guida N, Anzilotti S, Fiorino F, Severino B, Santagada V, Caliendo G, Di Renzo G, Annunziato L (2015) Pharmacological characterization of the newly synthesized 5-amino-N-butyl-2-(4-ethoxyphenoxy)-benzamide hydrochloride (BED) as a potent NCX3 inhibitor that worsens anoxic injury in cortical neurons, organotypic hippocampal cultures, and ischemic brain. ACS Chem Neurosci 6:1361-1370.
Shamsaei N, Khaksari M, Erfani S, Rajabi H, Aboutaleb N (2015) Exercise preconditioning exhibits neuroprotective ef f ects on hippocampal CA1 neuronal damage after cerebral ischemia. Neural Regen Res 10:1245-1250.
Sheng ZJ, Qin CJ, Wei CW, Miao LC, Hua ZG, Rui C, Lin L, Cai WM (2015)e ef f ect of aerobic exercise and Macrothele raven venom on tumor-bearing mice. Int J Sports Med 36:93-100.
Skovsted GF, Kruse LS, Berchtold LA, Grell AS, Warfvinge K, Edvinsson L (2017) Myocardial ischemia-reperfusion enhances transcriptional expression of endothelin-1 and vasoconstrictor ET(B) receptors via the protein kinase MEK-ERK1/2 signaling pathway in rat. PLoS One 12:e0174119.
Sun F, Mao X, Xie L, Ding M, Shao B, Jin K (2013) Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell 12:978-987.
Tao J, Chen B, Gao Y, Yang S, Huang J, Jiang X, Wu Y, Peng J, Hong Z, Chen L (2014) Electroacupuncture enhances hippocampal NSCs proliferation in cerebral ischemia-reperfusion injured rats via activation of notch signaling pathway. Int J Neurosci 124:204-212.
Valentino K, Newcomb R, Gadbois T, Singh T, Bowersox S, Bitner S, Justice A, Yamashiro D, Hof f man BB, Ciaranello R, et al. (1993) A selective N-type calcium channel antagonist protects against neuronal loss aer global cerebral ischemia. Proc Natl Acad Sci U S A 90:7894-7897.
Vinciguerra A, Formisano L, Cerullo P, Guida N, Cuomo O, Esposito A, Di Renzo G, Annunziato L, Pignataro G (2014) MicroRNA-103-1 selectively downregulates brain NCX1 and its inhibition by anti-miRNA ameliorates stroke damage and neurological def i cits. Moler 22:1829-1838.
Wang HL, Zhou QH, Xu MB, Zhou XL, Zheng GQ (2017a) Astragaloside IV for Experimental focal cerebral ischemia: preclinical evidence and possible mechanisms. Oxid Med Cell Longev 2017:8424326.
Wang L, Chopp M, Zhang RL, Zhang L, Letourneau Y, Feng YF, Jiang A, Morris DC, Zhang ZG (2009)e Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells aer stroke. Neuroscience 158:1356-1363.
Wang N, Zhang L, Lu Y, Zhang M, Zhang Z, Wang K, Lv J (2017b) Down-regulation of microRNA-142-5p attenuates oxygen-glucose deprivation and reoxygenation-induced neuron injury through up-regulating Nrf2/ARE signaling pathway. Biomed Pharmacother 89:1187-1195.
Wang YR, Liu RY, Wang LC, Mao HF, Chen JQ (2007) Effect of Huwentoxin-I on the Fas and TNF apoptosis pathway in the hippocampus of rat with global cerebral ischemia. Toxicon 50:1085-1094.
Wen Tao Z, Gu Yang T, Ying R, Mao Cai W, Lin L, Chi Miao L, Peng H, Joa Qin C (2011)e antinociceptive efficacy of HWTX-I epidurally administered in rheumatoid arthritis rats. Int J Sports Med 32:869-874.
Yenari MA, Palmer JT, Sun GH, de Crespigny A, Mosely ME, Steinberg GK (1996) Time-course and treatment response with SNX-111, an N-type calcium channel blocker, in a rodent model of focal cerebral ischemia using diffusion-weighted MRI. Brain Res 739:36-45.
Yenari MA, Minami M, Sun GH, Meier TJ, Kunis DM, McLaughlin JR, Ho DY, Sapolsky RM, Steinberg GK (2001) Calbindin d28k overexpression protects striatal neurons from transient focal cerebral ischemia. Stroke 32:1028-1035.
Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H (2008) Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol 210:585-591.
You K, Sun P, Yue Z, Li J, Xiong W, Wang J (2017) NOR1 promotes hepatocellular carcinoma cell proliferation and migration through modulating the Notch signaling pathway. Exp Cell Res 352:375-381.
Zhang H, Lai Q, Li Y, Liu Y, Yang M (2017) Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J Ethnopharmacol 196:225-235.
Zhang HP, Sun YY, Chen XM, Yuan LB, Su BX, Ma R, Zhao RN, Dong HL, Xiong L (2014)e neuroprotective ef f ects of isof l urane preconditioning in a murine transient global cerebral ischemia-reperfusion model: the role of the Notch signaling pathway. Neuromolecular Med 16:191-204.
Zhang L, Cao S, Deng S, Yao G, Yu T (2016) Ischemic postconditioning and pinacidil suppress calcium overload in anoxia-reoxygenation cardiomyocytes via down-regulation of the calcium-sensing receptor. PeerJ 4:e2612.
Zhao Y, Gu JH, Dai CL, Liu Q, Iqbal K, Liu F, Gong CX (2014) Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral def i cits in mice. Front Aging Neurosci 6:10.
Zhao Y, Deng B, Li Y, Zhou L, Yang L, Gou X, Wang Q, Chen G, Xu H, Xu L (2015) Electroacupuncture pretreatment attenuates cerebral ischemic injury via notch pathway-mediated up-regulation of hypoxia inducible factor-1alpha in rats. Cell Mol Neurobiol 35:1093-1103.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Jia-qin Chen, M.D., chenjiaqin2010@sina.com.
Jia-qin Chen, M.D., chenjiaqin2010@sina.com.
orcid: 0000-0002-2968-9193 (Jia-qin Chen)
10.4103/1673-5374.205099
Accepted: 2017-02-28
杂志排行
中国神经再生研究(英文版)的其它文章
- Recovery of multiply injured ascending reticular activating systems in a stroke patient
- Neuroprotective mechanism of Kai Xin San: upregulation of hippocampal insulin-degrading enzyme protein expression and acceleration of amyloid-beta degradation
- Mitomycin C induces apoptosis in human epidural scar fi broblasts after surgical decompression for spinal cord injury
- Exenatide promotes regeneration of injured rat sciatic nerve
- Recombinant human fi broblast growth factor-2 promotes nerve regeneration and functional recovery after mental nerve crush injury
- Ca2+involvement in activation of extracellular-signalregulated-kinase 1/2 and m-calpain after axotomy of the sciatic nerve
