Recovery of multiply injured ascending reticular activating systems in a stroke patient
2017-05-03SungHoJang,HanDoLee
Recovery of multiply injured ascending reticular activating systems in a stroke patient
Consciousness is controlled by activation of the ascending reticular activating system (ARAS).e ARAS consists mainly of the lower and upper parts between the thalamus and cerebral cortex (Edlow et al., 2012; Yeo et al., 2013; Jang et al., 2014). Because the ARAS is composed of several neuronal circuits connecting the brainstem to the cortex.ese neuronal connections begin from the reticular formation (RF) of the brainstem and the intralaminar nucleus of thalamus to the cerebral cortex (Gosseroes et al., 2011). In addition, the ARAS system also includes several brainstem nuclei (such as dorsal raphe, locus coeruleus, pedunculopontine nucleus, median raphe and parabrachial nucleus), non-specif i c thalamic nuclei, hypothalamus, and basal forebrain (Fuller et al., 2011).
Development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has enabled three-dimensional reconstruction and estimation of the ARAS of the live human brain (Yeo et al., 2013; Jang et al., 2014). Many studies using DTT have demonstrated injury of the ARAS in various brain pathologies including subarachnoid hemorrhage, cerebral infarction, traumatic brain injury, intracerebral hemorrhage, and hypoxic ischemic brain injury (Jang et al., 2015a, b; Jang and Kim, 2015; Jang and Lee, 2015a; Jang and Seo, 2015), while only a few studies have reported on the recovery of an injured ARAS in patients with brain injury (Jang et al., 2015c, 2016; Jang and Lee, 2015b).
In this study, we report on a stroke patient who showed recovery of a multiply injured ARAS using DTT.
A 57-year-old male patient with a spontaneous intraventricular hemorrhage (IVH) and lebasal ganglia hemorrhage (ICH) underwent bilateral frontal extraventricular drainage (EVD) for IVH (Figure 1A). One month from symptom onset, he was transferred to the department of rehabilitation. He exhibited impaired alertness, with a Glasgow Coma Scale (GCS) score of 9 (eye opening: 4, best verbal response: 1, and best motor response: 4) and a Coma Recovery Scale-Revised (CRS-R) score of 5 (auditory function: 0, visual function: 0, motor function: 3, verbal function: 0, communication: 0, and arousal: 2) (Teasdale and Jennett, 1974; Giacino et al., 2004). He showed quadriplegia of motor function (shoulder abductor: 0/0, elbow flexor: 0/0, fi nger extensor: 0/0, hip fl exor: 0/0, knee extensor: 0/0 and ankle dorsiflexor: 0/0). Brain MR images taken 1 month after onset showed multiple leukomalactic lesions in both frontal lobes, left subcortical white matter and basal ganglia (Figure 1A). He underwent comprehensive rehabilitative therapy, which included neurotropic drugs (pramipexole 3 mg, ropinirole 3 mg, amantadine 300 mg and levodopa 750 mg), physical therapy, occupational therapy until 7 months aer symptom onset. At 7 months aer onset, his GCS score had recovered to 15 (eye opening: 4, best verbal response: 5, and best motor response: 6) with a GRS-R score of 22 (auditory function: 4, visual function: 5, motor function: 6, verbal function: 3, communication: 1, arousal: 3). He presented some recovery of the lehemiplegia aer 7 months (shoulder abductor: 0/4–, elbow fl exor: 0/4–, fi nger extensor: 0/3, hip fl exor: 0/3, knee extensor: 0/3 and ankle dorsif l exor: 0/2+). However, we could not perform Mini-Mental State Exam (MMSE) evaluation at 1 and 7 months due to poor awareness and cognition. The patient’s wife provided signed, informed consent and our institutional review board approved the study protocol.
DTI data were acquired twice (1 and 7 months after onset) using a 6-channel head coil on a 1.5 T Philips Gyroscan Intera (Philips, Best, The Netherlands) with single-shot echo-planar imaging. For each of the 32 non-collinear diffusion sensitizing gradients. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed matrix = 192 × 192; fi eld of view = 240 × 240 mm2; repetition time = 10,726 ms; echo time = 76 ms;b= 1,000 s/mm2; number of excitations = 1; and a slice thickness of 2.5 mm with no gap. Analysis of diffusion-weighted imaging data was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Soware Library (FSL; www.fmrib.ox.ac.uk/fsl). Fiber tracking was performed using a probabilistic tractography method based on a multif i ber model, and applied in the current study utilizing tractography routines implemented in FMRIB diffusion (Behrens et al., 2007).
For the lower dorsal ARAS, the seed region of interest (ROI) was given at the RF of the pons and the target ROI was given at the thalamic intralaminar nucleus (ILN) (Yeo et al., 2013). For the lower ventral ARAS, the seed ROI was given at the pontine RF and the target ROI was given at the hypothalamus (Jang and Kwon, 2015). Finally, for the neural connectivity of the upper ARAS, the seed ROI was given at the ILN (Jang et al., 2014). Out of 5,000 samples generated from the seed voxel, results for contact were visualized at a threshold for the lower (dorsal andventral) ARAS of a minimum of 2, for upper neural connectivity of 15 streamlined through each voxel for analysis.
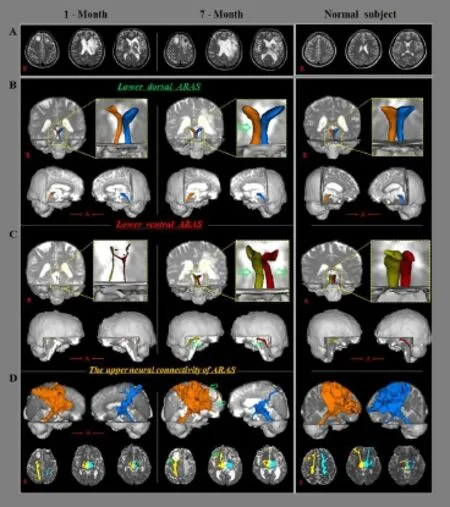
Figure 1 Brain magnetic resonance images (Mri) and diffusion tensor tractography (DTT) of a 57-year-old male patient with multiply injured ascending reticular activating systems (ArAs).
On 1-month DTT images, narrowing was observed in the right lower dorsal ARAS and bilateral lower ventral ARAS, the neural connectivity between the thalamic of ILN and the cerebral cortex was decreased in bilateral prefrontal cortices and basal forebrains (Figure 1B, C). By contrast, on 7-month DTT images, the three narrowed neural tracts of the lower dorsal and ventral ARAS were thickened, and the neural connectivity of the upper ARAS to the right prefrontal cortex and basal forebrain was increased (Figure 1B, C).
In this study, three portions of the ARAS (the lower dorsal and ventral ARAS, and upper ARAS) were evaluated in a stroke patient using DTT. Multiple injuries of the ARAS were observed on 1-month DTT images as follows: the right lower dorsal and bilateral lower ventral ARAS were narrowed, and the neural connectivity of the upper ARAS from the thalamic ILN to bilateral prefrontal cortices and basal forebrains was decreased. These fi ndings appear to suggest injury of the lower dorsal and ventral ARAS and the upper ARAS. Previous studies have reported on injury of lower ARAS in patients with stroke using DTT (Jang et al., 2015a, b; Jang and Seo, 2015). A few studies have demonstrated the injury of lower ARAS due to transtentorial herniation and Kernohan’s notch phenonmenon following ICH, respectively (Jang et al., 2015b; Jang and Seo, 2015). In a recent study reporting on an injury of the lower ARAS by IVH (Jang et al., 2015a), it appeared that injury of the upper ARAS was due to bilateral EVD and intracerebral hemorrhage. On 7-month DTT images, we found evidence indicating the recovery of the injured ARAS, which showed thickening of the narrowed lower dorsal and ventral ARAS and increased neural connectivity of the upper ARAS to the right forebrain and basal forebrain.ese fi ndings indicate recovery of lower dorsal and ventral ARAS and upper ARAS. At 7 months after onset, the patient showed marked recovery of consciousness to a nearly normal state (GCS: 9->15, CRS-R: 5->22) compared to 1 month aer onset. Our results on the neural connectivity of the upper ARAS indicate that the recovery of the upper ARAS connnectivity to the impotant areas (the prefrontal cortex and basal forebrain) only in one hemisphere might be suf fi cient for good consciousness in only one hemisphere and might be enough to achieve a nearly intact consciousness (Laureys et al., 2000; Schif f, 2008, 2010; Jang and Lee, 2015b).
In conclusion, recovery of a multiply injured ARAS with the recovery of consciousness was demonstrated in a stroke patient. Our results indicate that evaluation of the ARAS using DTT would be necessary in elucidating the state of the ARAS, particularly in stroke patients with multiple pathologies who undergo neuroinvasive neurosurgical procedures. However, because it is a case report, further studies including larger numbers of cases with multiple and various brain pathologies are warranted.
This work was supported by the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (MSIP), No. 2015R1A2A2A01004073.
sung ho Jang, han Do Lee*
Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daemyungdong, Namgu, Daegu, Republic of Korea
*Correspondence to:Han Do Lee, M.S., lhd890221@hanmail.net.
Accepted:2017-01-14
orcid:0000-0002-1668-2187 (Han Do Lee)
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fi bre orientations: What can we gain? Neuroimage 34:144-155.
Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, Folkerth RD (2012) Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 71:531-546.
Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J (2011) Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519:933-956.
Giacino JT, Kalmar K, Whyte J (2004) The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 85:2020-2029.
Gosseries O, Bruno MA, Chatelle C, Vanhaudenhuyse A, Schnakers C, Soddu A, Laureys S (2011) Disorders of consciousness: what’s in a name? NeuroRehabilitation 28:3-14.
Jang SH, Kim HS (2015) Aneurysmal subarachnoid hemorrhage causes injury of the ascending reticular activating system: relation to consciousness. AJNR Am J Neuroradiol 36:667-671.
Jang SH, Lee HD (2015a) The ascending reticular activating system in a patient with severe injury of the cerebral cortex: a case report. Medicine (Baltimore) 94:e1838.
Jang SH, Lee HD (2015b) Ascending reticular activating system recovery in a patient with brain injury. Neurology 84:1997-1999.
Jang SH, Seo YS (2015) Injury of the contralateral lower ascending reticular activating system by an intracerebral hemorrhage. AJNR Am J Neuroradiol 36:E58-59.
Jang SH, Kwon HG (2015) The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett 590:58-61.
Jang SH, Lim HW, Yeo SS (2014)e neural connectivity of the intralaminar thalamic nuclei in the human brain: a diffusion tensor tractography study. Neurosci Lett 579:140-144.
Jang SH, Lee J, Seo YS (2015a) Injury of the lower ascending reticular activating system in a patient with cerebral infarct. Int J Stroke 10:E72-73.
Jang SH, Lim HW, Yeo SS (2015b) Injury of the ascending reticular activating system by transtentorial herniation in a patient with intracerebral haemorrhage: a diffusion tensor tractography study. J Neurol Neurosurg Psychiatry 86:1164-1166.
Jang SH, Kim SH, Lim HW, Yeo SS (2015c) Recovery of injured lower portion of the ascending reticular activating system in a patient with traumatic brain injury. Am J Phys Med Rehabil 94:250-253.
Jang SH, Lee HD, Chang CH, Jung YJ (2016) Recovery of hypersomnia concurrent with recovery of an injured ascending reticular activating system in a stroke patient: a case report. Medicine (Baltimore) 95:e2484.
Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P (2000) Restoration of thalamocortical connectivity aer recovery from persistent vegetative state. Lancet 355:1790-1791.
Schif f ND (2008) Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci 1129:105-118.
Schif f ND (2010) Recovery of consciousness aer brain injury: a mesocircuit hypothesis. Trends Neurosci 33:1-9.
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81-84.
Yeo SS, Chang PH, Jang SH (2013)e ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci 7:416.
Copyedited by Li CH, Song LP, Zhao M
10.4103/1673-5374.205109
How to cite this article:Jang SH, Lee HD (2017) Recovery of multiplyinjured ascending reticular activating systems in a stroke patient. Neural Regen Res 12(4):671-672.
Open access statement:Tis is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/ have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due ef f orts will be made to conceal their identity, but anonymity cannot be guaranteed.
杂志排行
中国神经再生研究(英文版)的其它文章
- RhoA as a target to promote neuronal survival and axon regeneration
- The complexities underlying age-related macular degeneration: could amyloid beta play an important role?
- The reasons for end-to-side coaptation: how does lateral axon sprouting work?
- Axon degeneration: make the Schwann cell great again
- Neuroprotective mechanism of Kai Xin San: upregulation of hippocampal insulin-degrading enzyme protein expression and acceleration of amyloid-beta degradation
- Phosphatidylserine improves axonal transport by inhibition of HDAC and has potential in treatment of neurodegenerative diseases
