HBsAg阴性HBV相关肝细胞癌的临床特点
2017-04-24安林静曲建慧王春平杨永平
马 燕, 陈 艳, 安林静, 曲建慧, 王春平, 杨永平
(北京大学解放军三〇二医院教学医院 肝脏肿瘤诊疗与研究中心, 北京 100039)
论著/肝脏肿瘤
HBsAg阴性HBV相关肝细胞癌的临床特点
马 燕, 陈 艳, 安林静, 曲建慧, 王春平, 杨永平
(北京大学解放军三〇二医院教学医院 肝脏肿瘤诊疗与研究中心, 北京 100039)
目的 探讨HBsAg阴性HBV相关肝细胞癌(HCC)临床特点。方法 收集2005年1月-2012年1月于解放军三○二医院初诊为HCC患者。根据HBV相关血清学标志物的表现将患者分为HBsAg阴性HBV相关HCC组(HBsAg阴性组)和乙型肝炎相关HCC组(乙型肝炎组)。回顾性分析入选患者初次诊断时的临床数据,包括性别、年龄、HBV血清标志物、TBil、Alb、AFP、HBV DNA、BMI、饮酒史、糖尿病史、治疗方法以及随访结果。符合正态分布的计量资料2组间比较采用t检验;不符合正态分布的计量资料2组间比较采用Wilcoxon秩和检验;计数资料组间比较采用χ2检验。生存曲线统计采用log-rank检验,生存差异采用Kaplan-Meier检验。结果 HBsAg阴性组患者的平均(59.42±11.13)岁、年龄>60岁比例(46.85%)、体质量过轻比例(12.24%)、超重比例(20.98%)均明显高于乙型肝炎组和总体患者,差异有统计学意义(P值均<0.01)。HBsAg阴性组患者男女比例(2.86∶1)、每年参加健康体检定期筛查比例(10.41%)、HBV DNA阳性率(1.77%)、吸烟史、饮酒史比例均明显低于总体患者和乙型肝炎组,差异有统计学意义(P值均<0.01)。HBsAg阴性组患者中以抗-HBs、抗-HBe和抗-HBc均为阳性的患者居多(64.08%),抗-HBe和抗-HBc均为阳性次之(21.13%),仅抗-HBc阳性者最少(14.79%)。HBsAg阴性组患者的AFP水平明显低于总体患者和乙型肝炎组,差异有统计学意义(P=0.039)。且HBsAg阴性组患者初诊时的多发病灶所占比例、肿瘤平均最大直径以及肿瘤肝外转移例数均明显高于总体患者和乙型肝炎组,差异有统计学意义(P值均<0.001)。HBsAg阴性组患者初诊时ECOG-PS评分为0者占17.69%,高于乙型肝炎组患者,且BCLC分期中早期(A、B)患者占30.26%,明显低于乙型肝炎组(51.60%),其晚期(C、D)患者例数是乙型肝炎组的1.83倍,差异有统计学意义(P值均<0.001)。HBsAg阴性组患者中位生存期15.22个月,低于总体患者(25.98个月)和乙型肝炎组(26.55个月),差异有统计学意义(P=0.024、0.031)。预后方面,HBsAg阴性组的1、3年生存率(56.0%、24.0%)明显低于总体患者(61.7%、45.2%)、乙型肝炎组患者(66.2%、44.1%)(χ2=4.93、6.87,P=0.026、0.009)。结论 HBsAg阴性HBV相关HCC多发生于抗-HBs、抗-HBe和抗-HBc均为阳性的60岁以上人群,建议将60岁以上的HBV感染后人群确立为HCC高危险人群,推荐每6个月行HCC筛查,以提高HBsAg阴性HBV相关HCC的早期诊断率。
肝炎病毒, 乙型; 肝炎表面抗原, 乙型; 癌, 肝细胞; 疾病特征
肝细胞癌(HCC)是全球常见恶性肿瘤之一,其发生率逐年增高[1],约占全球的55%,5年生存率差[2-3]。我国约90%HCC患者有HBV感染的背景,HBV高病毒载量[4]及C型HBV[5]感染被视为慢性乙型肝炎是否会进展至HCC的主要预测因素[6]。在自然人群中将既往有急慢性乙型肝炎病史,且HBsAg阴性、抗-HBs阳性/阴性、抗-HBc 阳性、HBV DNA低于检测下限、ALT水平在正常范围者定义为乙型肝炎康复人群[7]。研究[8-10]发现该人群若干年后罹患HCC明显高于正常人群,认为HBV相关抗体与肝癌的发生相关。但关于HBsAg阴性HBV相关HCC的患病率及临床特征鲜有深入研究,笔者就此进行临床分析。1 资料与方法
1.1 研究对象 收集本院肝脏肿瘤诊疗与研究中心2005年1月-2012年1月住院初诊为HCC的患者。肝硬化诊断基于临床特点及肝脏影像学特征、食道胃内镜特点和生化指标与外周血常规。HCC的病因描述基于血清病毒抗原/抗体、自身免疫学抗体及酒精摄入史。参考2011年原发性肝癌诊疗规范[11]、2009年美国肝病学会原发性胆汁性肝硬化实践指南[12]、2010年酒精性肝病诊疗指南[13]等诊断标准确定诊断,并排除其他部位肿瘤肝脏转移及肝占位病变未确诊为HCC病例,就病因及临床特点进行初步探讨。1.2 研究方法 回顾性分析入选患者初次诊断时的临床数据,包括性别、年龄、HBV血清标志物、TBil、Alb、AFP、HBV DNA、BMI、饮酒史、糖尿病史和治疗方法等。BMI>25 kg/m2定义为超重,BMI<18.5 kg/m2为偏瘦。AFP超过20 μg/L定义为阳性。采用BCLC分期系统进行HCC分期、体力状况评分(ECOG-PS)评价患者的体力活动状态、MELD评分系统评价严重程度。患者的存活状态及死亡时间通过本中心肝癌患者随访系统获得,生存时间的计算为首次治疗日至死亡日(或最后随访截止日)。随访时间为2005年1月-2016年5月,随访率为70.9%。

2 结果
2.1 纳入、分组情况 共纳入患者5021例,其中乙型肝炎相关HCC患者4163例(82.91%)、HBsAg阴性HBV感染相关HCC患者286例(5.70%),丙型肝炎相关HCC患者350例(6.97%),HBV/HCV重叠感染相关HCC患者37例(0.74%)、原发性胆汁性肝硬化相关HCC患者10例(0.20%),酒精性HCC患者59例(1.18%),其他病因HCC患者116例(2.31%)。由于本研究中乙型肝炎相关HCC病例数远远大于HBsAg阴性HBV感染相关HCC样本量,为避免样本差异影响检验效能,采用SPSS24.0软件的SELECT CASES模块从HBsAg阳性HCC患者中随机抽样500例。根据HBV相关血清学标志物的表现将患者分为:(1)HBsAg阴性HBV相关HCC组(HBsAg阴性组),即HBsAg阴性、抗-HBc阳性,286例;(2)乙型肝炎相关HCC组(乙型肝炎组),即HBsAg阳性,500例。2.2 临床特点 HBsAg阴性组患者的平均年龄、年龄>60岁比例、体质量过轻比例、超重比例均明显高于乙型肝炎组,差异有统计学意义(P值均<0.01)。HBsAg阴性组患者男女比例(2.86∶1)、每年参加健康体检定期筛查比例、HBV DNA阳性率、吸烟史、饮酒史、HBV感染家族史比例均明显低于乙型肝炎组,差异有统计学意义(P值均<0.01)。2组患者在糖尿病史和肝癌家族史方面的差异无统计学意义(P值均>0.05)(表1)。2.3 血清病毒学标志物特点 乙型肝炎组患者中HBeAg阳性者占32.66%,HBeAg阴性者占67.34%;HBsAg阴性组患者中以抗-HBs、抗-HBe和抗-HBc均为阳性的患者居多(64.08%),抗-HBe和抗-HBc均为阳性次之(21.13%),仅抗-HBc阳性者最少(14.79%)。
2.4 肿瘤特性 HBsAg阴性组患者的AFP水平明显低于乙型肝炎组,差异有统计学意义(P=0.039)。且HBsAg阴性组患者初诊时以多发病灶所占比例、肿瘤平均最大直径以及肿瘤肝外转移例数均明显高于乙型肝炎患者,差异有统计学意义(P值均<0.001)(表2)。
2.5 肿瘤评分、分期及预后 HBsAg阴性组患者初诊时ECOG-PS评分为0者占17.69%,高于乙型肝炎组患者,且BCLC分期中早期(A、B)患者占30.26%,明显低于乙型肝炎组(51.60%),其晚期(C、D)患者例数是乙型肝炎组的1.83倍,差异有统计学意义(P值均<0.001)(表3)。MELD评分结果显示乙型肝炎组和HBsAg阴性组的生存率差异无统计学意义。HBsAg阴性组患者中位生存期15.22个月,乙型肝炎组26.55个月,差异有统计学意义(P=0.031);预后方面,HBsAg阴性组的1、3年生存率(56.0%、24.0%)明显低于乙型肝炎组(66.2%、44.1%)(χ2值分别为4.93、6.87,P值分别为0.026、0.009)(图1)。
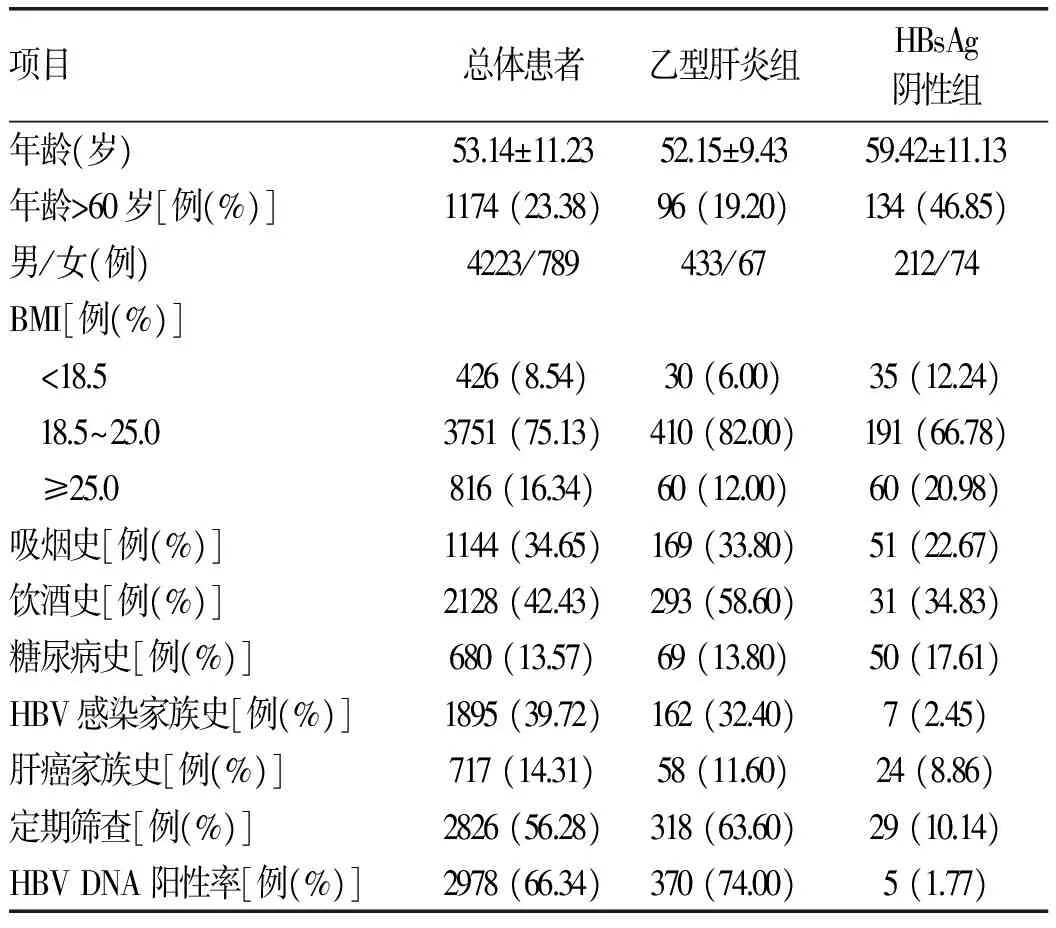
表1 患者临床特征比较
注:因部分患者临床数据缺失,故各项目间总例数存在差异
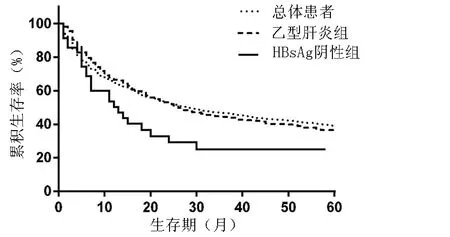
图1 HCC患者生存曲线
3 讨论
近年HCC的发病率呈持续上升趋势,Schutte等[14]研究结果提示HBV感染与HCC的发生发展密切相关,认为HBV感染是HCC发生的独立影响因素。目前,关于HBsAg阴性HBV感染者发生HCC的临床特点相关研究较少,本研究通过对5021例初诊HCC患者的临床资料进行分析发现,HBsAg阴性HBV相关HCC患者具有独特的临床特点,对该群体患者的HCC防治具有重要的指导意义。

表2 2组患者实验室检查及肿瘤相关指标比较

表3 2组患者肿瘤评分和分期比较
本研究中,HBsAg阴性HBV相关HCC患者在整体HCC患者构成中占5.7%,仅次于乙型肝炎相关性HCC和丙型肝炎相关性HCC,明显高于酒精性、脂肪肝性和代谢性疾病相关性HCC,提示在HBV感染高发地区应重视HBsAg阴性HBV感染患者的HCC防治。研究结果显示该人群发生HCC的平均年龄为59.42岁,高于乙型肝炎相关性HCC患者的平均年龄;64.08%的HBV感染相关HCC患者的抗-HBs、抗-HBe和抗-HBc均为阳性,男女比例为2.86∶1,低于吴孟超等[15]报道的7∶1与Shariff等[16]指出的5∶1,提示HBV感染被人体清除后,HBsAg阴性人群的HCC发生风险与雄激素等诱发肝癌因素的关联性降低。在HBsAg阴性HBV相关HCC患者中肥胖患者占20.98%,因此认为肥胖可能是发生HCC的促进因素。
初诊HCC时,早期HCC患者比例明显低于乙型肝炎相关性HCC患者,且晚期HCC患者较多(43.17%),肝外转移的患者高达30.28%,肿瘤平均直径较大,主要源于HBsAg阴性HBV感染后人群定期健康体检者比例明显低于慢性乙型肝炎患者(10.4% vs 63.60%),错过早期发现的机会,确诊时有条件接受根治性治疗(肝癌切除术、肝移植)的患者较少,导致该群体的平均生存期较短,1、3年的生存率较低,预后较差。
综上所述,HBsAg阴性HBV相关HCC多发生于抗-HBs、抗-HBe和抗-HBc均为阳性的60岁以上人群,在总体HCC患者构成中仅次于慢性HBV、HCV感染者。该群体定期筛查HCC较少,早期诊断率低,接受根治性治疗的机会少,预后差。建议将60岁以上的HBsAg阴性HBV感染后人群定义为HCC的高危险人群,每6个月定期筛查HCC,以提高早期诊断率,对延长该群体患者的中位生存期具有重要意义。
[1] CHEN WQ, ZHENG RS, ZHANG SW, et al. Report of cancer incidence and mortality in China, 2012[J]. China Cancer, 2016, 25(1): 1-8. (in Chinese) 陈万青, 郑荣寿, 张思维, 等. 2012年中国恶性肿瘤发病和死亡分析[J]. 中国肿瘤, 2016, 25(1): 1-8.
[2] MALUCCIO M, COVEY A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma[J]. CA Cancer J Clin, 2012, 62(6): 394.[3] BRUIX J, SHERMAN M. Management of hepatocellular carcinoma: an update[J]. Hepatology, 2011, 53(3): 1020-1022.[4] YANG T, LU JH, ZHAI J, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virus-related hepatocellular carcinoma after curative resection: a prospective cohort study[J]. Eur J Surg Oncol, 2012, 38(8): 683-691.
[5] WEN J, SONG C, JIANG D, et al. Hepatitis B virus genotype, mutations, human leukocyte antigen polymorphisms and their interactions in hepatocellular carcinoma: a multi-centre case-control study[J]. Sci Rep, 2015, 5: 16489.
[6] LU T, SETO WK, ZHU RX, et al. Prevention of hepatocellular carcinoma in chronic viral hepatitis B and C infection[J]. World J Gastroenterol, 2013, 19(47): 8887-8894.
[7] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B : a 2015 update[J]. J Clin Hepatol, 2015, 31(12): 1941-1960. (in Chinese) 中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2015年更新版) [J]. 临床肝胆病杂志, 2015, 31(12): 1941-1960.
[8] LIU J, YANG HI, LEE MH, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma[J]. Gut, 2014, 63(10): 1648-1657.
[9] KIM GA, LEE HC, KIM MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance[J]. J Hepatol, 2015, 62(5): 1092-1099.
[10] CHEN YC, JENG WJ, CHIEN RN, et al. Clinical outcomes after spontaneous and nucleos(t)ide analogue-treated HBsAg seroclearance in chronic HBV infection[J]. Aliment Pharmacol Ther, 2016, 43(12): 1311-1318.
[11] Ministry of Health of the People′s Republic of China. Diagnosis, management, and treatment of hepatocellular carcinoma (V2011) [J]. J Clin Hepatol, 2011, 27(11): 1141-1159. (in Chinese) 中华人民共和国卫生部. 原发性肝癌诊疗规范(2011年版)[J]. 临床肝胆病杂志, 2011, 27(11): 1141-1159.
[12] LINDOR KD, GERSHWIN ME, POUPON R, et al. Primary biliary cirrhosis[J]. Hepatology, 2009, 50(1): 291-308.
[13] Fatty liver and Alconholic Liver Disease Study Group of the Chinese Liver Disease Association, Chinese Medical Association. Guidelines for prevention and treatment of alcoholic liver disease (revised rersion 2010)[J]. J Clin Hepatol, 2010, 26(3): 229-232. (in Chinese) 中华医学会肝病学分会脂肪肝和酒精性肝病学组. 酒精性肝病诊疗指南(2010年修订版)[J]. 临床肝胆病杂志, 2010, 26(3): 229-232.
[14] SCHUTTE K, SCHULZ C, PORANZKE J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver[J]. BMC Gastroenterol, 2014, 14: 117.
[15] WU MC, CHEN H, SHEN F. Surgical treatment of primary liver cancer: report of 5524 cases [J]. Chin J Surg, 2001, 39(1): 24-27. (in Chinese) 吴孟超, 陈汉, 沈锋. 原发性肝癌的外科治疗——附5524例报告[J]. 中华外科杂志, 2001, 39(1): 24-27.
[16] SHARIFF MI, COX IJ, GOMAA AI, et al. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics[J]. Expert Rev Gastroenterol Hepatol, 2009, 3(4): 353-367.
引证本文:MA Y, CHEN Y, AN LJ, et al. Clinical features of HBsAg-negative HBV-related hepatocellular carcinoma[J]. J Clin Hepatol, 2017, 33(4): 674-678. (in Chinese) 马燕, 陈艳, 安林静, 等. HBsAg阴性HBV相关肝细胞癌的临床特点[J]. 临床肝胆病杂志, 2017, 33(4): 674-678.
(本文编辑:邢翔宇)
Clinical features of HBsAg-negative HBV-related hepatocellular carcinoma
MAYan,CHENYan,ANLinjing,etal.
(LiverCancerTreatmentandResearchCenter,TeachingHospitalof302HospitalofPLA,PekingUniversity,Beijing100039,China)
Objective To investigate the clinical features of HBsAg-negative HBV-related hepatocellular carcinoma (HCC). MethodsThe patients who were newly diagnosed with HCC from January 2005 to January 2012 were enrolled. According to the HBV-related serological markers, the patients were divided into HBsAg-negative HBV-related HCC group and hepatitis B-related HCC group. A retrospective analysis was performed for the clinical and laboratory examination data at initial diagnosis, including sex, age, hepatitis B virus markers, total bilirubin (TBil), albumin (Alb), alpha-fetoprotein (AFP), HBV DNA, body mass index (BMI), drinking history, history of diabetes, therapies, and follow-up results. Thet-test was used for comparison of normally distributed continuous data between groups; the non-normally distributed data were expressed as median and interquartile range (Q1 and Q3), and the Wilcoxon rank sum test was used for comparison of non-normally distributed continuous data between groups; the chi-square test or Fisher′s exact test were used for comparison of categorical data. The log-rank test was used to compare survival curves between the two groups, and the Kaplan-Meier method was used to calculate survival rates. Results Compared with the hepatitis B group and all patients, the HBsAg-negative group had a significantly higher mean age (59.42±11.13 years) and significantly higher proportions of patients with age >60 years (46.85%), low body weight (12.24%), and overweight (20.98%)(allP<0.01). Compared with all patients and the hepatitis B group, the HBsAg-negative group had a significantly lower male-to-female ratio (2.86∶1), a significantly lower proportion of patients regularly undergoing physical examination every year (10.41%), a significantly lower HBV DNA positive rate (1.77%), and significantly lower proportions of patients with smoking and drinking histories (allP<0.01). Of all patients in the HBsAg-negative group, 64.08% had positive anti-HBs, anti-HBe, and anti-HBc, 21.13% had positive anti-HBe and anti-HBc, and 14.79% only had positive anti-HBc. The HBsAg-negative group had a significantly lower AFP level than all patients and the hepatitis B group (P=0.039). Compared with the hepatitis B group and all patients, the HBsAg-negative group had a significantly higher proportion of multiple lesions, a significantly greater mean maximum tumor diameter, and a significantly higher number of patients with extrahepatic metastasis (allP<0.001). Compared with the hepatitis B group, the HBsAg-negative group had a higher proportion of patients with an Eastern Cooperative Oncology Group Performance Status score of 0 at initial diagnosis (17.69%) and a lower proportion of patients with early-to-medium Barcelona Clinic Liver Cancer stage (stages A and B) (30.26% vs 51.60%,P<0.001); the number of patients with stages C and D in the HBsAg-negative group was 1.83 times that in the hepatitis B group (P<0.001). The HBsAg-negative group had a significantly lower median survival time (15.22 months) than all patients and the hepatitis B group (P=0.024、0.031). Compared with all patients and the hepatitis B group, the HBsAg negative group had significantly lower 1- and 3-year survival rates (1-year survival rate: 56.0% vs 61.7% and 66.2%,χ2=4.93,P=0.026; 3-year survival rate: 24.0% vs 45.2% and 44.1%,χ2=6.867,P=0.009). Conclusion HBsAg-negative HBV-related HCC is commonly seen in patients aged >60 years with positive anti-HBs, anti-HBe, and anti-HBc. The population aged >60 years with HBV infection should be the high-risk population for HCC, and it is recommended to perform HCC screening every 6 months to increase the early diagnostic rate of HBsAg-negative HBV- related HCC.
hepatitis B virus; hepatitis B surface antigens; carcinoma, hepatocellular; disease attributes
10.3969/j.issn.1001-5256.2017.04.015
2016-11-24;
2016-12-21。
全军医学科技“十二五”科研重点项目(BWS11J074);国家自然基金课题(81272330)
马燕(1988-),女,主要从事肝脏肿瘤治疗方面的研究。
杨永平,电子信箱:yongpingyang@hotmail.com。
R512.62; R735.7
A
1001-5256(2017)04-0674-05
