白介素18在颞下颌关节炎中的作用研究
2017-04-10胡雅琳姜程今刘婧芳何韶衡李志刚
黄 芳,胡雅琳,姜程今,刘婧芳,何韶衡,李志刚
·论著·
白介素18在颞下颌关节炎中的作用研究
黄 芳1,胡雅琳1,姜程今1,刘婧芳1,何韶衡2*,李志刚3*
目的 探究白介素(IL)-18在颞下颌关节炎中的作用。方法 2015年11月—2016年4月,30只SPF级雄性BALB/c小鼠适应性喂养1周后,采用随机数字表法分为A、B、C、D、E组,每组6只。B组~E组在第1、21天时分别注射Ⅱ型胶原酶;从第1天开始,A组仅注射0.9%氯化钠溶液,B组注射0.9%氯化钠溶液,C组注射IL-18,D组注射IL-18结合蛋白(IL-18BP),E组注射IL-18、IL-18BP。第25天,采集各组小鼠外周血、组织灌洗液,采用流式细胞术检测外周血单核细胞/颞下颌关节组织灌洗液巨噬细胞中白介素18受体(IL-18R)阳性表达率,ELISA法检测外周血血浆/颞下颌关节组织灌洗液上清液中肿瘤坏死因子(TNF)-α、IL-1β水平。结果 B组、C组小鼠外周血单核细胞中IL-18R阳性表达率大于A组(P<0.05);C组小鼠外周血单核细胞中IL-18R阳性表达率大于B组、D组、E组(P<0.05)。B组~E组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率大于A组(P<0.05);C组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率大于B组、D组、E组(P<0.05)。B组~E组小鼠外周血血浆中TNF-α、IL-1β水平高于A组(P<0.05);C组小鼠外周血血浆中TNF-α、IL-1β水平高于B组、D组、E组(P<0.05)。B组~E组小鼠颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平高于A组(P<0.05);C组小鼠颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平高于B组、D组、E组(P<0.05);D组小鼠颞下颌关节组织灌洗液上清液中TNF-α水平低于B组(P<0.05);E组小鼠颞下颌关节组织灌洗液上清液中TNF-α水平高于D组(P<0.05)。结论 IL-18能通过其受体IL-18R刺激单核细胞/巨噬细胞产生TNF-α与IL-1β等炎性因子,从而参与颞下颌关节炎的发生发展,推测IL-18R可能成为颞下颌关节炎的治疗靶点。
颞下颌关节障碍;关节炎;白细胞介素18
黄芳,胡雅琳,姜程今,等.白介素18在颞下颌关节炎中的作用研究[J].中国全科医学,2017,20(12):1457-1462.[www.chinagp.net]
HUANG F,HU Y L,JIANG C J,et al.Role for IL-18 in temporomandibular joint arthritis[J].Chinese General Practice,2017,20(12):1457-1462.
1.JinzhouMedicalUniversity,Jinzhou121000,China
2.AllergyandClinicalImmunologyResearchCentre,theFirstAffiliatedHospitalofJinzhouMedicalUniversity,Jinzhou121000,China
3.DepartmentofProsthodontics,theSecondAffiliatedHospitalofJinzhouMedicalUniversity,Jinzhou121000,China
*Correspondingauthor:HEShao-heng,Professor;E-mail:shoahenghe@126.com
LIZhi-gang,Professor;E-mail:ZGLi700103@163.com
骨关节炎是临床常见病,其能导致各个关节(包括颞下颌关节)剧痛和功能障碍。颞下颌关节炎是颞下颌关节紊乱综合征的主要亚型之一,多发于20~30岁青壮年,93%的青少年自发性关节炎的早期症状可见于颞下颌关节[1]。研究发现,颞下颌关节炎与功能性运动综合征如张口受限、颞下颌关节移动弹响和下颌非对称移动存在着某种联系[2-5]。因此早期诊断和及时治疗颞下颌关节炎是至关重要的。
白介素(IL)-18是IL-1超因子家族的一员,是一种促炎性细胞因子,可由巨噬细胞、上皮细胞、T淋巴细胞、中性粒细胞、自然杀伤(NK)细胞和B淋巴细胞分泌,而单核细胞和巨噬细胞为其主要来源细胞[6-8]。IL-18在炎症发生和免疫应答中有促进作用[6-7]。研究表明,IL-18与多种炎性疾病,包括缺血性再灌注损伤、移植抑制和自体免疫性疾病等有关[9-11]。WANNER等[12]对90例肩部关节炎患者进行研究发现,IL-18水平在骨关节炎的早晚期均升高;李勇等[13]对30例膝关节炎患者的研究发现,IL-18水平的升高促进了炎性因子前列腺素E2(PGE2)水平的升高,参与了骨关节炎的发生发展;GOUDA等[14]提取44例类风湿关节炎患者滑膜液检测到IL-18在患者体内大量表达并促进干扰素-γ(IFN-γ)的产生。通过以上研究可推测出IL-18与关节炎有密切联系,但关于IL-18在颞下颌关节炎中的作用目前鲜有研究。白介素18受体(IL-18R)属于IL-1受体/Toll样受体(IL-1R/TLR)家族,是由IL-18Rα亚基和IL-18Rβ亚基组成的异质二聚体,其中IL-18Rα亚基直接与IL-18特异性结合。IL-18结合蛋白(IL-18BP)可由单核细胞与巨噬细胞等细胞产生[15],其通过与IL-18功能性结合,阻断IL-18与IL-18R的相互作用进而发挥抑制IL-18生理功能的作用[16]。因此,本实验通过检测IL-18对单核细胞/巨噬细胞中IL-18R阳性表达率及促炎性细胞因子水平的影响来探究其在颞下颌关节炎中的作用,以期为颞下颌关节炎的临床治疗提供借鉴及新靶点。
Selvester QRS积分系统在评估心肌梗死后心肌瘢痕负荷及预测远期预后方面具有较高的临床价值。本研究结果表明,Selvester QRS积分在评估急性STEMI患者再灌注治疗后3个月内时,前壁心梗组的积分是逐步下降的,而下壁心梗组的积分则较为稳定,因此,为了得到更准确的评估结果,对于急性前壁心肌梗死患者,可在再灌注治疗后3个月后实施,而对下壁心肌梗死的积分评估时间则无明确的限制。由于本研究为单中心研究,入选样本量相对较小,且多为男性患者,因此研究结果可能存在偏倚,尚有待多中心或大样本的临床研究进一步证实。
1 材料与方法
1.1 实验动物 SPF级雄性BALB/c小鼠30只,8周龄,体质量18~22 g,购自北京维通利华实验动物技术有限公司,批号:11400700056942。
1.2 实验药物 Ⅱ型胶原酶(北京索莱宝科技有限公司,批号:17101-015);IL-18(R&D Systems,批号:B004-5);IL-18BP(R&D Systems,批号:122-BP-100)。实验前称取Ⅱ型胶原酶1 g,完全溶于500 μl磷酸盐缓冲液(PBS)中,配制出浓度为2 g/ml的Ⅱ型胶原酶;称取IL-18、IL-18BP各1 μg,分别溶于1 ml PBS中,得到1 ml(1 μg/ml)原液,分别抽取1 μl原液加入99 μl PBS中,得到100 μl浓度为10 ng/ml的IL-18、IL-18BP,随用随配。
1.3 实验试剂 BV421-CD11b、APC/Cy7-Ly-6c、PE-F4-80、APC/Cy7-CD11c表面抗体(BioLegend),APC-IL-18R细胞内抗体(R&D Systems),肿瘤坏死因子(TNF)-α、IL-1β ELISA试剂盒(北京达科为生物技术有限公司),TNF-α抗体(Rockland公司),IL-1β抗体(美国PeproTech公司)。
1.4 实验仪器 酶标仪、普通台式离心机、全自动洗板机、-80 ℃冰箱(美国Thermo公司),流式细胞仪(美国BD公司),水浴锅(上海精宏实验设备有限公司)。
1.5 研究方法
1.5.1 小鼠分组及处理 2015年11月—2016年4月,将30只小鼠置于室内温度为23~28 ℃、相对湿度为60%~75%的环境中,自由摄取标准啮齿动物的食物和水,适应性喂养1周后,采用随机数字表法将其分为A、B、C、D、E组,各6只。B组~E组在第1、21天时分别注射Ⅱ型胶原酶100 μl;从第1天开始,A组仅注射0.9%氯化钠溶液100 μl,B组注射0.9%氯化钠溶液100 μl,C组注射IL-18 100 μl,D组注射IL-18BP 100 μl,E组注射IL-18、IL-18BP各100 μl;每3 d注射1次。具体操作如下:用2%异氟烷短暂麻醉后,修剪小鼠双侧颞下颌关节区域周围毛发,注射器针尖透过皮肤向前直达颧弓,然后逐渐向前插入颧弓后下边缘的下方,最终进入关节腔,注入不同刺激剂,注射速度约为2 μl/s[17]。第25天,B组~E组小鼠表现为明显的刮擦头面部及缩头反应,提示炎症出现,造模成功[18]。
1.5.2 标本采集
1.5.2.2 组织灌洗液采集 第25天采集小鼠颞下颌关节组织灌洗液200 μl,提取颞下颌关节组织灌洗液上清液,-80 ℃保存备用。
1.5.2.3 流式细胞术(flow cytometry,FCM)检测外周血单核细胞/颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率 取外周血/颞下颌关节组织灌洗液200 μl,加入BV421-CD11b、APC/Cy7-Ly-6c、PE-F4-80、APC/Cy7-CD11c表面抗体,室温避光孵育15 min,加入1.5 ml红细胞裂解液裂解红细胞,室温避光孵育12 min,1 200 r/min离心6 min(离心半径5 cm),弃上清液;加入1 ml 1×PBS洗掉被裂解过的破碎红细胞,1 200 r/min离心6 min(离心半径5 cm),弃上清液;加入250 μl透膜固定液重悬细胞,4 ℃避光孵育20 min,加入洗液500 μl,洗掉透膜固定液,1 200 r/min离心6 min(离心半径5 cm),弃上清液;加入100 μl洗液重悬细胞,加入APC-IL-18R细胞内抗体,4 ℃避光孵育30 min,加入洗液500 μl,1 200 r/min离心6 min(离心半径5 cm),弃上清液;加入300 μl 1×PBS重悬细胞沉淀,使细胞混匀,采用流式细胞仪检测单核细胞/巨噬细胞中IL-18R阳性表达率。实验独立重复6次。
1.5.2.4 ELISA法检测外周血血浆/颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平 设置标准孔、样本孔和空白孔(标准孔和空白孔均设1个复孔),加入100 μl提前稀释好的标准液与样本到各对应孔中,轻轻混匀,封板,室温孵育90 min;洗板3次,加入TNF-α、IL-1β一抗,轻轻混匀,封板,室温孵育60 min;洗板3次,每孔加入链霉亲和素标记的辣根过氧化物酶结合一抗抗体,轻轻混匀,封板,室温孵育30 min;洗板3次,各孔加入显色液,室温避光15 min,加入终止液,采用酶标仪检测450 nm处吸光度。实验独立重复6次。

2 结果
2.1 各组小鼠外周血单核细胞中IL-18R阳性表达率比较 5组小鼠外周血单核细胞中IL-18R阳性表达率比较,差异有统计学意义(P<0.05)。其中B组、C组小鼠外周血单核细胞中IL-18R阳性表达率均大于A组,差异有统计学意义(P<0.05);C组小鼠外周血单核细胞中IL-18R阳性表达率大于B组、D组、E组,差异有统计学意义(P<0.05,见表1)。
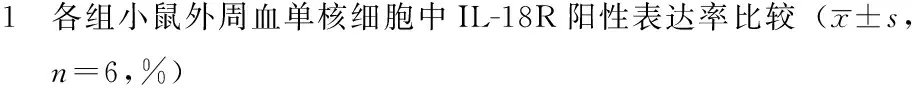
Table 1 Comparison of positive expression rate of IL-18R in peripheral monocytes of mice among the five groups

组别IL-18R阳性表达率A组0.30±0.06B组4.76±1.39aC组19.14±6.13abD组2.44±0.97cE组2.65±0.80cF值42.01P值<0.001
注:IL-18R=白介素18受体;与A组比较,aP<0.05;与B组比较,bP<0.05;与C组比较,cP<0.05
2.2 各组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率比较 5组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率比较,差异有统计学意义(P<0.05)。其中B组~E组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率大于A组,差异有统计学意义(P<0.05);C组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率大于B组、D组、E组,差异有统计学意义(P<0.05,见表2)。

Table 2 Comparison of positive expression rate of IL-18R in macrophages in temporomandibular joint lavage fluid of mice among the five groups
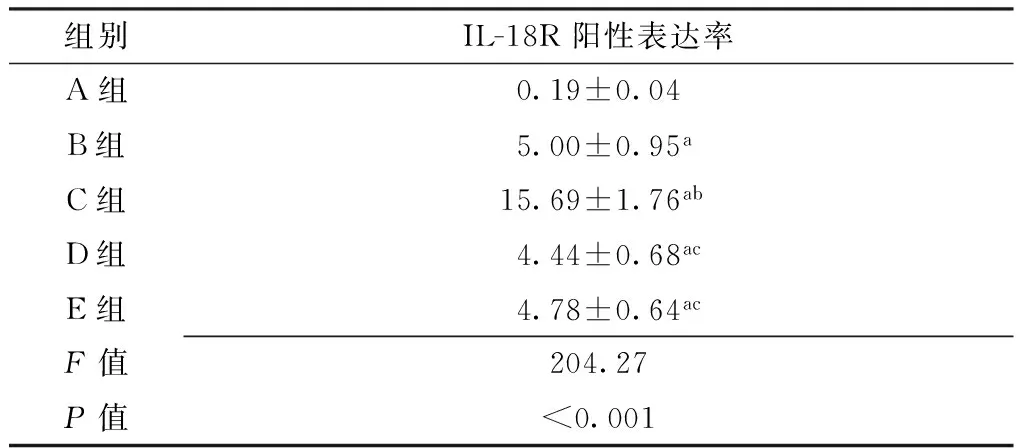
组别IL-18R阳性表达率A组0.19±0.04B组5.00±0.95aC组15.69±1.76abD组4.44±0.68acE组4.78±0.64acF值204.27P值<0.001
注:与A组比较,aP<0.05;与B组比较,bP<0.05;与C组比较,cP<0.05
2.3 各组小鼠外周血血浆中TNF-α、IL-1β水平比较 5组小鼠外周血血浆中TNF-α、IL-1β水平比较,差异有统计学意义(P<0.05)。其中B组~E组小鼠外周血血浆中TNF-α、IL-1β水平高于A组,差异有统计学意义(P<0.05);C组小鼠外周血血浆中TNF-α、IL-1β水平高于B组、D组、E组,差异有统计学意义(P<0.05,见表3)。
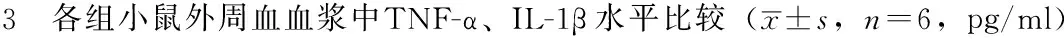
Table 3 Comparison of levels of TNF-α and IL-1β in peripheral plasma of mice among the five groups

组别TNF-αIL-1βA组26.0±2.84.3±2.5B组71.7±8.0a11.2±2.2aC组141.4±7.6ab23.6±4.9abD组69.3±8.5ac11.8±2.2acE组69.7±3.5ac12.4±1.8acF值239.7733.28P值<0.001<0.001
注:TNF=肿瘤坏死因子,IL=白介素;与A组比较,aP<0.05;与B组比较,bP<0.05;与C组比较,cP<0.05
2.4 各组小鼠颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平比较 5组小鼠颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平比较,差异有统计学意义(P<0.05)。其中B组~E组小鼠颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平高于A组,差异有统计学意义(P<0.05);C组小鼠颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平高于B组、D组、E组,差异有统计学意义(P<0.05);D组小鼠颞下颌关节组织灌洗液上清液中TNF-α水平低于B组,差异有统计学意义(P<0.05);E组小鼠颞下颌关节组织灌洗液上清液中TNF-α水平高于D组,差异有统计学意义(P<0.05,见表4)。

Table 4 Comparison of levels of TNF-α and IL-1β in liquid supernatant of temporomandibular joint lavage fluid of mice among the five groups
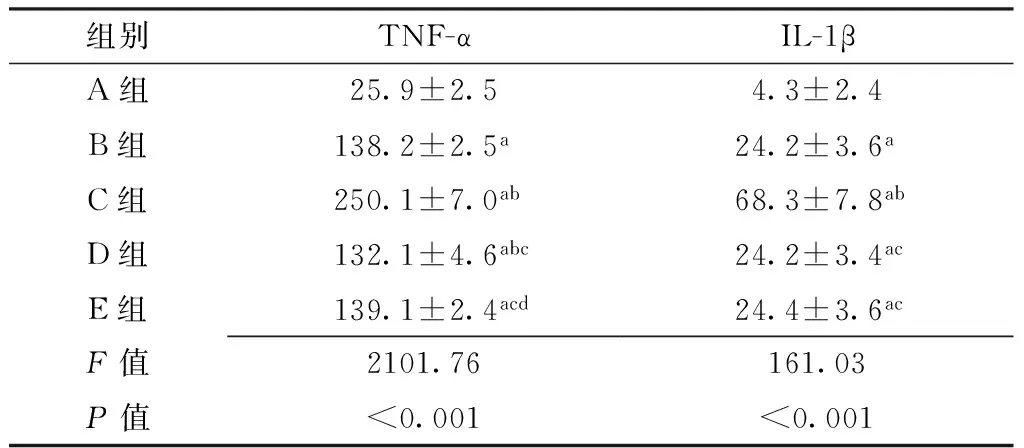
组别TNF-αIL-1βA组25.9±2.54.3±2.4B组138.2±2.5a24.2±3.6aC组250.1±7.0ab68.3±7.8abD组132.1±4.6abc24.2±3.4acE组139.1±2.4acd24.4±3.6acF值2101.76161.03P值<0.001<0.001
注:与A组比较,aP<0.05;与B组比较,bP<0.05;与C组比较,cP<0.05;与D组比较,dP<0.05
3 讨论
近年来越来越多研究显示,IL-18在炎症中有重要作用,在骨关节炎如肩关节炎[12]、膝关节炎[13]、类风湿关节炎[14]等中有重要作用。KANEYAMA等[19-20]、SUZUKI等[21]发现,颞下颌关节炎患者的颞下颌关节组织灌洗液中TNF水平升高。BRENNAN等[22]、WATKINS等[23]、DUFF[24]发现,在关节滑膜组织中,TNF可通过破坏软骨和骨组织、感受器致敏等途径来促进关节炎的发生发展。NORDAHL等[25-26]、NORTH等[27]发现,与检测不到IL-1β的慢性关节炎患者相比,可检测到IL-1β的慢性关节炎患者在X线片上的关节组织改变程度更大。KONTZIAS等[28]发现,IL-18对促炎性细胞因子的产生起到了至关重要的作用。
本研究结果显示,B组、C组小鼠外周血单核细胞和B组~E组小鼠颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率大于A组,表示Ⅱ型胶原酶可以引起小鼠颞下颌关节炎,造模成功。C组小鼠外周血单核细胞、颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率大于B组、D组、E组,表示IL-18在颞下颌关节炎中起到促进炎症的作用;E组小鼠外周血单核细胞、颞下颌关节组织灌洗液巨噬细胞中IL-18R阳性表达率与D组无明显差异,表示IL-18BP预处理小鼠后,即使再有IL-18的刺激也不会使IL-18R表达水平增高。B组、C组小鼠外周血血浆、颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平高于A组,C组小鼠外周血血浆、颞下颌关节组织灌洗液上清液中TNF-α、IL-1β水平高于B组、D组、E组,提示IL-18在炎症中起到激活单核细胞、巨噬细胞产生炎症因子的作用;B组、E组小鼠颞下颌关节组织灌洗液上清液中TNF-α水平高于D组,推测炎症产生后,即使加入IL-18BP,IL-18BP也未能竞争性结合IL-18,从而使IL-18在炎症中仍旧起到激活单核细胞/巨噬细胞产生炎性因子的作用。以上结果表示,IL-18不仅是促炎性细胞因子,更通过单核细胞/巨噬细胞上其相应的受体IL-18R激活单核细胞/巨噬细胞,从而产生TNF-α与IL-1β等炎性因子,促进并加快颞下颌关节炎的形成。
颞下颌关节炎是临床较常见的口腔疾病之一,其总体预后较差。有报道以不同的诊断方法对颞下颌关节炎进行评估发现,颞下颌关节炎在青少年中尤为普遍,而且17%~87%的人群有不同的亚型与分类[29-30]。颞下颌关节炎的治疗方法多种多样,如物理疗法、社会心理支持疗法、药物治疗、外科手术等。药物治疗包括非甾体抗炎药(NSAIDs)、皮质类固醇、抗风湿药物(DMARDs)等,均有疗效,但各有不足,如NSAIDs主要对少数严重关节炎有效[31];皮质类固醇的使用目前仅在兔身上做过实验,还未曾在人及其他动物身上获取可靠结果[32];DMARDs也不常应用于颞下颌关节炎的治疗。此外,还有地塞米松电离子透入疗法(IP),但会出现短暂性红斑等不良反应;TNF-α抑制剂可缓解颞下颌关节炎的疼痛,改善口腔功能[33],但如何根治还鲜有报道。也有医生采用手术或利用关节刺穿术治疗颞下颌关节炎,但手术治疗造成的失误[34]和关节炎晚期[35]使其均不能成为最佳治疗方法。本研究结果提示,可以通过特异性抑制或阻断IL-18,防止其与IL-18R结合,抑制单核细胞/巨噬细胞的激活,下调IL-18等促炎性细胞因子的水平,从而降低炎症发生率,推测IL-18可作为颞下颌关节炎的治疗新靶点。
综上所述,IL-18能通过其受体IL-18R刺激单核细胞/巨噬细胞产生TNF-α与IL-1β等炎性因子,从而参与颞下颌关节炎的发生发展,推测IL-18R可能成为颞下颌关节炎的治疗靶点。本研究为动物实验,今后应进一步行人体试验对本研究结果进行验证与探究;且IL-18是否能通过其他形式刺激炎症的产生,有待进一步研究。
作者贡献:黄芳、何韶衡、李志刚进行文章的构思与设计;黄芳进行研究的施行、数据整理、统计学处理、撰写论文;何韶衡、李志刚进行可行性分析;胡雅琳、姜程今、刘婧芳进行数据收集;何韶衡、李志刚对文章整体负责,监督管理。
本文无利益冲突。
[1]SAURENMANN R.Clinical diagnosis of temporomandibular joint arthritis:a difficult task[J].J Rheumatol,2014,41(9):1734-1736.
[2]WEISS P F,ARABSHAHI B,JOHNSON A,et al.High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis,as detected by magnetic resonance imaging but not by ultrasound[J].Arthritis Rheum,2008,58(4):1189-1196.
[3]OLSON L,ECKERDAL O,HALLONSTEN A L,et al.Craniomandibular function in juvenile chronic arthritis.A clinical and radiographic study[J].Swed Dent J,1991,15(2):71-83.
[4]TWILT M,MOBERS S M,ARENDS L R,et al.Temporomandibular involvement in juvenile idiopathic arthritis[J].J Rheumatol,2004,31(7):1418-1422.
[5]PEDERSEN T K,KÜSELER A,GELINECK J,et al.A prospective study of magnetic resonance and radiographic imaging in relation to symptoms and clinical findings of the temporomandibular joint in children with juvenile idiopathic arthritis[J].J Rheumatol,2008,35(8):1668-1675.
[6]DAO T,MEHAL W Z,CRISPE I N.IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells[J].J Immunol,1998,161(5):2217-2222.
[7]YOSHIMOTO T,OKAMURA H,TAGAWA Y I,et al.Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells[J].Proc Natl Acad Sci U S A,1997,94(8):3948-3953.
[8]KRISHNAN S M,SOBEY C G,LATZ E,et al.IL-1β and IL-18:inflammatory markers or mediators of hypertension?[J].Br J Pharmacol,2014,171(24):5589-5602.DOI:10.1111/bph.12876.
[9]VENKATACHALAM K,PRABHU S D,REDDY V S,et al.Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury[J].J Biol Chem,2009,284(12):7853-7865.DOI:10.1074/jbc.M808824200.
[10]DUDLER J,SIMEONI E,FLEURY S,et al.Gene transfer of interleukin-18-binding protein attenuates cardiac allograft rejection[J].Transpl Int,2007,20(5):460-466.
[11]SHIMIZU C,MATSUMOTO K,FUJITA T,et al.Imbalance of interleukin-18 and interleukin-18 binding protein in patients with IgA nephropathy implicating renal vasculopathy[J].Clin Lab,2015,61(1/2):23-30.
[12]WANNER J,SUBBAIAH R,SKOMOROVSKA-PROKVOLIT Y,et al.Proteomic profiling and functional characterization of early and late shoulder osteoarthritis[J].Arthritis Res Ther,2013,15(6):R180.DOI:10.1186/ar4369.
[13]李勇,江建明,杨德鸿,等.骨关节炎关节滑液中白细胞介素18及其他相关因子含量测定[J].南方医科大学学报,2009,29(4):729-731. LI Y,JIANG J M,YANG D H,et al.Determination of the concentrations of interleukin-18 and other cytokines in the synovial fluid in patients with osteoarthritis[J].Journal of Southern Medical University,2009,29(4):729-731.
[14]GOUDA E A,ABOULATA A A,ELHAROUN A S,et al.Interleukin-18 expression in rheumatoid artheritis synovial tissue and its relation to disease activity[J].Egypt J Immunol,2007,14(2):1-10.
[15]VEENSTRA K G,JONAK Z L,TRULLI S,et al.IL-12 induces monocyte IL-18 binding protein expression via IFN-gamma[J].J Immunol,2002,168(5):2282-2287.
[16]NOVICK D,KIM S H,FANTUZZI G,et al.Interleukin-18 binding protein:a novel modulator of the Th1 cytokine response[J].Immunity,1999,10(1):127-136.
[17]KIM S H,SON C N,LEE H J,et al.Infliximab partially alleviates the bite force reduction in a mouse model of temporomandibular joint pain[J].J Korean Med Sci,2015,30(5):552-558.DOI:10.3346/jkms.2015.30.5.552.
[18]李波,卢利,谭学新,等.代谢型谷氨酸受体5在大鼠颞下颌关节疼痛中的作用[J].中国医科大学学报,2012,41(10):912-914. LI B,LU L,TAN X X,et al.Function of metabtrophic glutamate receptor subtype 5 for temporomandibular joint pain in rats[J].Journal of China Medical University,2012,41(10):912-914.
[19]KANEYAMA K,SEGAMI N,SUN W,et al.Levels of soluble cytokine factors in temporomandibular joint effusions seen on magnetic resonance images[J].Oral Surg Oral Med Oral Pathol Oral Radiol Endod,2005,99(4):411-418.DOI:10.1016/j.tripleo.2004.08.012.
[20]KANEYAMA K,SEGAMI N,NISHIMURA M,et al.Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders[J].Br J Oral Maxillofac Surg,2002,40(5):418-423.
[21]SUZUKI T,SEGAMI N,NISHIMURA M,et al.Co-expression of interleukin-1beta and tumor necrosis factor alpha in synovial tissues and synovial fluids of temporomandibular joint with internal derangement:comparison with histological grading of synovial inflammation[J].J Oral Pathol Med,2002,31(9):549-557.
[22]BRENNAN F M,MAINI R N,FELDMANN M.Role of pro-inflammatory cytokines in rheumatoid arthritis[J].Springer Semin Immunopathol,1998,20(1/2):133-147.
[23]WATKINS L R,WIERTELAK E P,GOEHLER L E,et al.Characterization of cytokine-induced hyperalgesia[J].Brain Res,1994,654(1):15-26.
[24]DUFF G W.Cytokines and acute phase proteins in rheumatoid arthritis[J].Scand J Rheumatol Suppl,1994,100:9-19.
[25]NORDAHL S,ALSTERGREN P,ELIASSON S,et al.Radiographic signs of bone destruction in the arthritic temporomandibular joint with special reference to markers of disease activity.A longitudinal study[J].Rheumatology (Oxford),2001,40(6):691-694.
[26]NORDAHL S,ALSTERGREN P,ELIASSON S,et al.Interleukin-1beta in plasma and synovial fluid in relation to radiographic changes in arthritic temporomandibular joints[J].Eur J Oral Sci,1998,106(1):559-563.
[27]NORTH J,SITUNAYAKE R D,TIKLY M,et al.Interleukin 1 beta,hand and foot bone mineral content and the development of joint erosions in rheumatoid arthritis[J].Ann Rheum Dis,1994,53(8):543-546.
[28]KONTZIAS A,EFTHIMIOU P.Adult-onset Still′s disease:pathogenesis,clinical manifestations and therapeutic advances[J].Drugs,2008,68(3):319-337.[29]TWILT M,MOBERS S M,ARENDS L R,et al.Temporomandibular involvement in juvenile idiopathic arthritis[J].J Rheumatol,2004,31(7):1418-1422.
[30]PEDERSEN T K,KUSELER A,GELINECK J,et al.A prospective study of magnetic resonance and radiographic imaging in relation to symptoms and clinical findings of the temporomandibular joint in children with juvenile idiopathic arthritis[J].J Rheumatol,2008,35(8):1668-1675.
[31]HASHKES P J,LAXER R M.Medical treatment of juvenile idiopathic arthritis[J].JAMA,2005,294(13):1671-1684.
[32]STOUSTRUP P,KRISTENSEN K D,KUSELER A,et al.Reduced mandibular growth in experimental arthritis in the temporomandibular joint treated with intra-articular corticosteroid[J].Eur J Orthod,2008,30(2):111-119.
[33]LAMAZZA L,GUERRA F,PEZZA M,et al.The use of etanercept as a non-surgical treatment for temporomandibular joint psoriatric arthritis:a case report[J].Aust Dent J,2009,54(2):161-165.DOI:10.1111/j.1834-7819.2009.01110.x.
[34]DOLWICK M F,DIMITROULIS G.Is there a role for temporomandibular joint surgery?[J].Br J Oral Maxillofac Surg,1994,32(5):307-313.
[35]AL-BELASY F A,DOLWICK M F.Arthrocentesis for the treatment of temporomandibular joint closed lock:a review article[J].Int J Oral Maxillofac Surg,2007,36(9):773-782.
(修回日期:崔丽红)
Role for IL-18 in Temporomandibular Joint Arthritis
HUANGFang1,HUYa-lin1,JIANGCheng-jin1,LIUJing-fang1,HEShao-heng2*,LIZhi-gang3*
Objective To explore the role of interleukin-18 (IL-18) in temporomandibular joint arthritis.Methods This study was conducted from November 2015 to April 2016.After being adaptively fed for 1 week,30 male BALB/c mice (SPF grade) were randomized into 5 groups (group A,group B,group C,group D and group E) with 6 mice in each.Group B,group C,group D and group E were injected collagenase Ⅱ on the 1st and 21st days of intervention,respectively.From the 1st to the 24th days of intervention,group A and group B were injected 0.9% sodium chloride solution,group C,group D and group E were injected IL-18,IL-18 binding protein (IL-18BP),IL-18 and IL-18BP,respectively.On the 25th day of intervention,peripheral blood and temporomandibular joint lavage fluid of mice were collected.The positive expression rates of the interleukin-18 receptor (IL-18R) in monocytes in peripheral blood and macrophages in temporomandibular joint lavage fluid were detected by flow cytometry (FCM).The levels of TNF-α and IL-1β in peripheral plasma/liquid supernatant of temporomandibular joint lavage fluid were detected by enzyme-linked immunosorbent assay (ELISA).Results The positive expression rate of IL-18R in peripheral blood monocytes in group B and group C was significantly higher than that in group A (P<0.05);the positive expression rate of IL-18R in peripheral blood monocytes in group C was significantly higher than that in group B,group D and group E (P<0.05).The positive expression rate of IL-18R in macrophages in temporomandibular joint lavage fluid in group B,group C,group D and group E was higher than that in group A (P<0.05);the positive expression rate of IL-18R in macrophages in temporomandibular joint lavage fluid in group C was higher than that in group B,group D and group E (P<0.05).The levels of TNF-α and IL-1β in peripheral plasma in group B,group C,group D and group E were higher than those in group A (P<0.05);the levels of TNF-α and IL-1β in peripheral plasma in group C were higher than those in group B,group D and group E (P<0.05).The levels of TNF-α and IL-1β in liquid supernatant of temporomandibular joint lavage fluid in group B,group C,group D and group E were higher than those in group A (P<0.05);the levels of TNF-α and IL-1β in liquid supernatant of temporomandibular joint lavage fluid in group C were higher than those in group B,group D and group E (P<0.05);the level of TNF-α in liquid supernatant of temporomandibular joint lavage fluid in group D was lower than that in group B (P<0.05);the level of TNF-α in liquid supernatant of temporomandibular joint lavage fluid in group E was higher than that in group D (P<0.05).Conclusion IL-18 can produce pro-inflammatory cytokines such as TNF-α and IL-1β which may play an important role in the occurrence and development of temporomandibular joint arthritis through IL-18R stimulating monocytes/macrophages.IL-18R may be a novel therapy target for temporomandibular joint arthritis.
Temporomandibular joint disorders;Arthritis;Interleukin-18
国家自然科学基金资助项目(81471592)
R 782.6
A
10.3969/j.issn.1007-9572.2017.12.010
2016-09-12;
2016-12-30)
1.121000 辽宁省锦州市,锦州医科大学
2.121000 辽宁省锦州市,锦州医科大学附属第一医院变态反应与临床免疫研究中心
3.121000 辽宁省锦州市,锦州医科大学附属第二医院修复科
*通信作者:何韶衡,教授;E-mail: shoahenghe@126.com
李志刚,教授;E-mail:ZGLi700103@163.com
