小鼠黑素细胞中过表达Oct-1对毛色主效基因的影响
2017-04-08杨玉静聂瑞强谢建山范瑞文许冬梅董常生
杨玉静,聂瑞强,谢建山,2,范瑞文,许冬梅,董常生
(1山西农业大学动物科技学院,山西太谷 030801;2山西医科大学基础医学院,太原 030001)
小鼠黑素细胞中过表达Oct-1对毛色主效基因的影响
杨玉静1,聂瑞强1,谢建山1,2,范瑞文1,许冬梅1,董常生1
(1山西农业大学动物科技学院,山西太谷 030801;2山西医科大学基础医学院,太原 030001)
【目的】克隆小鼠八聚体结合转录因子1(octamer-binding transcription factor 1,Oct-1)基因序列,探讨八聚体结合转录因子1在小鼠黑素细胞中过表达对毛色主效基因表达的影响及在毛色形成中的作用。【方法】使用实验室冻存的第5代小鼠黑素细胞,通过普通PCR方法用引物以小鼠黑素细胞cDNA为模板克隆Oct-1基因cDNA序列, 构建小鼠Oct-1克隆载体和真核表达载体;通过KEGG PATHWAY、NCBI、Transfec等软件对获得的序列进行生物信息学分析;在细胞水平通过细胞转染技术过量表达小鼠Oct-1;转染后使用荧光显微镜观察细胞转染效率,采用分光光度计对小鼠黑素细胞中黑色素含量进行测定,并进行Real-time PCR实验检测转染后黑素细胞中毛色主效基因在mRNA水平表达量的变化,Western blot实验检测转染后细胞中MITF、TYR、TYRP-1和TYRP-2蛋白水平的变化。【结果】经测序和拼接最终获得长度为2 313 bp的小鼠Oct-1基因的cDNA 序列;成功构建真核表达载体, 载体上连有小鼠黑素细胞特异性TYRP-2基因启动子和一个启动报告基因绿色荧光蛋白;通过KEGG PATHWAY 分析获得与毛色形成有关的34个候选基因,NCBI查找出34各个基因的启动子,由Transfec启动子分析软件找出Oct-1可以调节的毛色主效基因;细胞转染后,在荧光显微镜下可观察到黑素细胞带有绿色荧光说明转染效率明显;分光光度计检测显示,转染后小鼠黑素细胞中黑色素含量减少(P<0.05);荧光定量检测结果显示,小鼠黑素细胞中Oct-1 mRNA表达量显著增加(P<0.001),表明小鼠Oct-1转染效率显著,MITF mRNA显著降低至0.70倍(P<0.01),TCF mRNA显著降低至0.66倍(P<0.01),Ras、Frizzled、ERK2和TYRP-2 mRNA的表达未见变化,TYR mRNA显著增加至7.69倍(P<0.01),TYRP-1 mRNA升高至3.11倍(P<0.01),αMSH mRNA显著增加至18.49倍(P<0.001),AC mRNA显著增加至6.88倍(P<0.01),c-kit mRNA显著增加至18.75倍(P<0.001),ET1 mRNA增加至1.50倍(P<0.05),ETB-R mRNA显著增加至13.47倍(P<0.001),CAM mRNA增加至1.46倍(P<0.05);蛋白免疫印迹结果显示,小鼠黑素细胞中Oct-1转染组MITF蛋白显著降低至0.67倍(P<0.01),TYR蛋白增加至1.16倍(P<0.05),TYRP-1蛋白升高至1.15倍(P<0.05),TYRP-2蛋白未见变化。【结论】通过PCR和克隆技术及核酸测序技术获得了小鼠Oct-1基因全长2 313 bp的CDS区,经生物信息学分析找出Oct-1作用的毛色主效基因,过表达Oct-1后使黑素细胞中MITF和TCF的表达降低,TYR、TYRP-1、αMSH、AC、c-kit、ET1、ETB-R和CAM的表达增加。证实Oct-1可调节毛色主效基因的表达,参与黑色素合成的调节,改变毛色。
八聚体结合转录因子1;黑色素;毛色主效基因;MITF;TYR
0 引言
【研究意义】动物毛色由毛发中沉着的黑色素含量及其种类决定,不同颜色的被毛是当今绿色纺织业的主要原料,黑色素在黑色素细胞中形成,包括真黑色素和伪黑色素两种色素成分,使被毛呈现黑色或金黄色[1]。在黑色素形成过程中,受诸多相关基因的调控。八聚体结合转录因子1(octamer-binding transcription factor 1,Oct-1),又称为POU2F1,属于POU反式作用因子家族成员,该家族成员都含有保守的功能域即POU结构域,包含两个螺旋-转角-螺旋DNA结合域,位于N端的POU特异结构域和位于C端的POU同源异形域,通过一个相对较短的连接器连接[2]。Oct-1蛋白对DNA八聚体元件有高亲和性,在组织细胞中广泛表达[3],参与调节多种组织特异性基因和管家基因的表达[4]。Oct-1蛋白不具有导肽,N端无信号肽,没有跨膜结构域,表现为亲水性,蛋白质二级结构主要结构元件是无规卷曲和α-螺旋。Oct-1作为一个转录抑制因子或作为一个转录激活因子取决于启动子序列,Oct-1可以直接结合到启动子或与其他转录因子互相作用结合到启动子上调节基因的表达[5]。【前人研究进展】关于Oct-1主要集中在癌细胞、亚型结构、2型糖尿病、与其他基因的相互作用及其过表达或敲除等方面的研究。癌症生物学研究Oct-1转录因子的两个相反功能显示,转录因子Oct-1浓度低时IFN-γ(γ干扰素)刺激膀胱癌细胞增殖,当转录因子过度积累时促进IFN-γ诱导细胞发生凋亡[6]。在胃癌细胞中,活化的CDC42相关激酶1(ACK1)磷酸化在细胞存活和凋亡中起重要作用的蛋白激酶B(PKB/AKT),激活AKT通路中转录因子Oct-1,结合靶基因蜕皮激素(ECD)的启动子区,调控ECD的表达[7]。过表达miR-449a(microRNA 449a)可下调钙蛋白酶6(CAPN6)和Oct-1,抑制肝肿瘤细胞增殖和诱导细胞凋亡[8]。p38蛋白激酶(酪氨酸磷酸蛋白激酶)抑制剂可抑制Oct-1结合能力[9]。在人乳腺癌细胞(MDA-MB-231)中,Oct-1和Oct-2可结合诱导型一氧化氮合酶(iNOS)启动子,形成一个高级复合体,使其不能结合RNA聚合酶II,导致iNOS转录不能进行[10]。在乳腺囊泡分泌上皮细胞(MECs)中,Oct-2与Oct-1形成一个异聚体,反式激活催乳激素(HP)诱发型β-酪蛋白基因表达[11]。ZHAO等研究表明Oct-1亚型的结构改变对其结合靶基因和组织特异性基因的转录活性发挥重要作用,Oct-1Z亚型是Oct-1基因选择性剪接形成,其蛋白C端较短,但仍可有效地结合到Oct-1基因结构寡核苷酸上发挥作用,像Oct-1B亚型,可激活鼠β-酪蛋白基因启动子活性[4]。KRYLOVA等发现Oct-1基因亚型Oct-1X的mRNA,在编码过程中这个亚型mRNA截去N-末端序列,经试验证明其在人类组织和细胞系中广泛表达,能激活管家基因(组蛋白H2B)和调控组织特异性基因(B29)的转录[12]。NG等研究2型糖尿临床基础病例中发现Oct-1基因的6个单核苷酸多态性,其中rs4657652,rs7532692,rs10918682和rs3767434在大部分2型糖尿病患者中发挥作用,rs3767434作用于家族性2型糖尿病患者,此外,rs10918682的G等位基因风险导致胰岛素治疗的使用量增加[13]。VOLETI 等研究发现在细胞中Oct-1作为C-反应蛋白(CRP)启动子的转录抑制因子,过表达Oct-1会抑制CRP的表达[5]。在人类的干细胞中,Oct-1过表达增强细胞因子诱导人诱导型一氧化氮合成酶(hiNOS)蛋白表达,当hiNOS启动子上Oct-1结构突变,会减少细胞因子诱导hiNOS启动子的活性[14]。THUM等研究表明敲除Oct-1基因可阻止氧化低密度脂蛋白(oxLDL)介导的细胞色素P450(CYP)基因沉默[15]。【本研究切入点】关于转录因子Oct-1在毛色方面的研究,有报道乙醇通过减少Oct-1与Slc7a11基因启动子的结合增加毛色基因Slc7a11的表达[16],然而,Oct-1在毛色形成过程中是否起作用尚不清楚。【拟解决的关键问题】本试验通过生物信息学方法预测到毛色生成主效基因中有Oct-1的靶基因,通过黑素细胞转染过表达Oct-1,探究Oct-1对黑色素形成过程中靶基因表达的影响,进而分析其对毛色形成的作用。
1 材料与方法
试验于2015年6月至2015年12月在山西农业大学羊驼生物工程实验室完成。
1.1 主要试剂和仪器
试剂:黑素细胞培养基(Scien Cell)、RIPA裂解液(碧云天)、TRIZOL(Invitrogen,美国)、反转录PCR试剂盒(TaKaRa,大连)、qRT- PCR kit(TaKaRa,大连)、MITF多克隆抗鼠 IgG抗体(abcam,艾博抗上海)、TYR多克隆抗鼠 IgG抗体(abcam,艾博抗上海)、T4 DNA Ligase(TaKaRa,大连)、蛋白marker(Fermentas公司);仪器:StepOne Fast Real time PCR System(Life technologies,美国)、高速低温冷冻离心机(Sigma;德国)、普通PCR仪(Bio RAD,美国)、紫外凝胶成像系统(WV-BP330,Panasonic公司,日本)、核酸蛋白测定仪(Nanodrop-1000,Thermo,美国)。
1.2 试验方法
1.2.1 Oct-1靶基因的预测和筛选 本研究根据KEGG PATHWAY database Melanogenesis(http://www. genome.jp/kegg/pathway.html)获得与毛色形成有关的34个候选基因,通过NCBI查找其各个基因的启动子,通过Transfec启动子分析软件找到了Oct-1对多个基因的启动子有作用,设计本试验方案对其是否有调节作用进行验证。
1.2.2 内源性Oct-1前体的克隆 使用GeneBank信息查询系统检索小鼠Oct-1 mRNA,找出CDS区的核酸序列。以cDNA为模版进行PCR扩增,产物电泳检测,切下目的条带,送公司进行测序,确定目的序列大小。小鼠Oct-1CDS区全长引物,由华大科技公司合成。Oct-1引物为F:5′-ATGAATAATCCATCAGA AACCAATAAATCATCTATGG-3′;R:5′-TCACTGTG CCTTGGAGGCAGCTGT-3′。
1.2.3 Oct-1真核表达载体的构建 小鼠Oct-1载体的构建方法为先用T-Vector pMD19载体试剂连接克隆载体,得到质粒,送公司测序,确定Oct-1序列连到克隆载体上,再进行真核表达载体的构建,将克隆载体质粒和实验室存有的慢病毒载体质粒一起用相同的限制性内切酶xbal、SalI进行双酶切和电泳,获得目的基因和真核表达载体的DNA片段,再用T4连接酶试剂进行连接,经过转化和涂板,挑菌摇菌,提出质粒并测序,确定表达载体是否构建成功。
1.2.4 小鼠黑色素细胞的复苏、培养与转染 将实验室冻存第5代小鼠黑素细胞从液氮中取出,迅速置于37℃水浴锅中进行解冻,在水中迅速摇动,直至全部溶解,接种于加有黑素细胞培养基的细胞培养板上,在37℃5%CO2饱和湿度培养箱中培养。在6孔板底壁长到60%—80%汇合时,通过转染连接有小鼠黑色素细胞特异性TYRP2启动子的GFP表达质粒,培养72 h,提取总蛋白和RNA进行测定。
1.2.5 黑色素测定 将空白、空载体和转染组的小鼠黑色素细胞收集起来,PBS冲洗2—3次后,用0.2 mol·L-1NaOH溶解黑色素细胞,进行黑色素含量的测定[17]。
1.2.6 总RNA的提取及反转录合成 总RNA的提取按照Trizol试剂盒说明书进行,然后用6210A反转录试剂盒对总RNA进行反转录合成长链cDNA,反应后,用核酸蛋白测定仪测定其浓度,保存后用于基因的扩增。
1.2.7 实时荧光定量PCR 通过Premier 5.0引物设计软件,根据KEGG通路中得知Oct-1的靶基因,结合NCBI确定靶基因的序列,设计靶基因的实时荧光定量PCR扩增引物,并通过NCBI和UCSC检测引物的特异性。引物见表1。
进行定量PCR反应后,先判定PCR反应的特异性,再用荧光曲线的CT值计算定量结果,相对表达量采用△△CT法计算[18]。所得数据用Microsoft Excel进行统计分析,结果用平均值±标准误(Means±SE)表示,每个基因通过内参基因β-actin校正后得出不同基因数据,采用Prism软件进行数据分析,判断每个基因的表达是否存在差异[19]。
1.2.8 Western Blot分析 按照总蛋白提取试剂盒说明书方法提取黑色素细胞总蛋白,然后进行SDS-PAGE电泳,当电泳将条带分散后,切出需要的蛋白大小的胶,进行转膜,转膜后将NC膜封闭1h(提前1 h配好封闭液),一抗4℃孵育过夜。孵育后用TBST洗膜,二抗37℃孵育1 h;二抗孵育后TBST洗膜30min,最后,在暗室曝光显现目的基因蛋白条带,得到有条带的胶片,标记大小。灰度值分析用Quantity one软件进行,同时通过β-actin作为内参,误差校正=目的蛋白灰度值/β-actin蛋白含量灰度值,所得值用Microsoft Excel进行统计,通过Prism软件进行数据分析,判断每个基因的表达是否存在差异。
2 结果
2.1 Oct-1的生物信息学预测和筛选
通过筛选发现MITF、TCF、Ras、Frizzled、ERK2、TYRP-2、TYR、TYRP-1、αMSH、AC、c-kit、ET1、ETB-R和CAM可能是Oct-1调节的靶基因,并且它们是黑色素合成与毛色形成过程中的关键基因,其直接参与了黑色素生成的调控过程,从而影响动物毛色的形成。

表1 引物序列Table 1 Primes in this experiment
2.2 Oct-1真核表达载体构建及测序
GeneBank中获得小鼠目的基因Oct-1的CDS区序列,扩增出单一条带用于构建真核表达载体(图1)。试验所用慢病毒载体,连有小鼠黑素细胞特异性TYRP-2基因启动子和一个启动报告基因绿色荧光蛋白,载体中已有酶切位点,用限制性内切酶XbaI和SalI切出载体,同样用XbaI和SalI从克隆载体中切出目的条带,进行连接反应。成功构建表达载体,质粒提取,公司测序后,在NCBI进行BLAST比对测序得到的结果和已知目的基因序列,得出序列大小完全匹配,为2 313 bp。
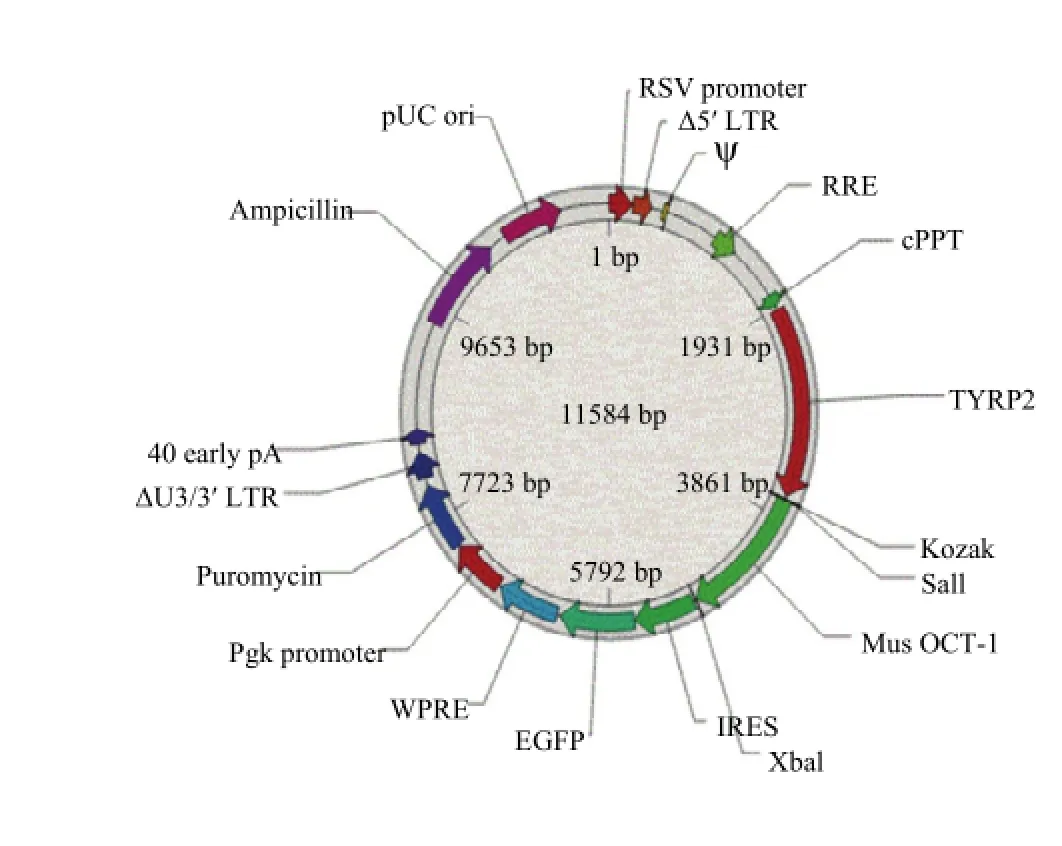
图1 小鼠Oct-1真核表达载体结构Fig. 1 Structure of over-expression vectiry Mus Oct-1
2.3 黑色素细胞的生长形态特征
小鼠黑色素细胞传代后正常培养6 h,可见活细胞贴壁伸展,再过18 h呈明显的树突状,生长良好。继续培养4 d后,在6孔培养板的每孔中铺满底壁的60%—80%时,可进行传代或转染试验(图2)。
2.4 Oct-1在黑色素细胞系的转染
2.4.1 黑色素细胞的转染效率观察 正常培养的小鼠色素细胞,长到对数生长期时即可进行转染试验,经过多次转染试验的探索,得出转染效率最高的是7.5 μgDNA/孔,最终确定该转染DNA浓度为转染试验的最佳浓度(图3)。

图2 小鼠黑色素细胞的形态Fig. 2 Morphology of melanocytes of mice
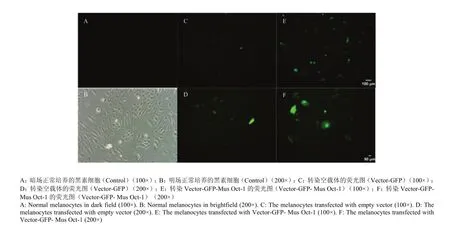
图3 小鼠Oct-1转染黑色素细胞的效率Fig. 3 The efficiency of transfected melanocytes by Mus Oct-1
2.4.2实时荧光定量PCR检测Oct-1在黑色素细胞系的转染效率试验设计小鼠黑色素细胞空白对照组、空载组和试验组,当细胞长到对数生长期进行转染试验,结果发现试验组小鼠Oct-1被极显著的提高(P<0.001)(图4)。
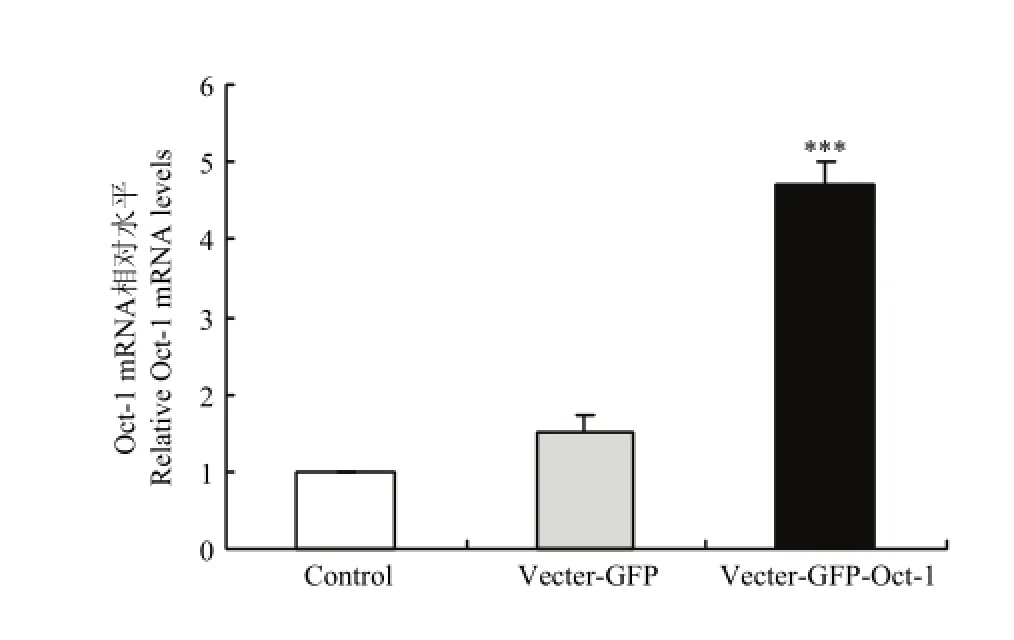
图4 转染小鼠Oct-1黑色素细胞Oct-1含量Fig. 4 Oct-1 mRNA levels in melanocytes transfected by the Mus Oct-1
2.4.3 转染后黑色素含量测定 小鼠黑色素细胞中黑色素含量通过分光光度法测定,测定空白组、空载体组和转染组,结果得出,转染后黑色素含量降低至0.87倍(P<0.05)(图5)。
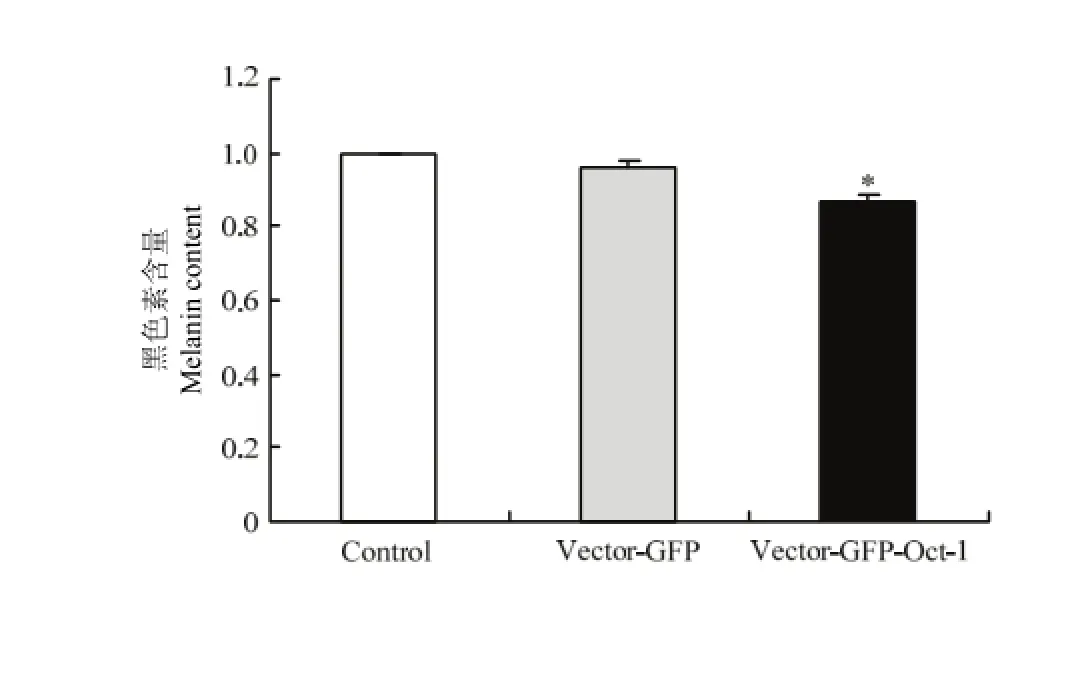
图5 小鼠Oct-1转染后黑色素细胞中黑色素mRNA水平Fig. 5 Melanin content in melanocytes transfected by the Mus Oct-1
2.4.4 转染黑色素细胞后主效基因mRNA的检测 通过RT-PCR的方法检测了小鼠Oct-1过表达对主效基因mRNA表达的影响。统计分析结果显示,与空载组相比,转染组的MITF mRNA显著降低至0.70倍(P<0.01),TCF mRNA显著降低至0.66倍(P<0.01),Ras mRNA未见变化,Frizzled mRNA未见变化,ERK2 mRNA未见变化,TYRP-2 mRNA未见变化;TYR mRNA显著增加至7.69倍(P<0.01),TYRP-1 mRNA升高至3.11倍(P<0.01),αMSH mRNA显著增加至18.49倍(P<0.001),AC mRNA显著增加至6.88倍(P<0.01),c-kit mRNA显著增加至18.75倍(P<0.001),ET1 mRNA增加至1.50倍(P<0.05),ETB-R mRNA显著增加至13.47倍(P<0.001),CAM mRNA增加至1.46倍(P<0.05),(图6)。由此可见,过表达Oct-1能改变毛色主效基因mRNA的表达,MITF和TCF表达量显著降低,Ras、Frizzled、ERK2和TYRP-2表达量未见变化,TYR、TYRP-1、αMSH、AC、c-kit、ET1、ETB-R和CAM表达量增加。
2.4.5 Western blot 检测MITF、TYR、TYRP-1和TYRP-2蛋白在转染后黑色素细胞中的表达 通过Western blot对所选主效基因进行检测,得出蛋白条带,经软件统计分析,结果显示,与空载组相比,转染组MITF蛋白显著降低至0.67倍(P<0.01),TYR蛋白升高至1.16倍(P<0.05),TYRP-1蛋白升高至1.15倍(P<0.05),TYRP-2蛋白没有变化。由此得出,过表达Oct-1可以显著减少MITF蛋白的表达,增加TYR和TYRP-1蛋白表达量,对TYRP-2蛋白表达未见影响(图7)。
3 讨论
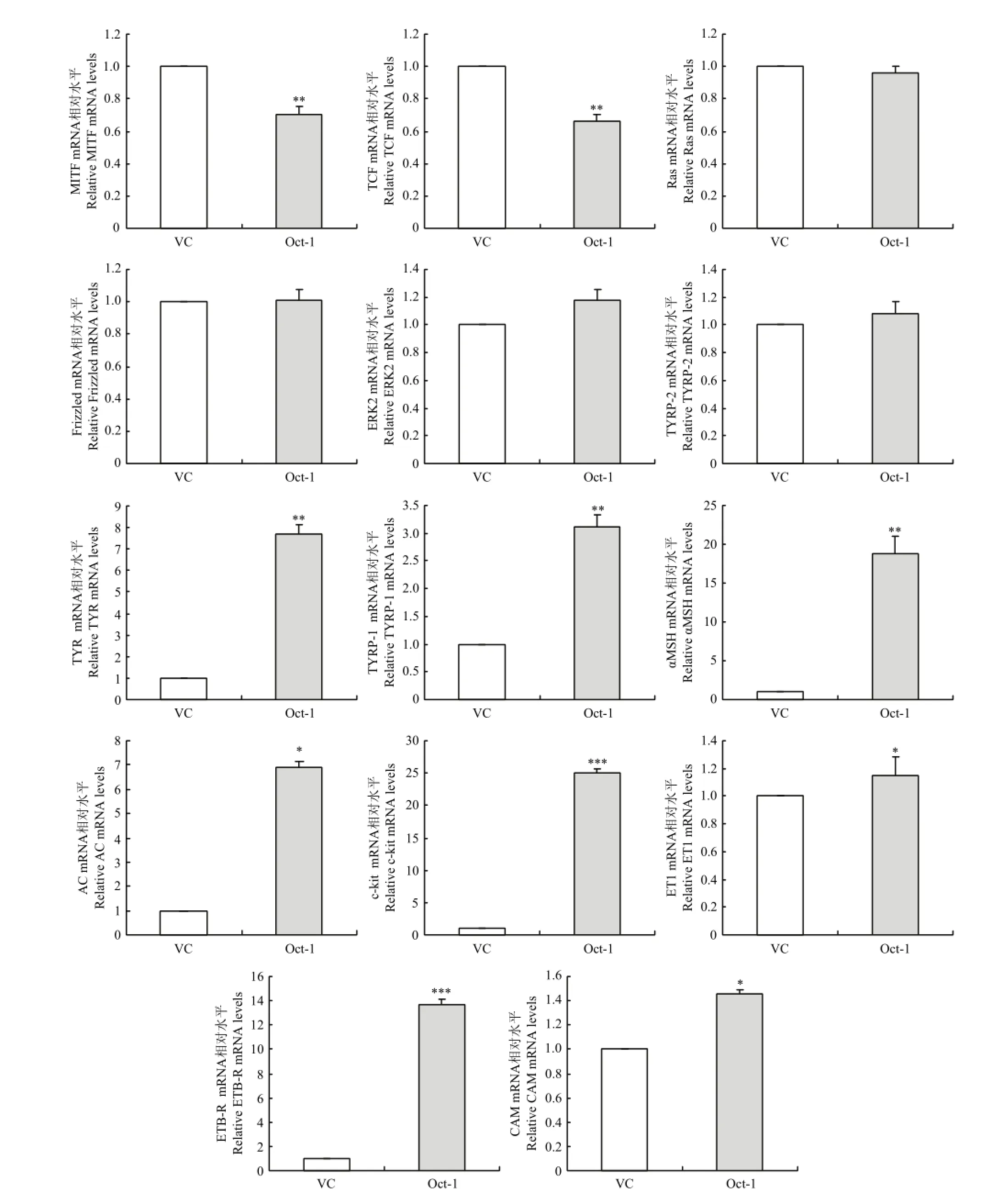
图6 RT-PCR检测转染小鼠Oct-1后黑色素细胞中主效基因mRNA的表达Fig. 6 Real-time PCR analysis of major genes expression in melanocytes transfected by the Mus Oct-1
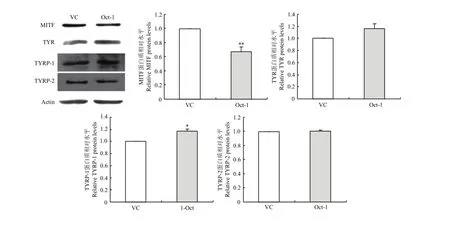
图7 Western blot 检测转染小鼠Oct-1后黑色素细胞中MITF、TYR、TYRP-1和TYRP-2蛋白的表达Fig. 7 Western blot analysis of MITF,TYR,TYRP-1 and TYRP-2 expression in melanocytes transfected by the Mus Oct-1
本试验通过构建小鼠八聚体结合转录因子(Oct-1)过表达载体,采用细胞转染技术过表达Oct-1,对影响黑色素沉着的调节机制进行研究。试验通过对黑色素细胞中黑色素含量进行测定,同时经RT-PCR以及Western bolt方法对黑色素细胞中毛色相关基因的表达进行检测。结果显示,Oct-1的过表达可使黑素细胞中黑色素含量减少;在mRNA水平,MITF和TCF表达量显著降低,Ras、Frizzled、ERK2和TYRP-2的表达未见变化,TYR、TYRP-1、αMSH、AC、c-kit、ET1、ETB-R和CAM表达量增加。在蛋白水平,MITF蛋白显著降低,TYR、TYRP-1蛋白增加,TYRP-2蛋白未见变化。有报道,减少Oct-1结合到Slc7a11基因,会增加毛色基因Slc7a11的表达[16],而Sla7a11基因可以使绵羊长出棕色/黄色的毛斑块[20]。在毛发和黑素细胞的研究中发现,Slc7a11基因是一个主要的褐黑色遗传控制器,对真黑素的影响最小或没有[1],当Slc7a11基因突变可使生成褐黑色素的途径受阻,转向真黑色素合成路径,导致黑色素细胞内真黑色素的生成量显著增加,然而,试验中小鼠毛色由红、黄色变为灰色,显著降低了毛发中褐黑色素含量,但是毛发中真黑色素含量没有显著差异[1]。此外,真/褐黑色素转换机制主要与TYR活性有关,高活性的TYR导致真黑色素产生,对于褐黑色素的生成,是TYR活性降低造成的[21],这也就说明了为什么Oct-1过表达后会出现一些主效基因表达降低,一些主效基因表达增加,还有一些基因未见变化的现象,Oct-1与黑色素合成通路中相关基因之间形成复杂的作用机制,本试验结果可为进一步研究Oct-1具体调控单个主效基因提供基础。在Oct-1过表达和缺失的相关报道中,胰岛素通过衰减Oct-1的抑制效应刺激碳水化合物反应元件结合蛋白(ChREBP)的表达,过表达Oct-1抑制内源性ChREBP mRNA和蛋白的表达,敲除Oct-1导致ChREBP mRNA和蛋白表达增加[22]。Oct-1过表达,人胃癌细胞增殖率导致胃腺癌变[23]。相反,在T细胞中,白介素17启动子有Oct-1结合位点, Oct-1基因缺失导致IL-17表达增加[24]。在头部和颈部癌症的研究中显示,敲除Oct-1显著降低同源异形盒蛋白D10(HOXD10)和同源异形盒蛋白D11(HOXD11)的表达,抑制头颈部鳞状细胞癌(HNSCC)增殖[25]。在初级人类CD4 T细胞中过表达Oct-1或Oct-2显示,Oct-1和Oct-2不能结合到HIV-1基因长末端重复序列(LTR),对HIV-1的转录没有影响[26]。在小鼠胚胎成纤维细胞和人类肺腺癌A549细胞,Oct-1的缺失在细胞生长或生存培养中有很小的影响[27]。此外,还有其他相关研究发现,在小鼠初级胰岛细胞,提高环磷酸腺苷(cAMP)会减少Oct-1的含量,这导致增加高血糖素原和胰岛素原mRNA的表达[28]。Oct-1通过O-乙酰葡糖胺(O-GlcNAc)的改变调控目的基因活性[27]。Oct-1和/或Oct-2缺陷,影响与II型糖尿病和代谢综合征有关的高迁移率族蛋白A1(HMGA1)基因的表达,从而导致胰岛素受体基因(INSR)功能缺失,阻碍INSR信号通路[29]。Oct-1充当细胞色素P450,家族2亚型,多肽4(CYP2D4)的活化剂,YY-1可能充当CYP2D4的抑制剂,YY-1结合结构的核心序列与Oct-1结合结构部分重叠,YY-1结合到神经表达调控元件(NERE),干扰Oct-1激活,从而调节CYP2D4基因[30]。在体外共转染Oct-1增加立即早期癌基因BRLF1结合到各种细胞EB病毒(EBV)启动子上,提高BRLF1的能力去激活细胞裂解基因表达,促进潜在病毒裂解。在EBV阳性B细胞和EBV阳性上皮细胞系,很低的内源性Oct-1表达降低细胞裂解EBV基因表达水平[31]。通过本试验可知,转录因子Oct-1在毛色形成中可能起到抑制的作用,然而具体的调控机制仍不清楚,由于在黑色素形成过程中存在真色素与褐黑色之间的转化,接下来有必要从真黑色和褐黑色两个方面分别进行深入探究。
4 结论
通过脂质体转染使小鼠八聚体结合转录因子1在小鼠黑色素细胞中过表达后,黑素细胞中黑色素含量减少,MITF和TCF表达量显著降低,Ras、Frizzled、ERK2和TYRP-2未见变化,TYR、TYRP-1、αMSH、AC、c-kit、ET1、ETB-R和CAM表达量增加,表明Oct-1可通过调控毛色主效基因表达的变化,参与调节黑色素的合成,影响毛色的变化。
[1] CHINTATA S, LI W, LAMOREUX M L, ITO S, WAKAMATSU K, SVIDERSKAYA E V, BENNETT D C, PARK Y M, GAHI W A, HUIZING M, SPRITZ R A, BEN S, NOVAK E K, TAN J, SWANK R T. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells.Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(31): 10964-10969.
[2] DOUCLEFF M, CLORE G M. Global jumping and domain-specific intersegment transfer between DNA cognate sites of the multidomain transcription factor Oct-1.Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(37): 13871-13876.
[3] KAMBE F, TSUKAHARA S, KATO T, SEO H. The POU-domain protein Oct-1 is widely expressed in adult rat organs.Biochimica et Biophysica Acta, 1993, 1171(3):307-310.
[4] ZHAO F Q, ZHENG Y, DONG B, OKA T. Cloning, genomic organization, expression, and effect on β-casein promoter activity of a novel isoform of the mouse Oct-1 transcription factor.Gene, 2004, 326: 175-187.
[5] VOLETI B, HAMMOND D J JR., THIRUMALAI A, AGRAWAL A. Oct-1 acts as a transcriptional repressor on the C-reactive protein promoter.Molecular Immunology, 2012, 52(3-4): 242-248.
[6] SZEKERES K, KOUL R, MAURO J, LLOYD M, JOHNSON J, BLANCK G. An Oct-1-based, feed-forward mechanism of apoptosis inhibited by co-culture with Raji B-cells: Towards a model of the cancer cell/B-cell microenvironment.Experimental and Molecular Pathology, 2014, 97(3):585-589.
[7] XU S H, HUANG J Z, XU M L, YU G, YIN X F, CHEN D, YAN G R. ACK1 promotes gastric cancer epithelial- mesenchymal transition and metastasis through AKT-POU2F1-ECD signalling.The Journal ofPathology, 2015, 236(2):175-185.
[8] LIU Y, WANG Y, SUN X, MEI C, WANG L, LI Z, ZHA X. miR-449a promotes liver cancer cell apoptosis by down-regulation of Calpain6 and POU2F1.Oncotarget, 2016, 7(12):13491-13501.
[9] LIN BR, NATARAJAN V. Negative regulation of human U6 snRNA promoter by p38 kinase through Oct-1.Gene, 2012, 497(2):200-207.
[10] BENTRARI F, CHANTOME A, KNIGHTS A, JEANNIN J F, PANCE A. Oct-2 forms a complex with Oct-1 on the iNOS promoter and represses transcription by interfering with recruitment of RNA PolII by Oct-1.Nucleic Acids Research, 2015, 43(20):9757-9765.
[11] QIAN X, ZHAO F Q. Collaborative interaction of Oct-2 with Oct-1 in transactivation of lactogenic hormones-induced beta-casein gene expression in mammary epithelial cells.General and Comparative Endocrinology, 2014, 204:185-194.
[12] KRYLOVA I D, PORTSEVA T N, GEORGIEVA S G, STEPCHENKO A G, PANKRATOVA E V. [The new isoform of Oct-1 transcription factor is transcribed from alternative promoter].Molekuliarnaia Biologiia, 2013, 47(4): 634-641.
[13] NG M C, LAM V K, TAM C H, CHAN A W, SO W Y, MA R C, ZEE B C, WAYE M M, MAK W W, HU C, WANG C R, TONG P C, JIA W P, CHAN J C. Association of the POU class 2 homeobox 1 gene (POU2F1) with susceptibility to Type 2 diabetes in Chinese populations.Diabetic Medicine, 2010, 27(12): 1443-1449.
[14] PARK K S, GUO Z, SHAO L, DU Q, GELLER D A. A Far-Upstream Oct-1 Motif Regulates Cytokine- Induced Transcription of the Human Inducible Nitric Oxide Synthase Gene.Journal of Molecular Biology, 2009, 390(4):595-603.
[15] THUM T, BORLAK J. LOX-1 receptor blockade abrogates oxLDL-induced oxidative DNA damage and prevents activation of the transcriptional repressor Oct-1 in human coronary arterial endothelium.The Journal of Biological Chemistry, 2008, 283(28): 19456-19464.
[16] LIN X, YANG H, ZHANG H, ZHOU L, GUO Z. A novel transcription mechanism activated by ethanol: induction of Slc7a11 gene expression via inhibition of the DNA-binding activity of transcriptional repressor octamer-binding transcription factor 1 (OCT-1).The Journal of Biological Chemistry, 2013, 288(21): 14815-14823.
[17] 马淑慧, 薛霖莉, 徐 刚, 侯亚琴, 耿建军, 曹 靖, 赫晓燕, 王海东,董常生. 黑色素细胞中过量表达miR-137对TYRP-1和TYRP-2的影响. 中国农业科学, 2013,46(16):3452-3459.
MA S H, XUE L L, XU G, HOU Y Q, GENG J J, GAO J, HE X Y, WANG H D, DONG C S. The influences of over-expressing miR-137on TYRP-1 and TYRP-2 in melanocytes.Scientia Agricultura Sinica, 2013, 46(16): 3452-3459. (in Chinese)
[18] 谢建山, 张丹瑾, 郭艳妮, 范瑞文, 许冬梅, 杨玉静, 聂瑞强, 于秀菊, 董常生. 突触融合蛋白4在不同毛色小鼠皮肤组织的差异表达.畜牧兽医学报, 2015, 46(6):957-964.
XIE J S, ZHANG D J, GUO Y N, FAN R W, XU D M, YANG Y Y, NIE R Q, YU X J, DONG C S.Differential expression of the syntaxin 4 in differential coat color mice skin.Acta Veterinaria et Zootechnica Sinica, 2015,46(6): 957-964. (in Chinese)
[19] 聂瑞强, 杨玉静, 谢建山, 范瑞文, 许冬梅, 于秀菊, 段志成, 董常生. Pax6 PAI 亚结构域在黑色素细胞中对MITF、TYR、TYRP1 和TYRP2 的影响. 中国农业科学, 2016, 49(17):3433-3442.
NIE R Q, YANG Y J, XIE J S, FAN R W, XU D M, YU X J, DUAN Z C, DONG C S. Influences of Pax6 PAI subdomain on MITF, TYR,TYRP1 and TYRP2 in melanocytes.Scientia Agricultura Sinica, 2016, 49(17): 3433-3442. (in Chinese)
[20] HE X, LI H, ZHOU Z, ZHAO Z, LI W. Production of brown/yellow patches in the SLC7A11 transgenic sheep via testicular injection of transgene.Journal of Genetics and Genomics, 2012, 39(6):281-285.
[21] ITO S, FUJITA K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography.Analytical Biochemistry, 1985, 144(2):527-536.
[22] SIREK A S, LIU L, NAPLES M, ADELI K, NG D S, JIN T. Insulin stimulates the expression of carbohydrate response element binding protein (ChREBP) by attenuating the repressive effect of Pit-1, Oct-1/Oct-2, and Unc-86 homeodomain protein octamer transcription factor-1.Endocrinolog, 2009, 150(8): 3483-3492.
[23] JEONG S H, LEE Y J, CHO B I, HA W S, CHOI S K, JUNG E J, JU Y T, JEONG C Y, KO G H, YOO J, HONG S C. OCT-1 overexpression is associated with poor prognosis in patients with well-differentiated gastric cancer.Tumour Biology, 2014, 35(6): 5501-5509.
[24] KIM LK, ESPLUGUES E, ZORCA CE, PARISI F, KLUGER Y, KIM T H, GALJART N J, FLAVELL R A. Oct-1 regulates IL-17 expression by directing interchromosomal associations in conjunction with CTCF in T cells.Molecular Cell, 2014, 54(1):56-66.
[25] SHARPE D J, ORR K S, MORAN M, WHITE S J, MCQUAID S, LAPPIN T R, THOMPSON A, JAMES J A. POU2F1 activity regulates HOXD10 and HOXD11 promoting a proliferative and invasive phenotype in head and neck cancer.Oncotarget, 2014, 5(18):8803-8815.
[26] ZHANG M, GENIN A, CRON R Q. Overexpression of octamer transcription factors 1 or 2 alone has no effect on HIV-1 transcription in primary human CD4 T cells.Virology, 2004, 321(2):323-331.
[27] KANG J, SHEN Z, LIM J M, HANDA H, WALLS L, TANTIN D. Regulation of Oct1/Pou2f1 transcription activity by O-GlcNAcylation.FASEB Journal, 2013, 27(7):2807-2817.
[28] WANG P, WANG Q, SUN J, WU J, LI H, ZHANG N, HUANG Y, SU B, LI R K, LIU L, ZHANG Y, ELSHOLTZ H P, HU J, GAISANO H Y, JIN T. POU homeodomain protein Oct-1 functions as a sensor for cyclic AMP.The Journal of Biological Chemistry, 2009, 284(39): 26456-26465.
[29] CHIEFARI E, ARCIDIACONO B, POSSIDENTE K, IIRITANO S, VENTURA V, PANDOLFO R, BRUNETTI F S, GRECO M, FOTI D, BRUNETTI A. Transcriptional regulation of the HMGA1 gene by octamer-binding proteins Oct-1 and Oct-2.PLoS One, 2013, 8(12): e83969.
[30] MIZUNO D, TAKAHASHI Y, HIROI T, IMAOKA S, KAMATAKI T, FUNAE Y. A novel transcriptional element which regulates expression of the CYP2D4 gene by Oct-1 and YY-1 binding.Biochimica et Biophysica Acta, 2003, 1627(2-3):121-128.
[31] ROBINSON A R, KWEK S S, HAGEMEIER S R, WILLE C K, KENNEY S C. Cellular transcription factor Oct-1 interacts with the Epstein-Barr virus BRLF1 protein to promote disruption of viral latency.Journal of Virology, 2011, 85(17):8940-8953.
(责任编辑 林鉴非)
The Influences of Over-Expression of Oct-1 on Major Genes of Coat Color in Melanocytes of Mice
YANG YuJing1, NIE RuiQiang1, XIE JianShan1,2, FAN RuiWen1, XU DongMei1, DONG ChangSheng1
(1College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu 030801, Shanxi;2School of Basic Medical Sciences, Shanxi Medical University, Taiyuan 030001)
Oct-1; melanin; major genes of coat color; MITF; TYR
10.3864/j.issn.0578-1752.2017.04.016
2016-01-26;接受日期:2016-12-20
国家高科技研究发展计划(“863”计划,2013AA102506)、公益性行业(农业)科研专项(201303119)、山西农业大学创新团队建设计划(CXTD201201)
联系方式:杨玉静,E-mail:15110679040@163.com。通信作者董常生,E-mail:cs_dong@sxau.edu.cn
Abstract:【Objective】The aim of the study was to clone the octamer-binding transcription factor 1, to investigate whether over-expression of Oct-1 regulated the major genes of coat color in melanocytes of mouse at the transcriptional levels and to explore its influence on the formation of melanin. 【Method】 The CDS region in Oct-1 gene were cloned from melanocytes of mouse by primers and PCR to build a mouse Oct-1 cloning vector and eukaryotic expression vector. The KEGG PATHWAY, NCBI, and Transfec softwares were adopted to analyze the biological information of the obtained sequence. Over-expression of Oct-1 was conducted in the melanocytes of the 5thgeneration mouse through the cell transfection technique and transfer efficiency was observed by fluorescence microscope. The content of melanin in melanocytes was detected by spectrophotometer. The level of major genes were detected using real-time PCR and the proteins of MITF, TYR, TYRP-1 and TYRP-2 were detected using western blot.【Result】Results showed that the 2 313 bp cDNA sequence of Oct-1 gene was obtained by sequencing and splicing. Eukaryotic expression vector was successfully constructed with specific TYRP-2 gene promoter of mouse and a startup report gene of green fluorescent protein. KEGG PATHWAY analysis obtained 34 candidate genes related with coat color, and the promoters of these 34 candidate genes were found by NCBI. The major genes of coat color regulated by Oct 1 was determined by using Transfec software. Under the fluorescence microscope, green fluorescence could be identified in melanocytes of mouse. The contents of melanin in melanocytes were reduced (P<0.05). Real-time PCR results showed that Oct-1 mRNA was significantly increased (P<0.001),witch indicated Oct-1 high transfection efficiency. MITF mRNA was significantly reduced to 0.70 times (P<0.01) and TCF mRNA was significantly reduced to 0.66 times (P<0.01). The expressions of Ras, Frizzled, ERK2 and TYRP-2 mRNA did not change. TYR mRNA was significantly increased to 7.69 times (P<0.01), TYRP-1 mRNA was significantly increased to 3.11 times (P<0.01), αMSH mRNA was significantly increased to 18.49 times (P<0.001), AC mRNA was significantly increased to 6.88 times (P<0.01), c-kit mRNA was significantly increased to 18.75 times (P<0.001), ET1 mRNA was increased to 1.50 times (P<0.05), ETB-R mRNA was significantly increased to 13.47 times (P<0.001), and CAM mRNA was increased to 1.46 times (P<0.05). Western blot results showed that MITF protein was significantly reduced to 0.67 times (P<0.01), TYR protein was increased to 1.16 times (P<0.05), TYRP-1 protein was increased to 1.15 times (P<0.05) and TYRP-2 protein did not change. 【Conclusion】 The 2 313 bp length CDS region of mouse Oct-1 gene was got by PCR, TA cloning and nucleic acid sequencing technology. Major genes of coat color regulated by Oct 1 was determined by bioinformatics analysis. Results of the study suggested that after over-expression of Oct-1, the expression of MITF and TCF was reduced, while that of TYR, TYRP-1, αMSH, AC, c-kit, ET1, ETB-R and CAM was increased. Therefore, the Oct-1 may mediate the alteration of coat color through regulating genes involved in the formation process of coat color.
