Protective effect of sodium ferulate against lipopolysaccharideinduced preterm delivery and intra-uterine fetal death in mice
2017-03-28LIXiaojunMAZhenguoGUOYuKOUHaoSUNRongzeJIZhengyuWANGHui
LI Xiao-jun,MA Zhen-guo,GUO Yu,3,KOU Hao,SUN Rong-ze,JI Zheng-yu,WANG Hui,3
(1.Department of Pharmacology,School of Pharmaceutical Sciences,South Central University for Nationalities,Wuhan 430074,China;2.Department of Pharmacology,School of Basic Medical Science,Wuhan University,Wuhan 430071,China;3.Hubei Provincial Key Laboratory of Developmentally Originated Disease,Wuhan 430071,China)
·ORIGINAL ARTICLES·
Protective effect of sodium ferulate against lipopolysaccharideinduced preterm delivery and intra-uterine fetal death in mice
LI Xiao-jun1,2,MA Zhen-guo2,GUO Yu2,3,KOU Hao2,SUN Rong-ze2,JI Zheng-yu2,WANG Hui2,3
(1.Department of Pharmacology,School of Pharmaceutical Sciences,South Central University for Nationalities,Wuhan 430074,China;2.Department of Pharmacology,School of Basic Medical Science,Wuhan University,Wuhan 430071,China;3.Hubei Provincial Key Laboratory of Developmentally Originated Disease,Wuhan 430071,China)
OBJECTIVETo investigate the effect of sodium ferulate(SF)on lipopolysaccharide (LPS)-induced preterm delivery and intra-uterine fetal death(IUFD).METHODSPregnant Kunming mice were subcutaneously pretreated with SF(25 or 50 mg·kg-1)from gestational day(GD)10 to GD 15 and with the single injection of LPS(150 μg·kg-1,ip)on GD15.5.The incidence of preterm delivery and IUFD was observed.HE staining was used for uterine and placental histological evaluation.The levels of thiobarbituric acid reactive substances(TBARS)and reduced glutathione(GSH)as well as the activities of glutathione S-transferase(GST)and glutathione peroxidase(GSH-Px)were detected in the maternal liver,placenta,and fetal liver using commercial kits.Interleukin-1β(IL-1β)and tumor necrosis factor-α(TNF-α)levels in amniotic fluid were evaluated by enzyme linked immunosorbent assay.RESULTSFor LPS group,the incidence of preterm was 47.8%,delivery time was(17.5±1.3)d, and the pups′survival rate was only 42.6%.Compared with LPS-treated group,SF 50 mg·kg-1group showed a lower incidence of preterm(14.3%,P<0.01),longer gestational days(18.4±0.5,P<0.05), and a higher pups′survival rate(75.6%,P<0.01).SF 50 mg·kg-1restored the LPS-induced GSH both in the maternal and fatal liver(a tendency without statistical significance),GST activity〔(163±82)kU·g-1proteinvs(95±90)kU·g-1protein,P<0.01)〕in the placenta,TBARS content〔(2.5±0.4)μmol·g-1proteinvs(3.1±0.6)μmol·g-1protein,P<0.01〕in the fetal liver,and TNF-α level〔(11±8)ng·L-1vs(20±8)ng·L-1,P<0.01〕in the amniotic fluid.SF also attenuated LPS-induced placental congestion and neutrophil infiltra⁃tion in the uterus.CONCLUSIONSF may protect against LPS-induced preterm delivery and IUFD, and anti-oxidation as well as anti-inflammation may contribute to these effects.
sodium ferulate;lipopolysaccharide;preterm delivery;intra-uterine fetal death
Preterm delivery,defined as birth before the completion of 37 weeks of gestation,accounts for as much as 70%of the perinatal mortality and nearly half of long-term neurologic morbidity[1]. Approximately 10%of parturitions worldwide are premature[2-3].Maternal infections are believed to be major causes of preterm delivery,and infec⁃tions are found in 30%-40%of women who deliv⁃ered prematurely[2,4-5].Lipopolysaccharide(LPS)has been commonly used to induce preterm delivery in animal models[6-7].LPS exposure has been associ⁃ated with adverse developmental outcomes,including preterm labor,intra-uterine fetal death(IUFD), and intra-uterine growth retardation(IUGR)[7]. These adverse effects of LPS appear to be medi⁃ated through a cascade of inflammatory cyto⁃kines,and possibly with the contributions of reac⁃tive oxygen species(ROS),which are character⁃ized by lipid peroxidation and depletion of reduced glutathione(GSH)[8].Several tocolytic agents have currently been applied to the cure of preterm labor, including magnesium sulfate,indometacin,nife⁃dipine,and atosiban.However,these agents were found to have limited effects and/or have signifi⁃cant maternal and fetal adverse reactions[9].Ferulic acid(FA)is widely distributed in plants and consti⁃tutes a bioactive ingredient of many staple foods, such as grain bran,whole grain foods,citrus fruits,and banana[10].Sodium ferulate(SF)has been clinically used for treatment of cardiovascular and cerebrovascular diseases in China[11].Our previous studies have demonstrated a broad spec⁃trum of biological activities of SF,e.g.protection on IUGR induced by prenatal tobacco/alcohol expo⁃sure,anti-inflammatory effect against osteoarthritis, and anti-oxidation as well as hepatic protective effect on various hepatic injury models[12-16].In view of the ubiquitous presence of SF and its antiinflammation as well as anti-oxidation effect,we evaluated the protective effects of SF on LPS-induced preterm delivery and IUFD in mice,and determined the effects of SF on LPS-caused tissue oxidative injury as well as levets of inflammation factors.
1 MATERIALS AND METHODS
1.1 Reagents
SF was purchased from Hainan Shuangcheng Pharmaceuticals Inc.(Hainan,China).LPS (Escherichia coliLPS,serotype 0127:B8)was purchased from Sigma-Aldrich Chemical Co.(St. Louis,MO,USA).GSH,glutathione peroxidase (GSH-Px),glutathione S-transferase(GST)and thiobarbituric acid reactive substances(TBARS) detection kits and bicinchoninic acid(BCA)protein assay kits were obtained from Nanjing Jiancheng Bioengineering Institute(Nanjing,China).Tumor necrosis factor-α(TNF-α)and interleukin-1β(IL-1β) assay kits were provided by eBioscience(San Diego,California,US).All other chemicals were of an analytical grade.
1.2 Animals and treatments
Kunming mice(8-9 weeks old;male:28-32 g; female:26-30 g)were purchased from Experi⁃mental Center of the Medical Scientific Academy of Hubei(China).Animal experiments were conducted in the Center for Animal Experiment of Wuhan University(Wuhan,China),which has been accredited by Association for Assessment and Accreditation of Laboratory Animal Care International(AAALAC International).All experimental procedures were approved and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee. The animals were allowed free access to food and water and maintained on a 12 h light/dark cycle in a controlled temperature(20-25)°C and humidity (50±5)%environment for one week before study. For mating purposes,two females were housed overnight with one male starting at 7:00 pm and the females were checked by 7:00 am the next morning. The presence of a vaginal plug was designated as gestational day 0(GD0).
1.3 Detection of delivery rate,gestation dura⁃tion,and intra-uterine fetal death
The pregnant mice were randomly assigned to four groups:normal control,LPS,LPS+SF 25 and LPS+SF 50 mg·kg-1.SF was administered subcutaneously(25 or 50 mg·kg-1)from GD9 to GD15(between 3∶00 pm to 4∶00 pm)and LPS-treated mice were given a single intraperitoneal injection of LPS(150 μg·kg-1),which was dis⁃solved in normal saline and administered at GD 15.5(10∶00 pm).The parturition of dams and the newborns was checked every 2 h after LPS injection.Preterm delivery was designated as thedeliveryoccurringbetweenGD15.5and GD17(12∶00 pm)while the term delivery was defined as birth on GD18-GD20.The pup survival rate was calculated by dividing the number ofsurvival pups in one group by the number of the total pups(both live and dead)in that group.SF 50 mg·kg-1was also administered subcutaneously to the normal pregnant mice,and there was no significant toxicity(data not shown).
1.4 Animal treatment
The pregnant mice were randomly assigned to three groups:normal control,LPS and LPS+SF 50 mg·kg-1.The treatment for each group was the same as described in1.3.All the dams were euthanized 6 h after LPS treatment.Uterus, amniotic fluid,maternal livers,placentas,and fetal livers were collected.Amniotic fluid,placentas and fetal livers were pooled from each dam and counted as one independent sample.
1.5 HE staining for morphological examination
The inflammatory responses to ascending infection fell into two components:the maternal and fetal inflammatory responses[17].Since placenta and uterus are very critical organs that account for the pathogenesis of preterm birth,morphological exami⁃nation was conducted in these three organs[17]. Placentas and uterus were fixed with 4%neutral formaldehyde.The fixed samples were processed by standard histological techniques and stained with hematoxylin and eosin(HE).The slides were observed under light microscopy,and the photo⁃micrographs were obtained by Photo Imaging System(Nikon,TE2000).The maternal and fetal liver histology was also checked with the above method.However,no significant changes were observed(data not shown).
1.6 Biochemical assays
For the preparation of liver homogenates, 0.1 g of the liver was homogenized in 1 mL of ice-cold homogenization buffer(mmol·L-1:Tris-HCl 50,KCl 180,ethylenediamine tetraacetic acid 10,pH 7.4).The homogenates were centri⁃fuged at a speed of 1000×gfor 10 min and the supernatants were collected.The contents of GSH,total protein,TBARS as well as the activi⁃ties of GSH-Px,GST in homogenates of the fetal liver,placenta,and maternal liver were detected by a series of commercial kits.
1.7 Determination of TNF-α and IL-1β in amni⁃otic fluid by ELISA
Commercial ELISA kits were used to determine levels of TNF-α and IL-1β in amniotic fluid according to the manufacturer′s protocols.
1.8 Statistical analysis
The concentrations of GSH and TBARS,and the activities of GSH-Px and GST were expressed asDifferent groups were compared by ANOVA with SPSS for Windows version 13(SPSS Science Inc,Chicago,Illinois).Preterm delivery rate and pup survival rate were compared using Fisher exact test.Statistical significance was attributed atP<0.05.
2 RESULTS
2.1 Effects of SF on LPS-induced preterm labor rate,IUFD and gestation duration
Under normal conditions,the rate of preterm delivery in mice is 0,however,LPS induced nearly half of dams(11/23,47.8%,P<0.01)preterm (Tab.1).Compared with LPS group,the mice treated with SF prior to LPS injection that a re⁃duced rate of preterm delivery,especially thedams given SF 50 mg·kg-1(4/28,P<0.05).SF pretreatment prolonged the shortening of gesta⁃tion duration induced by LPS.LPS induced 57.4%(78/136)of the fetal mortality.Treatment with SF 50 mg·kg-1significantly enhanced the survival rate of fetuses exposed to LPS(150/198vs58/136,P<0.05).
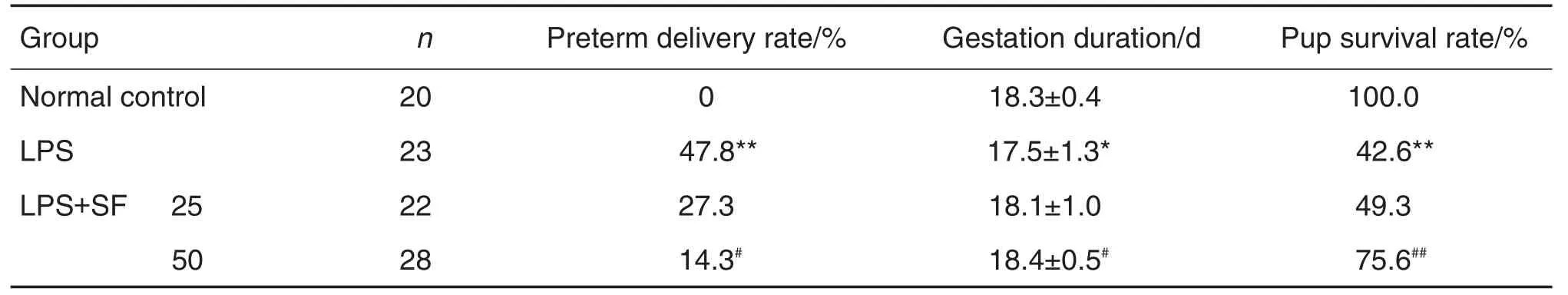
Tab.1 Effect of sodium ferulate(SF)on preterm delivery rate,gestation duration,and intra-uterine fetal death induced by lipopolysaccharide(LPS)in mice
2.2 Effects of SF on LPS-induced pathological alterations of placenta and uterus
The placental labyrinths in mice treated with LPS were congested,characterized by retained red blood cells in the labyrinths.SF 50 mg·kg-1pretreatment attenuated the pathological conges⁃ tion induced by LPS.Accumulation of neutrophils was observed in uterine tissues from LPS-treated mice,and SF 50 mg·kg-1pretreated mice showed lower numbers of neutrophils in uterine(Fig.1).
2.3 Effects of SF on LPS-induced redox func⁃tions in maternal and fetal livers
AS shown in Tab 2,SF pretreatment attenu⁃ated LPS-induced GSH depletion in both maternal and fetal livers.The data showed a tendency of no statisticalsignificance. SF pretreatment enhanced the activity of GSH-Px in maternal and fetal livers,especially in the maternal liver when compared with LPS alone group(P<0.01).SF50 mg·kg-1pretreatment also restored GST activity in placenta and TBARS contents in fetal liver(P<0.01).
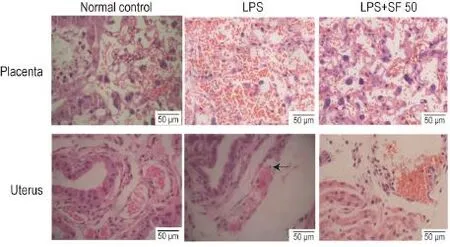
Fig.1 Effect of SF on placental and uterine of mice induced by LPS.See Tab.1 for the mouse treatment.Arrow indicates the neutrophil.
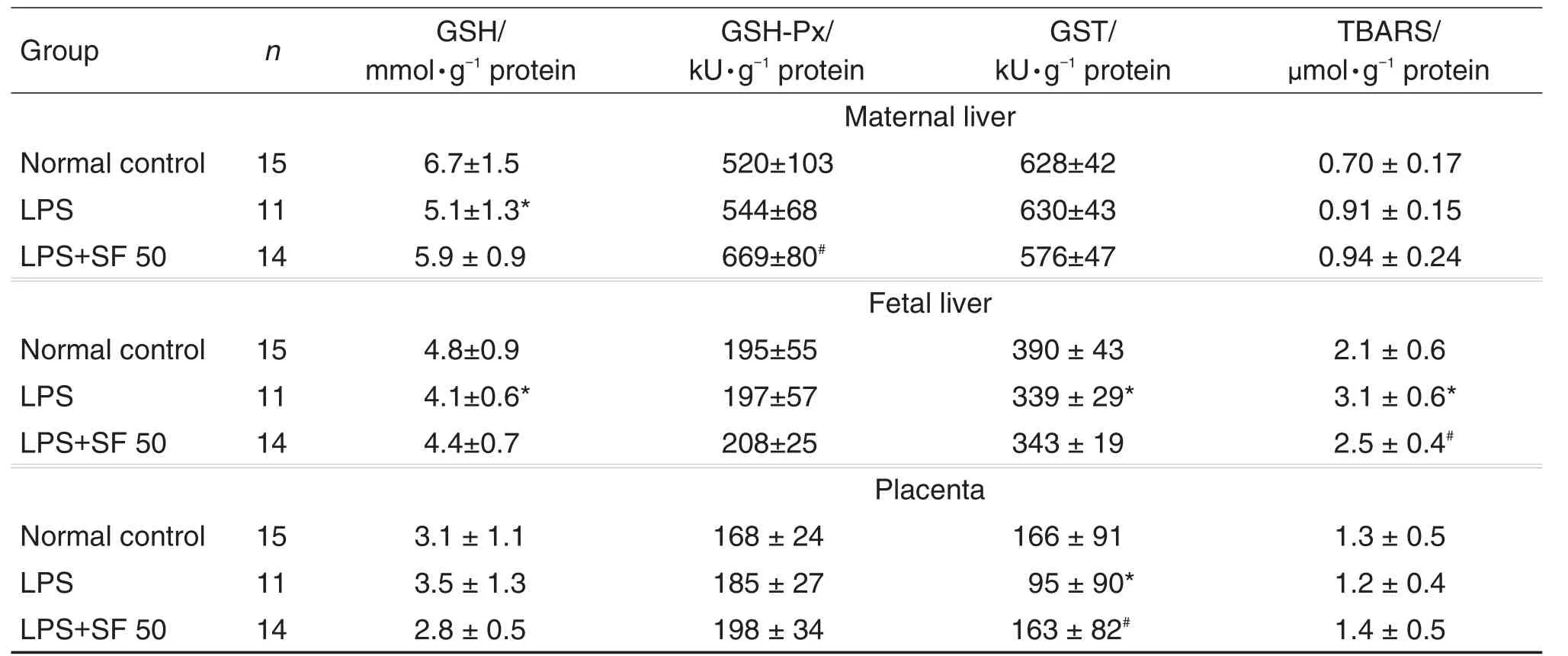
Tab 2.Effects of SF on activities of reduced glutathione(GSH),glutathione peroxidase(GSH-Px),glutathione S-transferase(GST)and thiobarbituric acid reactive substances(TBARS)in maternal liver,placenta,and fetal liver induced by LPS
2.4 Effects of SF on TNF-α and IL-1β in amniotic fluid induced by LPS in mice
Compared with the LPS group,SF 50 mg·kg-1significantly down-regulated the level of TNF-α in amniotic fluid(P<0.05).However,the levels of IL-1β showed no difference between the ese groups (Tab.3).
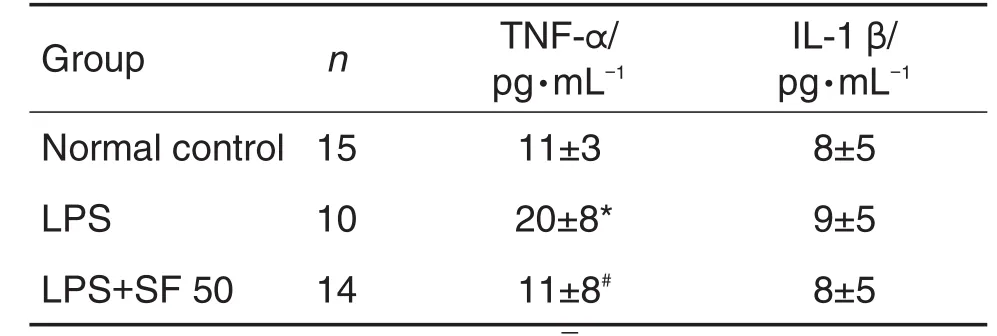
Tab 3.Effect of SF on production of tumor necrosis factor-α(TNF-α)and interleukin-1β(IL-1β)in amniotic fluid induced by LPS in mice.
3 DISCUSSION
In the present study,LPS induced 49%of the preterm delivery and 57.4%of the fetal death.SF showed a potent protective effect that significantly reduced therates of LPS-induced premature delivery and IUFD.
So far,there has been no thoroughly clarified mechanism for LPS-induced premature delivery and IUFD.After LPS enters the abdominal cavity,it combines with Toll-like receptors on macrophages to produce inflammatory factors.Alternatively,LPS interacts with membrane lipids,resulting in formation of ROS[18].Upregulation of inflammatory factors and/or ROS can lead to contraction of uterus ultimately[19].
Levels of ROS can be regulated by endoge⁃nous antioxidants.It has been reported that maternal exposure to LPS resulted in GSH deple⁃tion and lipid peroxidation in LPS-induced terato⁃genesis in mice[20].It was supported by the evidence that antioxidants,such as melatonin, ascorbic acid and N-acetylcysteine,can signifi⁃cantly attenuate LPS-induced fetal death[8,21-22]. In our study,SF pretreatment restored TBARS contents in the fetal liver.The protective effect may be partly attributed to the anti-oxidative property of SF as evidenced by the elevation of GSH content as well as GST activity in maternal and/or fetal livers.The anti-oxidative effect of SF was consis⁃tent with our previous studies on hepatic,renal and placental oxidative injuries[16,23-24].
Inflammation also plays a key role in the process of LPS-induced adverse pregnant outcomes. An increased TNF-α production was found in adverse pregnant outcomes induced by LPS[25-26].It was reported that LPS injection on GD15.5 increased the concentration of TNF-α in amniotic fluid,and pentoxifylline,an inhibitor of TNF-α synthesis, significantly inhibited TNF-α production and attenu⁃ated LPS-induced IUFD[27].In addition,TNF-α blocker could decrease preterm delivery and IUFD in an LPS-induced preterm delivery model[28]. However,as for IL-1β,LPS elevated TNF-α level in amniotic fluid without notably altering IL-1β[29]. In our study,we also found that SF significantly down-regulated the level of TNF-α in amniotic fluid induced by LPS.SF showed no remarkable effect on IL-1β.Congestion and neutrophil infiltra⁃tion are indicators of inflammatory response eval⁃uated in the present study.We found that SF pretreat⁃ment reduced placental congestion and neutro⁃phil infiltrate in uterus induced by LPS.That effect was consistent with a recent study,which reported that SF could decrease inflammatory cell numbers in lungs from LPS-treated mice[30]. Thus,the protection of SF against LPS-induced adverse pregnant outcome is also attributed to its anti-inflammation property.
In summary,the protective effects of SF against LPS-induced preterm delivery and IUFD were observed in the present study.Taking into consideration,the low cost,ready availability,ab⁃sence of obvious adverse effects[13,31],and signifi⁃cant protection against LPS,SF has the poten⁃tial to be developed into a useful therapeutic drug for preventing preterm birth and/or IUFD.
REFERENCES
[1] Rush RW,Keirse MJ,Howat P,Baum JD,Anderson AB,Turnbull AC.Contribution of preterm delivery to perinatal mortality[J].Br Med J,1976,2(642):965-968.
[2]Goldenberg RL,Hauth JC,Andrews WW.Intrauterine infection and preterm delivery[J].N Engl J Med,2000,342(20):1500-1507.
[3]Wen SW,Smith G,Yang Q,Walker M.Epidemiology of preterm birth and neonatal outcome[J].Semin Fetal Neonatal Med,2004,9(6):429-435.
[4]GrigsbyPL,NovyMJ,AdamsWaldorfKM,Sadowsky DW, GravettMG.Choriodecidual inflammation:a harbinger of the preterm labor syndrome[J].Reprod Sci,2010,17(1):85-94.
[5]Gibbs RS,Romero R,Hillier SL,Eschenbach DA,Sweet RL.A review of premature birth and subclinical infection[J].Am J Obstet Gynecol,1992,166(5):1515-1528.
[6]Leazer TM,Barbee B,Ebron-Mccoy M,Henry-Sam GA,Rogers JM.Role of the maternal acute phase response and tumor necrosis factor alpha in the developmental toxicity of lipopolysaccharide in the CD-1 mouse[J].Reprod Toxicol,2002,16(2):173-179.
[7]Chen YH,Xu DX,Zhao L,Wang H,Wang JP,Wei W.Ascorbic acid protects against lipopolysaccharideinduced intra-uterine fetal death and intra-uterine growth retardation in mice[J].Toxicology,2006,217(1):39-45.
[8]Belt AR,Baldassare JJ,Molnár M,Romero R,Hertelendy F.The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expres⁃sion of cyclooxygenase-2 in human myometrial cells[J].Am J Obstet Gynecol,1999,181(2):359-366.
[9]Giles W,Bisits A.Preterm Labour.the present and future of tocolysis[J].Best Pract Res Clin Obstet Gynaecol,2007,21(5):857-868.
[10]Zhao Z,Moghadasian MH.Chemistry,natural sources,dietary intake and pharmacokinetic prop⁃erties of ferulic acid:A review[J].Food Chem ,2008,109(4):691-702.
[11]Li X,Shang L,Wu Y,Abbas S,Li D,Netter P,et al. Identification of the human UDP-glucuronosyltrans⁃ferase isoforms involved in the glucuronidation of the phytochemical ferulic acid[J].Drug Metab Pharma⁃cokinet,2011,26(4):341-350.
[12]Li Y,Yan YE,Wang H.Enhancement of placental antioxidative function and P-gp expression by sodium ferulate mediated its protective effect on rat IUGR induced by prenatal tobacco/alcohol exposure[J]. Environ Toxicol Pharmacol,2011,32(3):465-471.
[13]Shang L,Qin J,Chen LB,Liu BX,Jacques M,Wang H.Effects of sodium ferulate on human osteo⁃arthritic chondrocytes and osteoarthritis in rats[J].Clin Exp Pharmacol Physiol,2009,36(9):912-918.
[14]Wang H,Peng RX.Sodium ferulate alleviated paracetamol-induced liver toxicity in mice[J].Acta Pharmacol Sin(中国药理学报),1994,15(1):81-83.
[15]Wu DF,Peng RX,Wang H.Sodium ferulate allevi⁃ates prednisolone induced liver toxicity in mice[J]. Acta Pharm Sin(药学学报),1995,30(11):801-805.
[16]Wang H,Peng RX,Li QX,Chen JH,Fu LS,Kong R. Antagonizing effect of sodium ferulate on the changes of hepatic antioxidative function induced by ethanol in mice[J].Acta Acad Med Hubei(湖北医科大学学报),1996,17(1):23-26.
[17]Erdener O,Preterm Birth[M].Croatia:InTech,2013:109-134.
[18]Bhattacharyya S, Dudeja PK, Tobacman JK. Lipopolysaccharide activates NF-kappaB by TLR4-Bcl10-dependent and Independent pathways in colonic epithelial cells[J].Am J Physiol Gastrointest Liver Physiol,2008,295(4):G784-G790.
[19]Slater D,Allport V,Bennett P.Changes in the expression of the type-2 but not the type-1 cycloox⁃ygenase enzyme in chorion-decidua with the onset of labour[J].Br JObstet Gynaecol,1998,105(7):745-748.
[20]Zhao L,Chen YH,Wang H,Ji YL,Ning H,Wang SF,et al.Reactive oxygen species contribute to lipo⁃polysaccharide-induced teratogenesis in mice[J]. Toxicol Sci,2008,103(1):149-157.
[21]Buhimschi IA,Buhimschi CS,Weiner CP.Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation[J]. Am J Obstet Gynecol,2003,188(1):203-208.
[22]Chen YH,Xu DX,Wang JP,Wang H,Wei LZ,Sun MF,et al.Melatonin protects against lipopolysaccharideinduced intra-uterine fetal death and growth retardation in mice[J].J Pineal Res,2006,40(1):40-47.
[23]Wang H,Peng RX,Wang RK,Kong R.Antagonizing effect of sodium ferulate on the changes of hepatic antioxidative function induced by ethanol in mice[J]. Acta Pharm Sin(药学学报),1997,32(7):511-514.
[24]Liao ZX,Wang H,Peng RX,Kong R.Antagonistic effect of sodium ferulate on glycerol-induced renal oxidative injury in mice[J].Acta Pharm Sin(药学学报),2003,38(12):900-903.
[25]Gendron RL,Nestel FP,Lapp WS,Baines MG. Lipopolysaccharide-induced fetal resorption in miceis associated with the intrauterine production of tu⁃mour necrosis factor-alpha[J].J Reprod Fertil,1990,90(2):395-402.
[26]Silver RM,Lohner WS,Daynes RA,Mitchell MD,Branch DW.Lipopolysaccharide-induced fetal death:the role of tumor necrosis factor alpha[J].Biol Reprod,1994,50(5):1108-1112.
[27]Xu DX,Chen YH,Wang H,Zhao L,Wang JP,Wei W. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice[J].Toxicol Lett,2006,163(1):20-29.
[28]Holmgren C,Esplin MS,Hamblin S,Molenda M,Simonsen S,Silver R.Evaluation of the use of anti-TNF-alpha in an LPS-induced murine model[J].J Reprod Immunol,2008,78(2):134-139.
[29] Xu DX,Wang H,Ning H,Zhao L,Chen YH. Maternally administered melatonin differentially regulates lipopolysaccharide-induced proinflammatory and anti-inflammatory cytokines in maternal serum,amniotic fluid,fetal liver,and fetal brain[J].J Pineal Res,2007,43(1):74-79.
[30]Yuan X,Wang Y,Du D,Hu Z,Xu M,Xu M,et al. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice[J].Inflammation,2012,35(3):1161-1168.
[31]Wang BH,Ouyang JP.Pharmacological actions of sodium ferulate in cardiovascularsystem[J]. Cardiovasc Drug Rev,2005,23(2):161-172.
阿魏酸钠对脂多糖引起小鼠早产和死胎的防治作用
李小军1,2,马振国2,郭 喻2,3,寇 皓2,孙荣则2,纪振宇2,汪 晖2,3
(1.中南民族大学药学院药理系,湖北武汉 430074;2.武汉大学基础医学院药理系,湖北武汉 430071;3.发育源性疾病湖北省重点实验室,湖北武汉 430071)
目的研究阿魏酸钠(SF)对脂多糖导致早产和死胎的防治作用。方法昆明种孕小鼠妊娠10~15 d(GD10~GD15)每天sc给予SF 25或50 mg·kg-1,GD15.5 ip给予脂多糖(LPS)150 μg·kg-1造成早产及死胎模型,观察早产和死胎的发生率;利用HE染色,观察胎盘和子宫组织病理学变化;通过商用试剂盒测定母肝、胎盘和胎肝中硫代巴比妥酸反应产物(TBARS)及还原型谷胱甘肽(GSH)浓度含量、谷胱甘肽巯基转移酶(GST)及谷胱甘肽过氧化物酶(GSH-Px)活性;酶联免疫吸附测定羊水中白细胞介素1β(IL-1β)和肿瘤坏死因子α(TNF-α)的水平。结果LPS组早产发生率为47.8%,妊娠天数为(17.5±1.3)d,活胎率为42.6%;与之相比,SF 50 mg·kg-1可以显著降低LPS引起早产发生率(14.3%,P<0.01),延长妊娠时间〔(18.4±0.5)d,P<0.05〕,并提高活胎率(75.6%,P<0.01)。与LPS组相比,SF 50 mg·kg-1具有逆转母肝和胎肝GSH降低的趋势;恢复胎盘GST活性〔(163±82)kU·g-1蛋白质vs(95±90)kU·g-1蛋白质,P<0.01〕以及胎肝TBARS的含量水平〔(2.5±0.4)μmol·g-1蛋白质vs(3.1±0.6)μmol·g-1蛋白质,P<0.01〕;降低羊水TNF-α表达水平〔(11±8)ng·L-1vs(20±8)ng·L-1,P<0.01〕;抑制胎盘充血和子宫粒细胞浸润。结论SF可以防治LPS导致的早产和死胎,其作用机制可能与抗氧化和抑制炎症有关。
阿魏酸钠;脂多糖;早产;宫内胎儿死亡
2016-06-02接受日期:2016-10-08)
国家自然科学基金(81220108026);国家自然科学基金(81430089);国家教育部回国人员科研启动基金资助(20111020);国家大学生创新创业训练项目(1210486080)
汪晖,Tel:(027)68758665;E-mail:wanghui19@whu.edu.cn
R99
A
1000-3002-(2017)01-0028-07
10.3867/j.issn.1000-3002.2017.01.003
(本文编辑:乔 虹)
Foundation item:The project supported by National Natural Science Foundation of China(81220108026);National Natural Science Foundation of China(81430089);Scientific Research Foundation for the Returned Overseas Chinese Scholars,State Ministry of Education(20111020);and Project of Innovation and Entrepreneurship Training of National Undergraduate(1210486080)
Biography:LI Xiao-jun,male,Doctor degree,lecturer,main research field is ethnodrugs against alcoholic liver diseases. Corresponding author:WANG Hui,Tel:(027)68758665,E-mail:wanghui19@whu.edu.cn
