亚精胺对鼠免疫器官指数及炎症因子表达的影响
2017-03-06徐麒麟姜冬梅魏春叶贾医林易治鑫陈咨余
徐麒麟,康 波,姜冬梅,魏春叶,贾医林,易治鑫,陈咨余
(四川农业大学 动物科技学院/畜禽遗传资源发掘与创新利用四川省重点实验室, 四川 成都 611130)
亚精胺对鼠免疫器官指数及炎症因子表达的影响
徐麒麟,康 波*,姜冬梅*,魏春叶,贾医林,易治鑫,陈咨余
(四川农业大学 动物科技学院/畜禽遗传资源发掘与创新利用四川省重点实验室, 四川 成都 611130)
为阐明亚精胺对鼠免疫器官指数、胸腺和脾脏组织炎症因子表达的影响,采用0、0.05、0.10和0.15 mg·g-1(体质量)亚精胺腹腔注射6周龄昆明小白鼠,测定鼠胸腺和脾脏指数,并定量检测胸腺和脾脏组织TNF-α、IL-1β、IL-6和IFN-γ表达量。结果表明:注射0.15 mg·g-1亚精胺组鼠胸腺指数和脾脏指数显著(P<0.05)低于对照组;0.10 mg·g-1亚精胺组胸腺组织IFN-γ表达量显著(P<0.05)高于对照组;0.05 mg·g-1亚精胺组鼠脾脏组织TNF-α和IL-1β表达量显著(P<0.05)低于对照组,0.10 mg·g-1亚精胺组脾脏组织IL-6和IFN-γ表达量均显著(P<0.05)高于对照组,0.15 mg·g-1组脾脏组织TNF-α表达量显著(P<0.05)低于对照组。由此可见,亚精胺对炎症因子表达具有组织特异性和剂量依赖性。低浓度亚精胺能抑制炎症因子表达发挥抗炎作用,随着亚精胺浓度增加则表现为诱导炎症。
亚精胺;胸腺指数;脾脏指数;炎症因子
多胺主要包括腐胺、亚精胺和精胺,在细胞生长、衰老、自噬和神经退行性疾病中扮演重要角色[1-2]。作为内源性的免疫调节分子,多胺与炎症密切相关,炎症组织中内源性多胺水平显著升高表明多胺在炎症反应中发挥直接作用[3]。创面坏死组织中大量产生的腐胺和尸胺经吸收入血后会显著提高血清中炎症因子TNF-α、IL-1β、IL-6的水平并诱导机体发生炎症[4]。Soulet等[5]研究发现小鼠神经胶质细胞炎症反应伴随着多胺水平升高,进一步研究发现多胺水平降低后,炎症因子TNF-α和TLR2表达也显著降低。多胺具有重要的抗氧化和抗炎功能,外源性多胺能够抑制炎症介质,如促炎细胞因子和趋化因子的表达,进而发挥抗炎作用[6-7]。研究发现,精胺能够通过增加抗炎因子IL-10合成和抑制促炎因子IL-12和IFN-γ的表达进而保护巨噬细胞免受炎症损伤[8]。
胸腺和脾脏是机体重要的免疫器官,胸腺指数和脾脏指数可以反映机体的免疫水平,同时免疫器官又是机体免疫细胞来源的重要场所[9]。然而,目前尚未见亚精胺对小鼠免疫器官指数及免疫器官炎症因子表达的影响的报道,鉴于此,本实验通过研究不同浓度亚精胺对鼠免疫器官指数和胸腺脾脏中TNF-α、IL-1β、IL-6和IFN-γ基因表达的影响,为研究亚精胺对机体免疫功能的作用机制奠定基础。
1 材料与方法
1.1 实验动物分组与实验设计
选取同批昆明小白鼠32只,常规方式分笼饲养。6周龄时记录小鼠体重并采用随机分配编号法编号,将小鼠随机分为4组,每组8只小鼠。参考周岳平等[10]腐胺安全性试验的结果,设定注射剂量分别为0.05、0.10和0.15 mg·g-1的亚精胺低、中、高剂量组,对照组注射生理盐水。试验开始的当天上午9:00腹腔单次单点注射,注射剂量为300 μL。
1.2 样品采集及处理
注射24 h后颈部脱臼处死小鼠,迅速采集小鼠的胸腺和脾脏组织,手术剪剪去表面脂肪和系膜后,用生理盐水洗净,滤纸吸干后电子天平称量并记录,胸腺和脾脏样品置于-80 ℃冰箱保存备用。
1.3 免疫器官指数
各组所有胸腺脾脏称量记录后计算免疫器官指数,免疫器官指数计算公式如下:免疫器官指数(g·kg-1)=免疫器官质量(g)/体质量(kg)。
1.4 实时荧光定量PCR
按照Trizol试剂盒(TaKaRa,大连)操作说明提取所采集的胸腺和脾脏组织总RNA。参照反转录试剂盒(TaKaRa,大连)说明书将总RNA反转录成cDNA模板,反应体系20 μL:包括9.0 μL RNase Free dH2O,1.0 μL Oligo dT Primer,1.0 μL Random 6 mers,4.0 μL 5×PrimeScript Buffer,1.0 μL PrimeScript RT Enzyme Mix,4.0 μL总RNA。反应条件为:37 ℃ 15 min,85 ℃ 5 s和4 ℃ 5 min,反应结束后将cDNA模板置于-20 ℃冰箱中保存备用。应用实时荧光定量PCR检测各处理组胸腺、脾脏样品组织中TNF-α、IL-1β、IL-6和IFN-γ基因表达量(引物信息参见表1)。实时荧光定量PCR反应体系为10 μL∶SYBR Green 5.0 μL,上、下游引物(10 μmol·L-1)各0.2 μL,cDNA模板0.5 μL,RNase Free dH2O 4.1 μL。反应条件为:95 ℃预变性3 min;95 ℃变性10 s,58 ℃退火30 s(退火温度如表1所示),72 ℃延伸30 s(采集荧光),39个循环;95 ℃ 10 s,然后以0.05 ℃·s-1的速率从65 ℃缓慢升温至95 ℃,绘制熔解曲线。
1.5 统计分析
实验数据采用SAS 9.2软件进行统计分析,结果用mean ± SE表示。荧光定量采用2-ΔΔCt法处理数据,用GAPDH作为内参基因,每个样品3个重复,以对照组胸腺和脾脏组织基因表达量为1,计算TNF-α、IL-1β、IL-6和IFN-γ基因的相对表达量,应用SAS 9.2统计分析软件中的MEANS过程进行描述性统计分析、单因素方差分析和Duncan’s多重比较,P<0.05表示差异显著。
表1 鼠实时荧光定量PCR引物信息表
Table 1 Real-time PCR information of primers for mice

引物名称Primername引物序列Primersequence(5'-3')退火温度Annealingtemperature/℃片段大小Size/bpTNF-αF:CACGTCGTAGCAAACCACCAAGTGGR:GATAGCAAATCGGCTGACGGTGTGG65210IL-1βF:AATGCCTCGTGCTGTCTGACCR:TTGTCGTTGCTTGTCTCTCCTTG55118IL-6F:AGTTGCCTTCTTGGGACTGAR:CAGAATTGCCATTGCACAAC64191IFN-γF:TAACTCAAGTGGCATAGATGTG-GAAGR:GACGCTTATGTTGTTGCTGATGG64169GAPDHF:AACGACCCCTTCATTGACR:TCCACGACATACTCAGCAC58191
2 结果与分析
2.1 亚精胺对小鼠免疫器官指数的影响
如图1所示,注射0.15 mg·g-1亚精胺组鼠胸腺指数和脾脏指数显著(P<0.05)低于对照组;0.05 mg·g-1和0.10 mg·g-1亚精胺组鼠的胸腺指数和脾脏指数与对照组相比均没有明显变化(P>0.05)。
2.2 亚精胺对小鼠胸腺炎症因子表达的影响
如图2所示,0.05 mg·g-1、0.10 mg·g-1和0.15 mg·g-1亚精胺组胸腺组织中TNF-α、IL-1β和IL-6基因的表达与对照组相比差异不显著(P>0.05),0.10 mg·g-1亚精胺组胸腺组织中IFN-γ基因表达量显著(P<0.05)高于对照组。

同一免疫器官无相同小写字母表示差异显著(P <0.05);下图同In the same immune organ, the bar graphs without the same lower letters indicated significant differences at P <0.05. The same as below图1 亚精胺对小鼠免疫器官指数的影响Fig.1 Effects of spermidine on mice immune organ index
2.3 亚精胺对小鼠脾脏炎症因子表达的影响
如图3所示,0.05 mg·g-1亚精胺组鼠脾脏组织中TNF-α和IL-1β基因表达显著(P<0.05)低于对照组,0.10 mg·g-1亚精胺组脾脏组织IL-6和IFN-γ基因的表达均显著(P<0.05)高于对照组,0.15 mg·g-1组脾脏组织TNF-α基因的表达显著(P<0.05)低于对照组。
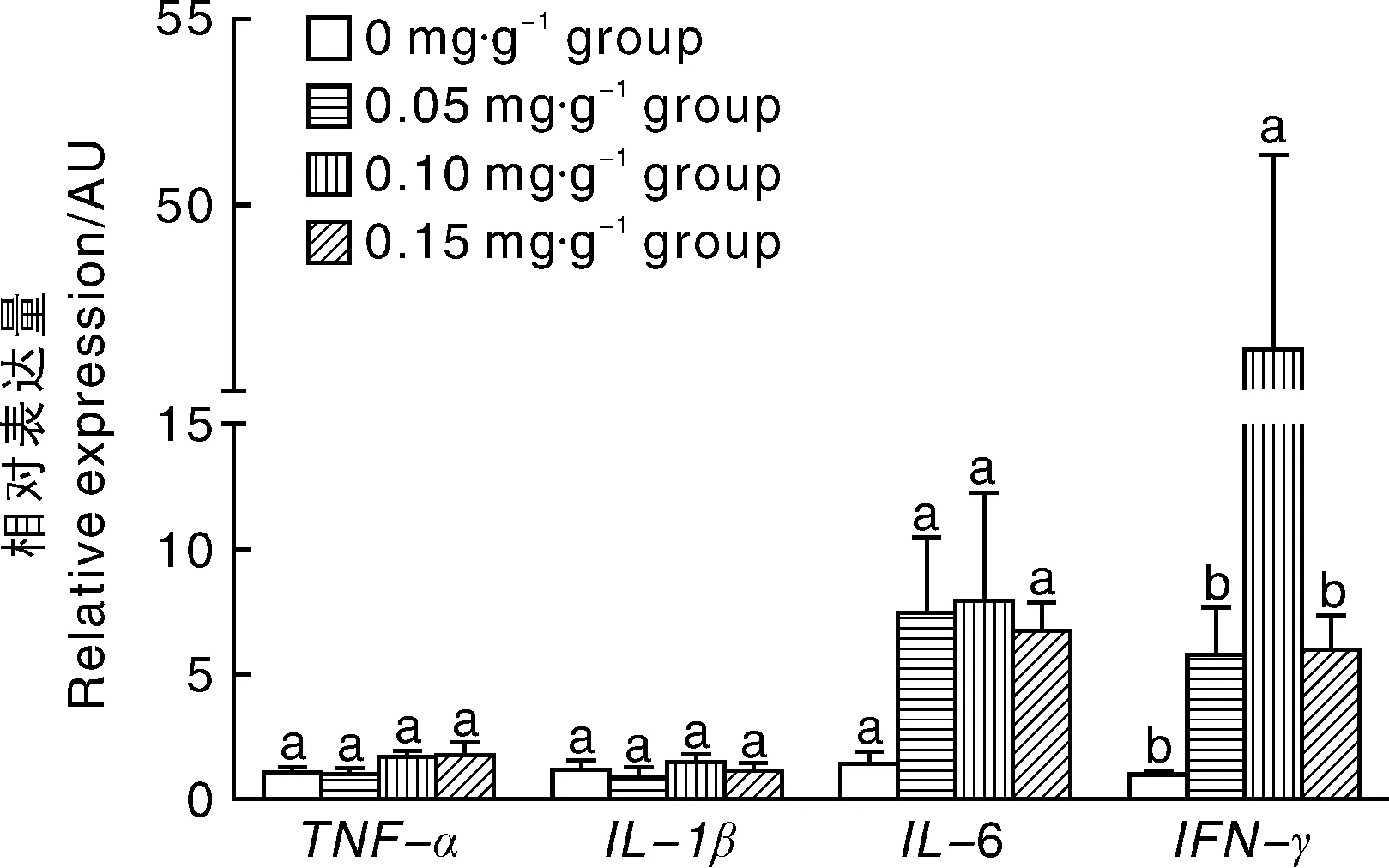
图2 亚精胺对小鼠胸腺炎症因子表达的影响Fig.2 Effects of spermidine on expression of mice thymus inflammatory cytokines
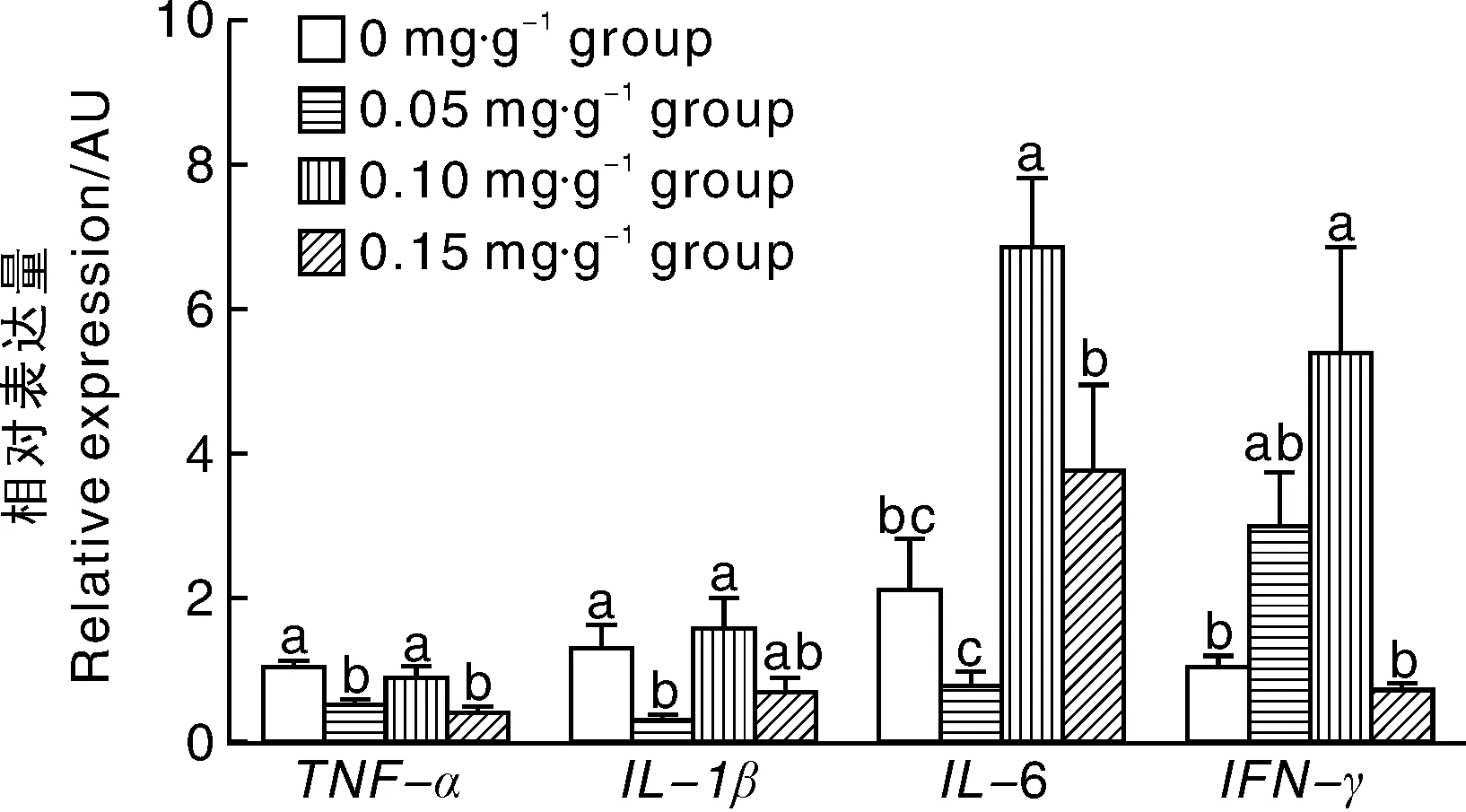
图3 亚精胺对小鼠脾脏炎症因子表达的影响Fig.3 Effects of spermidine on expression of mice spleen inflammatory cytokines
3 讨论
胸腺和脾脏是机体重要的免疫器官,免疫器官指数的高低可在一定程度上反映机体免疫水平的强弱[11]。一定浓度的多胺能够促进动物机体细胞增殖、分化和生长,赵宏涛等[12]研究发现,仔猪补充12和15 mg·kg-1外源性精胺能够通过增加脾脏相对质量进而提高仔猪免疫能力,多胺过量或耗竭会影响DNA和蛋白质、蛋白质和蛋白质的相互作用及线粒体完整,最终导致细胞凋亡[13],同时,多胺代谢产生的过氧化氢和丙烯醛也能破坏细胞DNA、蛋白质,并最终诱导细胞凋亡[14]。本实验发现,0.15 mg·g-1浓度的亚精胺处理会降低小鼠的胸腺指数和脾脏指数,致使小鼠发生免疫抑制,推测可能是由于亚精胺过量诱导产生活性氧并促进细胞凋亡,最终影响了免疫器官指数,抑制了机体的免疫功能,但其仍有待进一步研究证实。
机体摄入抗氧化物质可影响免疫反应和免疫状态[15]。个体水平和细胞水平均发现多胺具有重要的抗氧化功能[16-17],同时也是免疫功能的重要调节剂。炎症因子能够反映机体的炎症水平,炎症因子水平越高,表明炎症损伤越严重[18]。多胺能通过抑制炎症因子表达发挥抗炎作用[17,19-20]。促炎细胞因子如TNF-α、IL-1β和IL-6等能够活化NF-κB并激活炎症和凋亡通路[21]。TNF-α是机体中重要的促炎细胞因子,其水平是评价机体免疫状况的重要指标。IL-1β能够促进成纤维细胞因子、趋化因子、一氧化氮合酶释放增加并促进炎症的发展[22]。IFN-γ是一种二聚体糖蛋白,在炎症反应中扮演促炎因子角色[23]。本实验发现:各亚精胺组胸腺组织中TNF-α、IL-1β和IL-6基因的表达与对照组相比差异不显著,0.10 mg·g-1亚精胺组胸腺组织中IFN-γ的表达显著高于对照组,表明此剂量亚精胺能通过促进IFN-γ表达诱导胸腺组织发生炎症反应。Choi等[24]研究发现,外源性添加0.5和1 mmol·L-1的亚精胺可抑制LPS诱导的鼠小胶质细胞TNF-α和IL-6的转录,并降低培养液中炎症因子TNF-α和IL-6水平。Merentie等[25]研究发现,亚精胺能够抑制大鼠急性胰腺炎模型中血清炎症因子TNF-α和IL-6水平升高,本实验发现脾脏组织中,0.05 mg·g-1亚精胺组鼠TNF-α和IL-1β基因表达显著低于对照组,说明0.05 mg·g-1浓度亚精胺具有抗炎效果,此浓度下胸腺组织中TNF-α和IL-1β的表达与脾脏组织存在差异也说明亚精胺对小鼠炎症因子的表达具有组织差异性。Jamwal等[26]研究发现灌胃5和10 mg·kg-1外源性亚精胺能够抑制3-硝基丙酸诱导的鼠大脑纹状体中炎症因子IL-6的表达,同时该作者发现5和10 mg·kg-1外源性亚精胺预处理也能降低喹啉酸诱导的鼠大脑纹状体中炎症因子IL-6的表达[27],本实验研究发现,0.10 mg·g-1浓度亚精胺组IL-6表达显著高于对照组,提示高浓度的亚精胺可能通过促进脾脏IL-6的表达诱发脾脏发生炎症反应。IFN-γ等可通过一氧化氮合成酶诱导产生大量NO并最终诱导炎症反应,多胺能够通过降低NO水平进而保护巨噬细胞免受炎症损伤[28]。本实验研究发现,0.10 mg·g-1浓度亚精胺组IFN-γ表达显著高于对照组,提示0.10 mg·g-1亚精胺可能通过促进脾脏IFN-γ表达诱发脾脏发生炎症反应。
4 结论
亚精胺可通过介导鼠胸腺、脾脏指数及炎症因子表达来参与调节机体免疫功能。亚精胺对鼠胸腺和脾脏组织炎症因子表达具有组织特异性和剂量依赖性。本实验发现:0.05 mg·g-1剂量亚精胺可通过抑制脾脏TNF-α和IL-1β表达发挥抗炎作用;0.10 mg·g-1剂量亚精胺能够促进胸腺IFN-γ、脾脏IL-6和IFN-γ表达并最终诱导炎症反应;0.15 mg·g-1剂量亚精胺则可抑制鼠免疫器官功能。
[1] MINOIS N, CARMONA-GUTIERREZ D, MADEO F. Polyamines in aging and disease [J].Aging(AlbanyNY), 2011, 3(8):716-732.
[2] MILLER-FLEMING L, OLIN-SANDOVAL V, CAMPBELL K, et al. Remaining mysteries of molecular biology: the role of polyamines in the cell [J].JournalofMolecularBiology, 2015, 427(21):3389-3406.
[4] 樊桂成, 荣新洲, 王学敏, 等. 外源性腐胺和尸胺对兔外周血炎症因子的影响 [J]. 中华烧伤杂志,2012,28(6):451-454. FAN G C, RONG X Z, WANG X M, et al. Influence of exogenous putrescine and cadaverine on pro-inflammatory factors in the peripheral blood of rabbits [J].ChineseJournalofBurns, 2012, 28(6):451-454. (in Chinese with English abstract)
[5] SOULET D, RIVEST S. Polyamines play a critical role in the control of the innate immune response in the mouse central nervous system [J].TheJournalofCellBiology, 2003, 162(2):257-268.
[6] TER STEEGE J C, FORGET P P, BUURMAN W A. Oral spermine administration inhibits nitric oxide-mediated intestinal damage and levels of systemic inflammatory mediators in a mouse endotoxin model [J].Shock, 1999, 11(2):115-119.
[7] ZHANG M, CARAGINE T, WANG H, et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response [J].TheJournalofExperimentalMedicine, 1997, 185(10):1759-1768.
[9] 张少帅, 甄龙, 冯京海, 等. 持续偏热处理对肉仔鸡免疫器官指数、小肠形态结构和黏膜免疫指标的影响 [J]. 动物营养学报,2015,27(12):3887-3894. ZHANG S S, ZHEN L, FENG J H, et al. Effect of continuous partial heart treatment on immune organ indexes, small intestinal morphology and mucosal immunity indexes of broiler chickens [J].ChineseJournalofAnimalNutrition, 2015, 27(12):3887-3894. (in Chinese with English abstract)
[10] 周岳平,肖能坎,荣新洲,等. 外源性腐胺对正常大鼠肾功能和细胞凋亡的影响[J]. 南方医科大学学报,2012,32(11):1651-1654. ZHOU Y P, XIAO N K, RONG X Z, et al. Exogenous putrescine causes renal function impairment and cell apoptosis in rats [J].JournalofSouthernMedicalUniversity, 2012, 32(11):1651-1654. (in Chinese with English abstract)
[11] 郑灿财,黄艳玲,肖芳. 铬源与铬水平对常规饲养肉仔鸡生长性能、免疫器官指数及肉品质的影响 [J]. 动物营养学报,2015,27(8):2378-2387. ZHENG C C, HUANG Y L, XIAO F. Effect of chromium sources and chromium levels on growth performance, immune organ indexes and meat quality of broilers under normal rearing condition [J].ChineseJournalofAnimalNutrition, 2015, 27(8):2378-2387. (in Chinese with English abstract)
[12] 赵宏涛,何余湧,陆伟. 外源精胺对断奶仔猪血液精胺含量、脏器发育和生产性能的影响 [J]. 饲料工业,2015,36(11):15-17. ZHAO H T, HE Y Y, LU W. Effect of exogenous spermine on the level of spermine on blood, organ development and performance of weaner piglets [J].FeedIndustry, 2015, 36(11):15-17. (in Chinese with English abstract)
[13] THOMAS T, THOMAS T J. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications [J].CellularandMolecularLifeSciences, 2001, 58(2):244-258.
[14] WOOD P L, KHAN M A, MOSKAL J R. The concept of “aldehyde load” in neurodegenerative mechanisms: cytotoxicity of the polyamine degradation products hydrogen peroxide, acrolein, 3-aminopropanal, 3-acetamidopropanal and 4-aminobutanal in a retinal ganglion cell line [J].BrainResearch, 2007, 1145:150-156.
[15] KUANG S Y, XIAO W W, FENG L, et al. Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinuscarpiovar. Jian) [J].Fish&ShellfishImmunology, 2012, 32(5):629-636.
[16] WEI C, WANG Y, LI M, et al. Spermine inhibits endoplasmic reticulum stress-induced apoptosis: a new strategy to prevent cardiomyocyte apoptosis [J].CellularPhysiologyandBiochemistry, 2016, 38(2):531-544.
[17] KUMURA S, TERATANI T, FUJIMOTO Y, et al. Oral administration of polyamines ameliorates liver ischemia/reperfusion injury and promotes liver regeneration in rats [J].LiverTransplantation, 2016, 22(9):1231-1244.
[18] 刘玲,赵弘卿,王昕华,等. 重症肺炎患者干扰素-γ, IL-6和TNF-α含量变化研究 [J]. 现代生物医学进展,2015,15(36):7107-7110. LIU L, ZHAO H Q, WANG X H, et al. Study on the changes of interferon-γ, IL-6 and TNF-α of patients with severe pneumonia [J].ProgressinModernBiomedine, 2015, 15(36):7107-7110. (in Chinese with English abstract)
[19] YIM C Y, BASTIAN N R, SMITH J C, et al. Macrophage nitric oxide synthesis delays progression of ultraviolet light-induced murine skin cancers [J].CancerResearch, 1993, 53(22):5507-5511.
[20] PAUL S, KANG S C. Natural polyamine inhibits mouse skin inflammation and macrophage activation [J].InflammationResearch, 2013, 62(7):681-688.
[21] THAKUR P, NEHRU B. Anti-inflammatory properties rather than anti-oxidant capability is the major mechanism of neuroprotection by sodium salicylate in a chronic rotenone model of Parkinson’s disease [J].Neuroscience, 2013, 231:420-431.
[22] YE L, HUANG Y, ZHAO L, et al. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase [J].JournalofNeurochemistry, 2013, 125(6):897-908.
[23] 殷银霞,许雅清,李海龙,等. IL-1、IL-6、TNF-α及 IFN-γ在脾肾阳虚型溃疡性结肠炎模型大鼠血清及组织中的表达 [J]. 中国实验动物学报, 2015, 23(2):139-142. YIN Y X, XU Y Q, LI H L, et al. Expression and significance of IL-1, IL-6, TNF-α and INF-γ in serum and colon tissue in the rat models of ulcerative colitis with spleen and kidney yang deficiency [J].ActaLaboratoriumAnimallisScientiaSinica, 2015, 23(2):139-142. (in Chinese with English abstract)
[24] CHOI Y H, PARK H Y. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells [J].JournalofBiomedicalScience, 2012, 19(1):31.
[25] MERENTIE M, UIMARI A, PIETIL M, et al. Oxidative stress and inflammation in the pathogenesis of activated polyamine catabolism-induced acute pancreatitis [J].AminoAcids, 2007, 33(2):323-330.
[26] JAMWAL S,KUMAR P. Spermidine ameliorates 3-nitropropionic acid (3-NP)-induced striatal toxicity: possible role of oxidative stress, neuroinflammation, and neurotransmitters [J].Physiology&Behavior, 2016, 155:180-187.
[27] JAMWAL S, SINGH S, KAUR N, et al. Protective effect of spermidine against excitotoxic neuronal death induced by quinolinic acid in rats: possible neurotransmitters and neuroinflammatory mechanism [J].NeurotoxicityResearch, 2015, 28(2):171-184.
[28] SZABO C, SOUTHAN G J, WOOD E, et al. Inhibition by spermine of the induction of nitric oxide synthase in J774. 2 macrophages: requirement of a serum factor [J].BritishJournalofPharmacology, 1994, 112(2):355-356.
(责任编辑 卢福庄)
Effects of spermidine on immune organ index and expression of inflammatory cytokines in mice
XU Qilin, KANG Bo*, JIANG Dongmei*, WEI Chunye, JIA Yilin, YI Zhixin, CHEN Ziyu
(CollegeofAnimalScienceandTechnology,SichuanAgriculturalUniversity/FarmAnimalGeneticResourcesExplorationandInnovationKeyLaboratoryofSichuanProvince,Chengdu611130,China)
To clarify the effects of exogenous spermidine on mice immune organs index and expression of inflammatory cytokines in thymus and spleen, 0, 0.05, 0.10 and 0.15 mg·g-1(body weight) spermidine were intraperitoneal injected for the six-week Kunming mice. The thymus and spleen index were measured after 24 h and the expression ofTNF-α、IL-1β、IL-6 andIFN-γin thymus and spleen were assessed by quantitative real-time PCR. The results showed that, compared with the control group, intraperitoneal injection of 0.15 mg·g-1spermidine group significantly (P<0.05) decreased the thymus index and the spleen index. 0.1 mg·g-1spermidine group enhanced the thymusIFN-γexpression (P<0.05). In the spleen, the expressions ofTNF-αandIL-1βin 0.05 mg·g-1spermidine group were significantly (P<0.05) lower than those of the control group, but the expressions of theIL-6 andIFN-γgene in 0.1 mg·g-1spermidine group were significantly higher than those of the control group (P<0.05). The expression ofTNF-αin 0.15 mg·g-1spermidine group was significantly (P<0.05) lower than that of the control group. Thus, the spermidine showed tissue specific and dose dependent manner on the expression of inflammatory cytokines in mice. Low concentration of spermidine had anti-inflammatory effect by inhibiting the expression of inflammatory cytokines, but high-dose spermidine could also induce inflammation.
spermidine; thymus index; spleen index; inflammatory cytokines
10.3969/j.issn.1004-1524.2017.02.04
2016-08-22
国家自然科学基金资助项目(31201798);四川省教育综合改革试点项目“构建服务现代农业发展的产学研协同创新体系”
徐麒麟(1992—),男,四川崇州人,硕士研究生,主要从事动物卵泡发育机理研究。E-mail: albertxql@163.com
*通信作者,康波,E-mail: bokang@sicau.edu.cn;姜冬梅,E-mail:jiangdm9277@163.com
Q26
A
1004-1524(2017)02-0200-06
