N,N-二甲基乙酰胺促进的羧酸肉桂酯的合成及其促进机理
2017-02-24祝显虹周安西罗年华曾显柱郑大贵
祝显虹, 周安西, 罗年华, 彭 亮, 曾显柱, 郑大贵
(上饶师范学院 江西省普通高校应用有机化学重点实验室,江西 上饶 334001)
·研究论文·
N,N-二甲基乙酰胺促进的羧酸肉桂酯的合成及其促进机理
祝显虹, 周安西, 罗年华, 彭 亮, 曾显柱, 郑大贵*
(上饶师范学院 江西省普通高校应用有机化学重点实验室,江西 上饶 334001)
在N,N-二甲基乙酰胺(DMAc)促进下,羧酸(1a~1v)依次与SOCl2和肉桂醇反应合成了22个羧酸肉桂酯(2a~2v,其中2j, 2k, 2o, 2t和2v为新化合物),其结构经1H NMR,13C NMR, IR和MS(EI)表征。以巴豆酸肉桂酯(2i)的合成为例,研究了巴豆酸(1i)用量,肉桂醇用量,SOCl2用量,DMAc用量对2i产率的影响和醇的加入方式对产物组成的影响。结果表明:在最优合成条件(1i 1.0 eq.,肉桂醇1.3 eq., SOCl21.3 eq., DMAc 2 mL, CH2Cl26 mL,酰氯化后加入苄醇)下,2i产率82.2%。采用1H NMR跟踪反应,确证了DMAc促进反应的机理。
N,N-二甲基乙酰胺; 肉桂醇; 肉桂酯; 合成; 促进机理
羧酸酰氯化合成羧酸酯或酰胺是常用的有机合成方法。羧酸酰氯化常用的氯代试剂有:SOCl2、(COCl)2、PCl3、POCl3、PCl5和三聚氯乙腈等[1-3]。使用促进剂促进“氯代试剂”,可将羧基活化制得活性中间体AG, AG与Cl-反应生成酰氯(Scheme 1)。相对于单独使用氯代试剂的方法,用“促进剂-氯代试剂”的方法反应条件更温和、反应效果更好。
“PPh3-氯代试剂”是应用最广泛的“促进剂-氯代试剂”组合。其中氯代试剂包括CCl4[4]、三聚氯乙腈[5]、四甲基-α-氯烯胺[6]、Cl3CCCl3[7]、CCl3CN[8-9]和Cl3CCONH2[10]等。这类组合与羧酸作用后均可制得活性中间体AG-1。通过“苯并三氮唑-SOCl2”能将羧酸高效转化为酰氯[11],其原理为:苯并三氮唑先与SOCl2作用制得中间产物,再与羧酸反应得到活性中间体AG-2;“取代环丙烯酮-草酰氯”[12]和“环庚三烯酮-草酰氯”[13]分别使羧基活化制得中间体AG-3和AG-4。以上方法均能较大地提高羧基碳的电正性,有利于Cl-的亲核进攻,加速酰氯形成。此外,还可采用“(N,N-二烷基取代酰胺)-氯代试剂”组合,先相互作用生成Vilsmeier盐;羧酸与Vilsmeier盐反应制得类似于AG-5的活性中间体,如“DMAc-SOCl2”[14-18]、 “DMF-邻苯二甲酰氯”[16]、“四甲基脲(或1,3-二甲基-2-咪唑烷酮)-邻苯二甲酰氯”[16]等。
本文在DMAc促进下,羧酸(1a~1v)依次与SOCl2和肉桂醇反应合成了22个羧酸肉桂酯(2a~2v,其中2j, 2k, 2o, 2t和2v为新化合物),其结构经1H NMR,13C NMR, IR和MS(EI)表征。以巴豆酸肉桂酯(2i)的合成为例,研究了巴豆酸(1i)用量,肉桂醇用量,SOCl2用量,DMAc用量对2i产率的影响和醇的加入方式对产物组成的影响。结果表明:在最优合成条件(1i 1.0 eq.,肉桂醇1.3 eq., DMAc 2 mL, CH2Cl26 mL,酰氯化后加入苄醇)下,2i产率82.2%。采用1H NMR跟踪反应,确证了DMAc促进反应的机理。
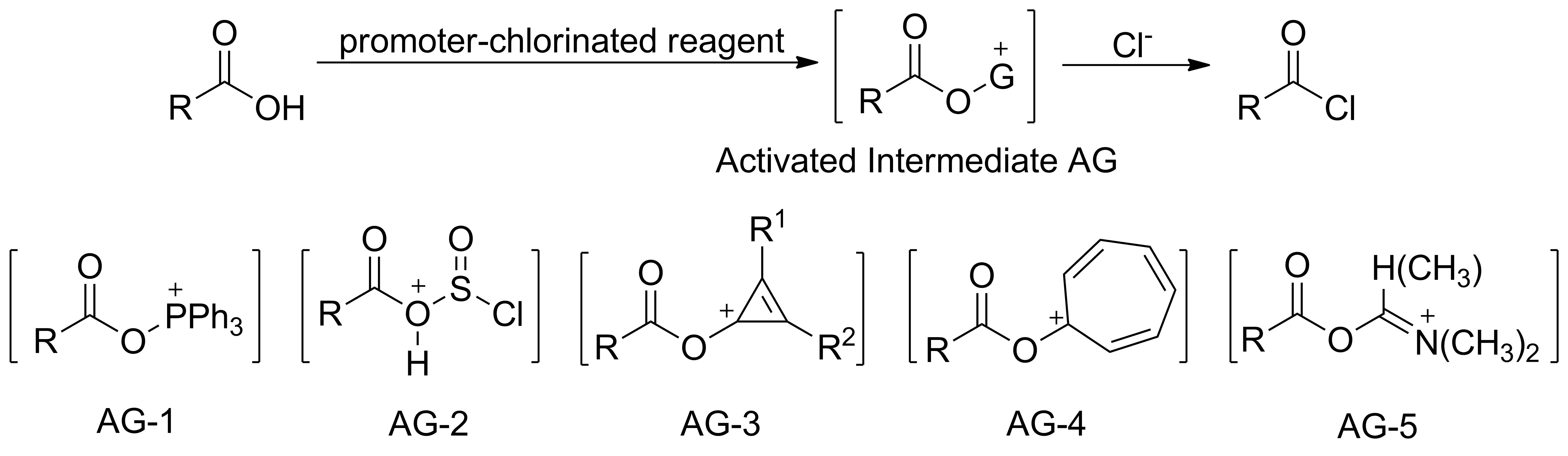
Scheme 1

Scheme 2
1 实验部分
1.1 仪器与试剂
X-6型显微熔点仪(温度未校正);Bruker Avance 400型核磁共振仪(CDCl3为溶剂,TMS为内标);Bruker Tensor 27型红外光谱仪(KBr压片);Agilent Techologies 5975C型质谱仪;Bruker Daltonics APEX II 47e型高分辨质谱仪。
1a~1v和肉桂醇(纯度≥98%),阿拉丁试剂公司;正己氧羰基丙烯酸按文献[19]方法合成;SOCl2,工业品,用前重蒸;DMAc,分析纯,用前经预处理;其余所用试剂均为分析纯。
1.2 2a~2i的合成(以2i为例)
在反应瓶中依次加入1a 0.861 g(10 mmol, 1.0 eq.), DMAc 2 mL和CH2Cl26 mL,搅拌使其溶解;冷却至0 ℃,缓慢滴加SOCl20.94 mL(13 mmol, 1.3 eq.),滴毕,反应2 h。加入肉桂醇1.745 g(13 mmol, 1.3 eq.),于25 ℃反应5 h。依次加入蒸馏水40 mL和乙酸乙酯40 mL,分液,水层用乙酸乙酯(2×40 mL)萃取,合并有机层,依次用饱和NaHCO3溶液(30 mL)和饱和食盐水洗至无DMAc(TLC跟踪),无水Na2SO4干燥,旋蒸除溶,残余物经硅胶柱层析[洗脱剂:石油醚,洗脱副产物肉桂基氯;洗脱剂:V(乙酸乙酯) ∶V(石油醚)=1 ∶50]纯化,旋蒸除溶后真空干燥得2i。
用类似的方法合成2a~2h, 2j~2v。
辛酸肉桂醇酯(2a)[20]: 无色油状液体,产率68.2%;1H NMRδ: 7.39~7.25(m, 5H), 6.65(d,J=16.0 Hz, 1H), 6.28(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.73(dd,J=6.4 Hz, 1.2 Hz, 2H), 2.34(t,J=7.6 Hz, 2H), 1.68~1.61(m, 2H), 1.31~1.25(m, 8H), 0.87(t,J=6.8 Hz, 3H);13C NMRδ: 173.6, 136.3, 134.1, 128.6, 128.0, 126.6, 123.4, 64.8, 34.4, 31.7, 29.1, 28.9, 25.0, 22.6, 14.1; IRν: 3 028, 2 957, 2 929, 2 857, 1 736, 1 499, 1 450, 1 380, 966, 745, 692 cm-1; MS(EI)m/z: 260.1[M+], 127.1[C7H15CO+]。
癸酸肉桂醇酯(2b)[21]: 无色油状液体,产率91.7%;1H NMRδ: 7.40~7.25(m, 5H), 6.64(d,J=16.0 Hz, 1H), 6.28 (dt,J=16.0 Hz, 6.4 Hz, 1H), 4.73(dd,J=6.4 Hz, 1.2 Hz, 2H), 2.34(t,J=7.6 Hz, 2H), 1.68~1.61 (m, 2H), 1.30~1.25(m, 12H), 0.87(t,J=6.8 Hz, 3H);13C NMRδ: 173.6, 136.3, 134.0, 128.6, 128.0, 126.6, 123.4, 64.8, 34.4, 31.9, 29.4, 29.3, 29.3, 29.2, 25.0, 22.7, 14.1; IRν: 3 028, 2 949, 2 926, 2 855, 1 736, 1 496, 1 450, 1381, 965, 745, 692 cm-1; MS(EI)m/z: 288.2[M+], 155.1[C9H19CO+]。
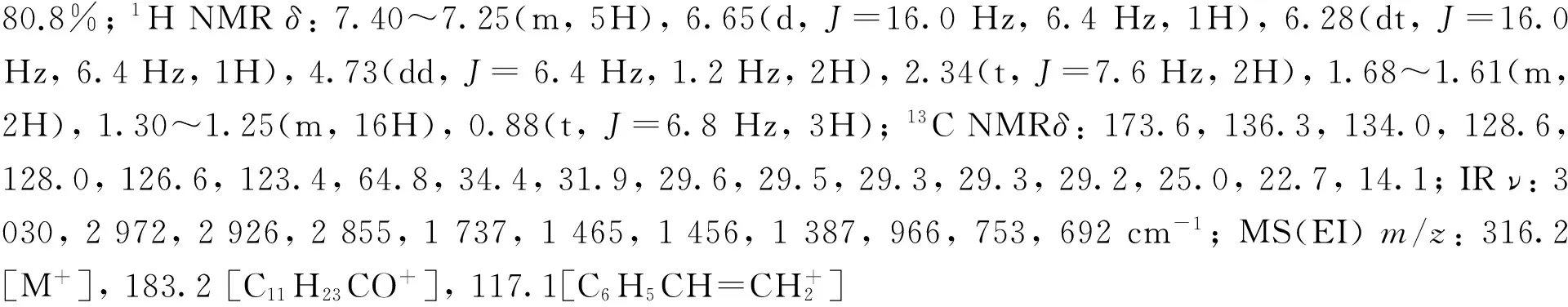
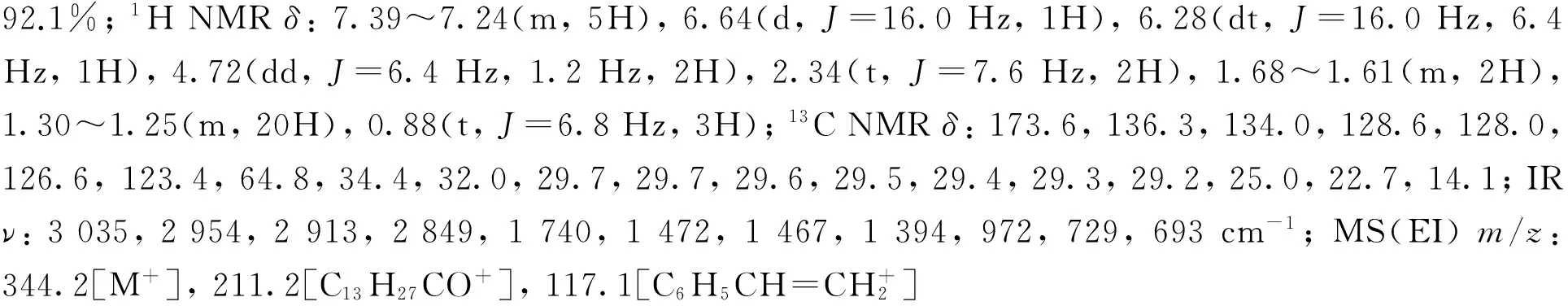
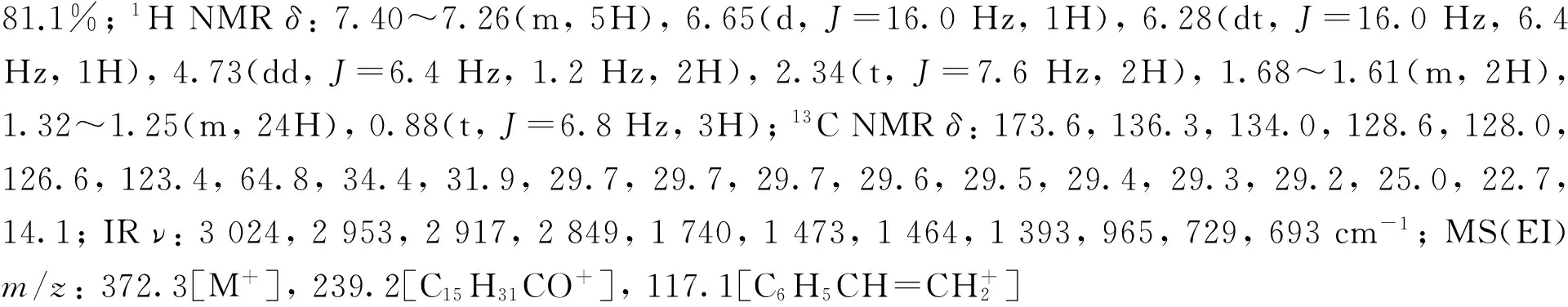
2-甲基丙酸肉桂醇酯(2f)[26]: 无色油状液体,产率82.4%;1H NMRδ: 7.40~7.25(m, 5H), 6.64(d,J=16.0 Hz, 1H), 6.28(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.72(dd,J=6.4 Hz, 1.2 Hz, 2H), 2.59(hept,J=6.8 Hz , 1H), 1.19(d,J=6.8 Hz, 6H);13C NMRδ: 176.9, 136.3, 133.9, 128.6, 128.0, 126.6, 123.5, 64.9, 34.1, 19.0; IRν: 3 028, 2 975, 2 937, 1 734, 1 493, 1 489, 1 468, 1 389, 965, 742, 691 cm-1; MS(EI)m/z: 204.1[M+], 71.1[C3H7CO+, 100]。

肉桂酸肉桂酯(2h)[23,27]: 白色固体,m.p.45.4~46.2 ℃(42~43 ℃[28]),产率86.9%;1H NMRδ: 7.73(d,J= 16.0 Hz, 1H), 7.54~7.52(m, 2H), 7.42~7.26(m, 8H), 6.71(d,J=16.0 Hz, 1H), 6.49(d,J=16.0 Hz, 1H), 6.36(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.87(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 166.7, 145.1, 136.3, 134.4, 134.3, 130.3, 128.9, 128.6, 128.1, 128.1, 126.7, 123.4, 118.0, 65.1; IRν: 3 027, 2 934, 1 715, 1 637, 1 496, 1 449, 969, 768, 750, 708, 686 cm-1; MS(EI)m/z: 264.1[M+], 131.1[C8H7CO+]。
巴豆酸肉桂醇酯(2i)[27]: 无色油状液体,产率82.2%;1H NMRδ: 7.40~7.25(m, 5H), 7.02(dq,J=16.0 Hz, 6.8 Hz, 1H), 6.65(d,J=16.0 Hz, 1H), 6.30(dt,J=16.0 Hz, 6.4 Hz, 1H), 5.89(dq,J=16.0 Hz, 1.6 Hz, 1H), 4.78(dd,J=6.4 Hz, 1.2 Hz, 2H), 1.89(dd,J=6.8 Hz, 1.6 Hz, 3H);13C NMRδ: 166.2, 145.0, 136.3, 134.0, 128.6, 128.0, 126.6, 123.5, 122.6, 64.7, 18.0; IRν: 3 022, 2 943, 2 915, 1 720, 1 658, 1 496, 1 446, 968, 749, 692 cm-1; MS(EI)m/z: 202.1[M+], 69.0[C3H5CO+]。
正己氧羰基丙烯酸肉桂醇酯(2j): 无色油状液体,产率88.3%;1H NMRδ: 7.41~7.26(m, 5H), 6.89(s, 2H), 6.70(d,J=16.0 Hz, 1H), 6.31(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.86(dd,J=6.4 Hz, 1.2 Hz, 2H), 4.20(t,J=6.8 Hz, 2H), 1.71~1.64(m, 2H), 1.32~1.30(m, 6H), 0.89(t,J=6.8 Hz, 3H);13C NMRδ: 165.0, 164.8, 136.0, 134.9, 134.1, 133.3, 128.7, 128.6, 128.3, 126.7, 125.7, 122.4, 77.2, 31.4, 28.5, 25.5, 22.5, 14.0; IRν: 3 027, 2 957, 2 932, 2 859, 1 724, 1 650, 1 451, 980, 748, 689 cm-1; HR-MS(ESI)m/z: Calcd for C19H24O4{[M+NH4]+}334.201 3, found 334.201 5。
环戊基甲酸肉桂醇酯(2k): 无色油状液体,产率80.7%;1H NMRδ: 7.39~7.24(m, 5H), 6.63(d,J=16.0 Hz, 1H), 6.28(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.72(dd,J=6.4 Hz, 1.2 Hz, 2H), 2.77(quint,J=8.0 Hz, 1H), 1.89~1.56(m, 8H);13C NMRδ: 176.5, 136.4, 133.9, 128.6, 128.0, 126.6, 123.6, 64.9, 43.9, 30.1, 25.9; IRν: 3 027, 2 958, 2 871, 1 731, 1 495, 1 449, 968, 745, 692 cm-1; HR-MS(ESI)m/z: Calcd for C15H18O2{[M+Na]+}253.119 9, found 253.119 8。
苯甲酸肉桂醇酯(2m)[21,27,30]: 白色固体, m.p.38.4~39.8 ℃(m.p.38~39 ℃[28]),产率69.7%;1H NMRδ: 8.14~8.07(m, 2H), 7.51~7.22(m, 8H), 6.70(d,J=16.0 Hz, 1H), 6.37(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.95(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 166.4, 136.3, 134.3, 133.1, 130.6, 130.3, 129.7, 128.9, 128.7, 128.5, 128.2, 126.7, 123.4, 65.6; IRν: 3 058, 3 028, 2 943, 2 870, 1 717, 1 600, 1 491, 966, 748, 712, 692 cm-1; MS(EI)m/z: 238.1[M+], 105.1[C6H5CO+]。
2-甲基苯甲酸肉桂醇酯(2n)[28,30]: 无色油状液体,产率68.2%;1H NMRδ: 7.97~7.95(m, 1H), 7.41~7.22(m, 8H), 6.72(d,J=16.0 Hz, 1H), 6.39(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.94(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 167.4, 140.3, 136.4, 134.3, 132.1, 131.8, 130.7, 129.7, 128.7, 128.1, 126.7, 125.8, 123.5, 65.3, 21.8; IRν: 3 060, 3 026, 2 930, 2 870, 1 717, 1 602, 1 494, 966, 742, 692 cm-1; MS(EI)m/z: 252.1[M+], 119.1[C7H7CO+]。
3-甲基苯甲酸肉桂醇酯(2o): 无色油状液体,产率60.7%;1H NMRδ: 7.95~7.87(m, 2H), 7.41~7.23(m, 7H), 6.73(d,J=16.0 Hz, 1H), 6.40(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.96(d,J=6.4 Hz, 2H), 2.39(s, 3H);13C NMRδ: 166.6, 138.2, 136.3, 134.3, 133.8, 130.2, 130.2, 128.6, 128.3, 128.1, 126.9, 126.7, 123.4, 65.5, 21.3; IRν: 3 082, 3 027, 2 946, 2 873, 1 720, 1 608, 1 495, 1 449, 964, 743, 692 cm-1; HR-MS(ESI)m/z: Calcd for C17H16O2{[M+K]+}291.078 2, found 291.078 3。
4-甲基苯甲酸肉桂醇酯(2p)[21,28]: 白色固体,m.p.24.3~26.0 ℃,产率57.0%;1H NMRδ: 7.98~7.96(m, 2H), 7.40~7.20(m, 7H), 6.70(d,J=16.0 Hz, 1H), 6.38(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.94(dd,J=6.4 Hz, 1.2 Hz, 2H), 2.37(s, 3H);13C NMRδ: 166.5, 143.7, 136.4, 134.2, 129.8, 129.2, 128.7, 128.1, 127.6, 126.7, 123.5, 65.4, 21.7; IRν: 3 082, 3 059, 2 946, 2 878, 1 716, 1 612, 1 509, 1 449, 966, 841, 753, 692 cm-1; MS(EI)m/z: 252.1[M+], 119.1[C7H7CO+]。
2-氯苯甲酸肉桂醇酯(2q)[27]: 无色油状液体,产率72.1%;1H NMRδ: 7.86~7.84(m, 1H), 7.44~7.37(m, 4H), 7.33~7.24(m, 4H), 6.74(d,J=16.0 Hz, 1H), 6.38(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.97(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 165.5, 136.2, 134.7, 133.8, 132.6, 131.5, 131.2, 130.2, 128.7, 128.2, 126.7, 126.6, 122.8, 66.1; IRν: 3 060, 3 027, 2 946, 2 879, 1 731, 1 592, 1 495, 1 436, 967, 747, 692 cm-1; MS(EI)m/z: 272.0[M+], 139.1[C7H4COCl+]。
3-氯苯甲酸肉桂醇酯(2r)[27]: 淡黄色油状液体,产率79.0%;1H NMRδ: 8.05~8.04(m, 1H), 7.96~7.94(m, 1H), 7.52~7.50(m, 1H), 7.41~7.30(m, 6H), 6.72(d,J=16.0 Hz, 1H), 6.38(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.97(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 165.2, 136.1, 134.8, 134.6, 133.0, 132.0, 129.8, 129.7, 128.7, 128.2, 127.8, 126.7, 122.9, 66.0; IRν: 3 060, 3 027, 2 946, 2 881, 1 720, 1 576, 1 496, 1 448, 965, 748, 692 cm-1; MS(EI)m/z: 272.0[M+], 139.1[C7H4COCl+]。
4-氯苯甲酸肉桂醇酯(2s)[21]: 白色固体,m.p.43.4~44.5 ℃(m.p. 44~46 ℃[27]),产率78.0%;1H NMRδ: 8.02~8.00(m, 2H), 7.42~7.25(m, 7H), 6.73(d,J=16.0 Hz, 1H), 6.39(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.97(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 165.5, 139.5, 136.2, 134.6, 131.1, 128.8, 128.7, 128.7, 128.2, 126.7, 123.0, 65.8; IRν: 3 081, 3 027, 2 954, 2 875, 1 715, 1 593, 1 487, 1 448, 969, 760, 692 cm-1; MS(EI)m/z: 272.0[M+], 139.1[C7H4ClCO+]。
2-甲氧基苯甲酸肉桂醇酯(2t): 淡黄色固体,m.p.47.7~48.5 ℃,产率76.8%;1H NMRδ: 7.86~7.83(m, 1H), 7.46~7.24(m, 6H), 7.00~6.96(m, 2H), 6.75(d,J=16.0 Hz, 1H), 6.39(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.96(dd,J=6.4 Hz, 1.2 Hz, 2H), 3.90(s, 3H);13C NMRδ: 165.9, 159.3, 136.4, 134.0, 133.6, 131.7, 128.6, 128.0, 126.7, 123.5, 120.2, 120.1, 112.1, 65.3, 56.0; IRν: 3 055, 3 016, 2 976, 2 923, 2 830, 1 712, 1 594, 1 492, 1 434, 972, 745, 692 cm-1; HR-MS(ESI)m/z: Calcd for C17H16O3{[M+H]+}269.117 2, found 269.117 1。
2-硝基苯甲酸肉桂醇酯(2u)[31]: 白色固体,m.p.59.3~61.4 ℃(m.p.63 ℃[32]),产率59.5%;1H NMRδ: 7.91~7.90(m, 1H), 7.77~7.75(m, 1H), 7.68~7.59(m, 2H),7.42~7.26(m, 5H), 6.72(d,J=16.0 Hz,1H), 6.34(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.98(dd,J=6.4 Hz, 1.2 Hz, 2H);13C NMRδ: 165.2, 148.2, 136.1, 135.3, 132.9, 131.8, 129.9, 128.7, 128.3, 127.7, 126.8, 124.0, 122.0, 67.0; IRν: 3 100, 3 042, 2 952, 2 886, 1 725, 1 577, 1 530, 1 484, 967, 873, 691 cm-1; MS(EI)m/z: 283.0[M+], 151.0[C6H4NO2CO+]。
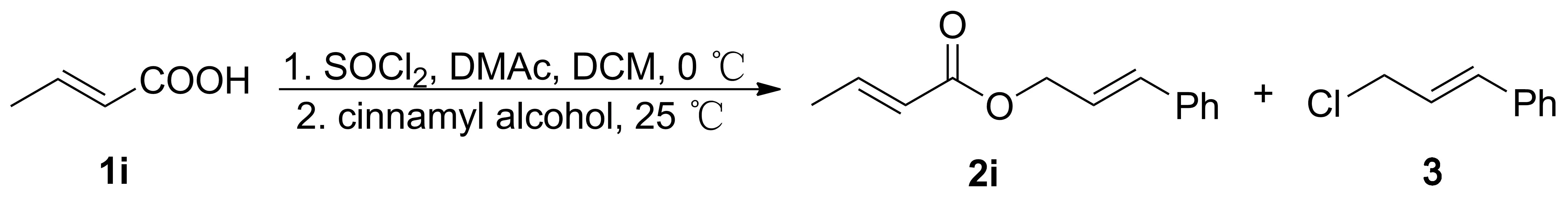

Scheme 3表1 2i的合成条件优化Table 1 Optimization of the synthesis reaction conditions for 2i
a1: 条件同1.2; 2: 0 ℃下,1i和肉桂醇同时加入DMAc, SOCl2和CH2Cl2中,反应2 h;于25 ℃反应5 h;b分离产率。
2-乙酰基苯甲酸肉桂醇酯(2v): 无色油状液体,产率46.3%;1H NMRδ: 7.91~7.90(m, 1H), 7.73~7.72(m, 1H), 7.60~7.54(m, 2H), 7.31~7.26(m, 5H), 6.47(d,J=16.0 Hz, 1H), 6.16(dt,J=16.0 Hz, 6.4 Hz, 1H), 4.08~4.03(m, 1H), 3.75~3.71(m, 1H), 1.90(s, 3H);13C NMRδ: 168.0, 147.9, 136.4, 134.6, 133.0, 130.7, 128.5, 127.9, 127.4, 126.5, 125.6, 124.5, 122.3, 108.5, 65.0, 25.7; IRν: 3 061, 3 026, 2 994, 2 936, 2 864, 1 771, 1 466, 1 284, 968, 890, 768, 695 cm-1; HR-MS(ESI)m/z: Calcd for C18H16O3{[M+NH4]+}298.143 8, found 298.143 7。
2 结果与讨论
2.1 2的合成条件优化
以2i的合成(Scheme 3)为例,研究了1i用量,肉桂醇用量,SOCl2用量,DMAc用量对2i产率的影响和醇的加入方式对产物组成的影响,结果见表1。
由表1可见,Entry 1和Entry 2~7为DMAc用量对产率的影响,当DMAc用量为2 mL时(Entry 3),产率比完全不用DMAc(Entry 1)提高50%以上。继续增大DMAc用量,产率基本稳定,其可能原因为:反应温度较低时,油状DMAc会影响反应传质。这在另外的实验中得以证实(将CH2Cl2用量从6 mL减少至3 mL,其余反应条件同Entry 11,产率仅70.3%)。此外,DMAc用过过多还会增加产物分离纯化过程中水洗的次数。因此,DMAc的适宜用量为2 mL。
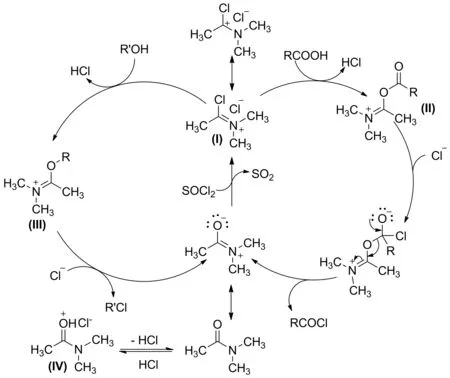
Scheme 4
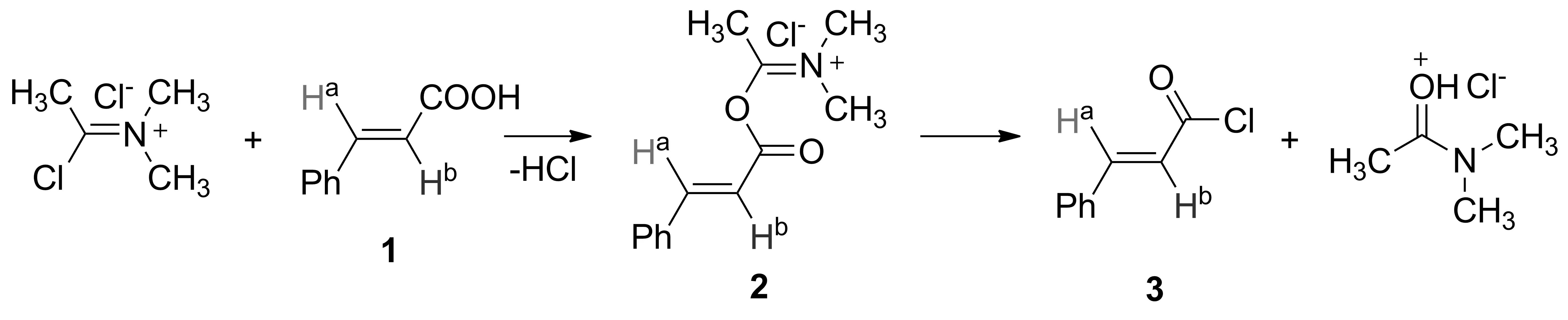
Scheme 5
Entry 8~13为SOCl2用量对产率的影响。SOCl2用量从1.0 eq.增加到1.3 eq.,产率提高,副产物肉桂基氯(3)产率约10%。当SOCl2用量增加到1.4 eq.(Entry 12),产率反而下降,3产率明显提高。其可能原因为:肉桂醇加入反应体系后,过量SOCl2和过量DMAc仍在反应生成Vilsmeier盐,由于与Vilsmeier盐反应以及与SOCl2反应的活性均是醇大于羧酸,醇更容易转变为氯代烃。通过改变投料方式,即将肉桂醇和1i同时加入DMAc, SOCl2和CH2Cl2中(Entry 13和Entry 14), 3产率明显提高,这一结果验证了上述分析。
综上,合成2i的最佳条件为:1i 1.0 eq.,肉桂醇1.3 eq., DMAc 2 mL, SOCl21.3 eq., CH2Cl26 mL,酰氯化后加入苄醇。
2.2 DMAc促进反应机理
DMAc可能从三个方面协同促进酯的合成(Scheme 4):(1)作催化剂,DMAc与SOCl2反应生成Vilsmeier盐(Ⅰ)[33]; Ⅰ与羧酸反应生成中间产物(Ⅱ),由于Ⅱ的羰基碳比羧酸的羰基碳电正性更强,容易接受Cl-的亲核进攻,加速了酰氯的生成(与此相似,Ⅰ与醇反应生成Ⅲ,加速了氯代烃的生成);(2)作为碱,DMAc吸收副产物HCl,生成DMAc盐酸盐(Ⅳ),也促进反应朝生成酰氯(或氯代烃)的方向进行。当酰氯生成后加入醇成酯,Ⅳ释放出的质子又能起到催化作用[34]。
为验证上述机理,我们在本课题组前期工作[16]的基础上,以1h为例,用1H NMR跟踪反应,确证了Ⅱ的存在。实验方法为:冰水浴冷却下,DMAc 1 mL(10.8 mmol), SOCl20.8 mL(11.0 mmol)在CDCl310 mL中反应70 min(生成Ⅰ[16])。加入肉桂酸1.778 g(12 mmol),每隔10 min取样,用1H NMR跟踪反应。加入1h 40 min后,反应混合液中同时检测到1h, Ⅱ和.3(Scheme 5)。
图1为反应混合液的1H NMR 谱图。由图1可见,1h, Ⅱ和3双键碳上所连aH的δ分别为7.77(d,J=16.0 Hz), 7.86(d,J=16.0 Hz)和7.85(d,J=15.6 Hz)。bH的δ分别为6.47(d,J=16.0 Hz), 6.54(d,J=16.0 Hz)和6.66(d,J=15.6 Hz)。此外,还能观察到Ⅳ的存在,其δ分别为12.35(s, 1H), 3.25(s, 6H), 2.64(s, 3H)。相关氢的积分比(0.99 ∶0.89=1.11 ∶1.00)与1h和DMAc的投料比(12 ∶11=1.09 ∶1.00)基本吻合。
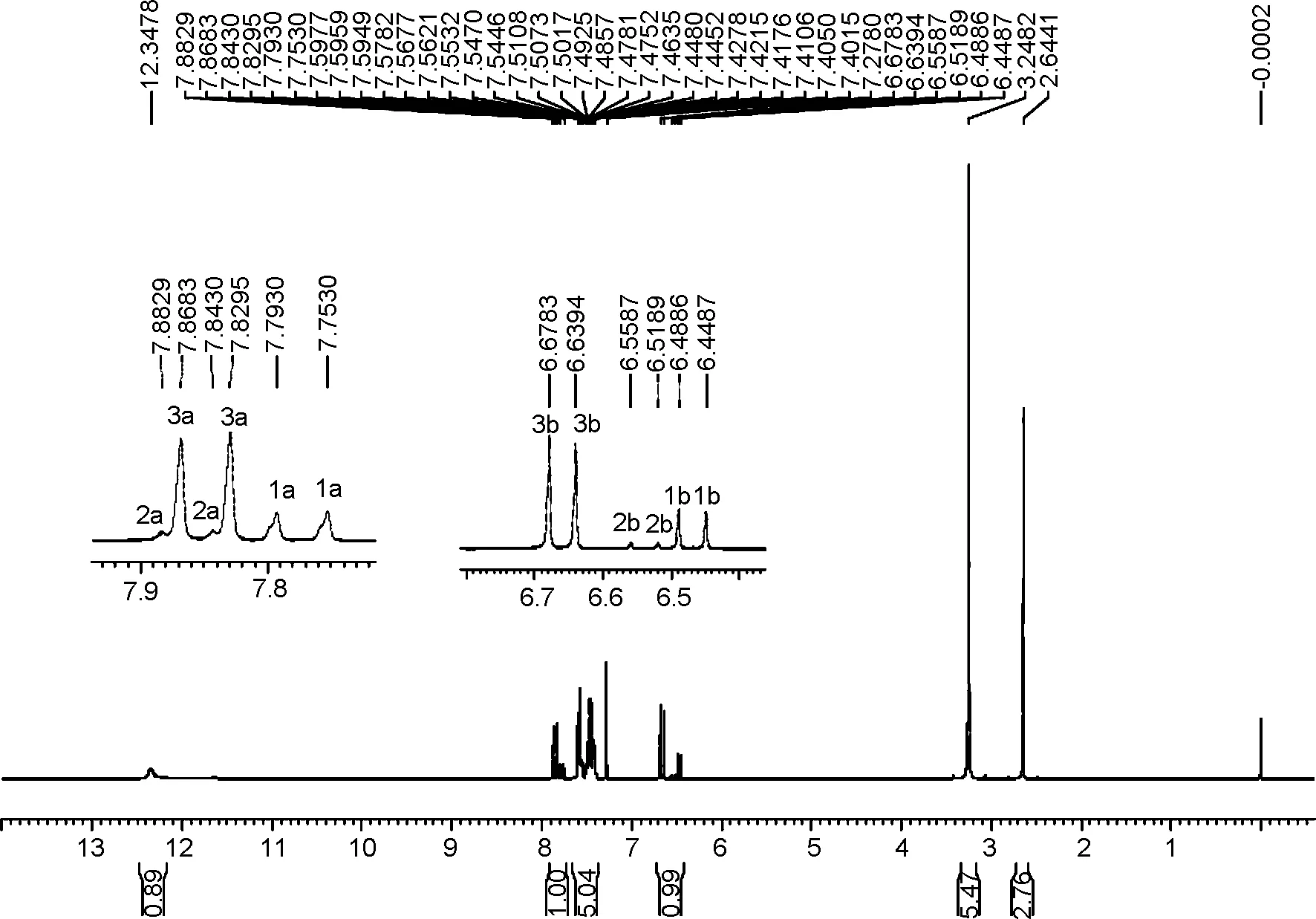
δ图1 DMAc-SOCl2-1h的1H NMR谱图Figure 1 1H NMR spectrum of the DMAc-SOCl2-1h
报道了一种N,N-二甲基乙酰胺促进的羧酸肉桂酯的合成方法。该方法具有反应条件温和、操作简单、底物适用范围较广等优点。
[1] Montalbetti C A G N, Falque V. Amide bond formation and peptide coupling[J].Tetrahedron,2005,61(46):10827-10852.
[2] Rayle H L, Fellmeth L.Development of a process for triazine-promoted amidation of carboxylic acids[J].Org Process Res Dev,1999,3(3):172-176.
[3] 李德江,龙德清,付和清. 肉桂酸苄酯的合成[J].合成化学,2004,12(4):378-380.
[4] Harrison C R, Hodge P, Hunt B J,etal. Preparation of alkyl chlorides,acid chlorides, and amides using polymer-supported phosphines and carbon tetrachloride:Mechanism of these reactions[J].J Org Chem,1983,48(21):3721-3728.
[5] Venkataraman K, Wagle D R.Cyanuric chloride:A useful reagent for converting carboxylic acids into chloride,esters,amides and peptides[J].Tetrahedron Lett,1979,20(32):3037-3040.
[6] Devos A, Remion J, Frisque-Hesbain A M,etal. Synthesis of acyl halides under very mild conditions[J].J Chem Soc Chem Commun,1979,(24):1180-1181.
[7] Villeneuve G B, Chan T H. A rapid,mild and acid-free procedure for the preparation of acyl chlorides including formyl chloride[J].Tetrahedron Lett,1997,38(37):6489-6492.
[8] Jang D O, Park D J, Kim J. A mild and efficient procedure for the preparation of acid chlorides from carboxylic acids[J].Tetrahedron Lett,1999,40(29):5323-5326.
[9] Jang D O, Cho D H, Kim J G. One-pot preparation of esters from carboxylic acids using the PPh3-CCl3CN system[J].Synth Commun,2003,33(16):2885-2890.
[10] Chantarasriwong O, Jang D O, Chavasiri W.Cl3CCONH2/PPh3:A versatile reagent for synthesis of esters[J].Synth Commun,2008,38(16):2845-2856.
[11] Chaudhari S S, Akamanchi K G. Thionyl chloride-benzotriazole in methylene chloride:A convenient solution for conversion of alcohols and carboxylic acids expeditiously into alkyl chlorides and acid chlorides by simple titration[J]. Synlett,1999,10(11):1763-1765.
[12] Hardee D J, Kovalchuke L, Lambert T H. Nucleophilic acyl substitution via aromatic cation activation of carboxylic acids:Rapid generation of acid chlorides under mild conditions[J].J Am Chem Soc,2010,132(14):5002-5003.
[13] Nguyen T V, Bekensir A. Aromatic cation activation:Nucleophilic substitution of alcohols and carboxylic acids[J].Org Lett,2014,16(6):1720-1723.
[14] Cvetovich R J, DiMichele L. Formation of acrylanilides,acrylamides,and amides directly from carboxylic acids using thionyl chloride in dimethylacetamide in the absence of bases[J].Org Process Res Dev,2006,10(5):944-946.
[15] Kimura Y, Matsuura D. Novel synthetic method for the Vilsmeier-Haack reagent and green routes to acid chlorides,alkyl formates,and alkyl chlorides[J].Int J of Org Chem,2013,3:1-7.
[16] Kimura Y, Matsuura D, Hanawa T,etal. New preparation method for Vilsmeier reagent and related imidoyl chlorides[J].Tetrahedron Lett,2012,53(9):1116-1118.
[17] 罗年华,郑大贵,祝显虹,等.N,N-二甲基乙酰胺促进N-取代肉桂酰胺的合成[J].合成化学,2016,24(5):445-449.
[18] 罗年华,郑大贵,周安西,等. DMAc促进取代肉桂酰吗啉的合成[J].精细化工,2016,33(8):956-960.
[19] 祝显虹,郑大贵,张小兰,等. 富马酸单正烷基酯的合成和表征[J].化学研究与应用,2012,24(9):1413-1417.
[20] Grossman R F. Mixed metal vinyl stabilizer synergism III:Reactions of mixed metals and phosphites[J].J Vinyl Technol, 1992,14(1):11-15.
[21] Dinesh M, Archana S, Ranganathan R,etal. Bis azide-triphenylphosphine as a reagent for esterification at room temperature[J].Tetrahedron Lett,2015,56(50):6975-6979.
[22] Yadav G D, Dhoot S B.Immobilized lipase-catalysed synthesis of cinnamyl laurate in non-aqueous media[J]. J Molecular Catalysis B:Enzymatic,2009,57(1-4):34-39.
[23] Huang X J, Fulton B, White K,etal. Metal-free,regio- and stereoselective synthesis of linear (E)-allylic compounds using C,N,O,and S nucleophiles[J].Org Lett,2015,17(11):2594-2597.
[24] Gonella M T, Abbattista F G. Esters of cinnamic alcohol with fatty acids[J].Gazz Chim Ital,1955,85:561-568.
[25] Vosmann K, Weitkamp P, Weber N. Solvent-free lipase-catalyzed preparation of long-chain alkyl phenylpropanoates and phenylpropyl alkanoates[J].J Agric Food Chem,2006,54(8):2969-2976.
[26] Naik S, Kavala V, Gopinath R,etal. 1,1′-(Ethane-1,2-diyl)dipyridinium bistribromide(EDPBT) as a recyclable catalyst for acylation[J].Arkivoc,2006,(Xi):21-36.
[27] Maji B, Vedachalan S, Ge X,etal.N-heterocyclic carbene-mediated oxidative esterification of aldehydes:Ester formation and mechanistic studies[J].J Org Chem,2011,76(9):3016-3023.
[28] Jia X S, Wang H L, Huang Q,etal. A fast and simple method for the acylation of alcohols with acid chlorides promoted by metallic samarium[J].J Chem Res,2006,(2):135-138.
[29] Liu C, Tang L W, Liu D,etal. Covalently bound benzyl ligand promotes selective palladium-catalyzed oxidative esterification of aldehydes with alcohols[J].Angew Chem Int Ed,2012,51(23):5662-5666.
[30] Sawant Y S, Wagh D N, Dhake K P,etal. Allylation of 1-phenyl-1-propyne withN- andO-pronucleophiles using polymer supported triphenylphosphine palladium complex as a heterogeneous and recyclable catalyst[J].Tetrahedron Lett,2011,52(8):5676-5679.
[31] Hossian A, Singha S, Jana R. Palladium(0)-catalyzed intramolecular decarboxylative allylation oforthonitrobenzoic esters[J].Org Lett,2014,16(15):3934-3937.
[32] Jacquemain R, Moskovits A. Some compounds obtained by the aid of complex iodosilvernitrobenzoates[J].Compt Rend,1937,204:134-136.
[33] 郑大贵,周安西,祝显虹,等. 二甲基乙酰胺高效促进由醇制备氯代烃[J].有机化学,2016,36(1):137-142.
[34] Horrobin D F, Knowles P, Manku M S. Fatty acid derivatives:US 5847000[P].1998.
N,N-Dimethylacetamide-promoted Synthesis of Cinnamyl Carboxylate and The Co-promoted Mechanism
ZHU Xian-hong, ZHOU An-xi, LUO Nian-hua,PENG Liang, ZENG Xian-zhu, ZHENG Da-gui*
(Key Laboratory of Applied Organic Chemistry, Higher Institutions of Jiangxi Province,Shangrao Normal University, Shangrao 334001)
Twenty two cinnamyl carboxylates(2a~2v, among them 2j, 2k, 2o, 2t and 2v were novel compounds) were synthesized by theN,N-dimethylacetamide(DMAc)-promoted reaction of carboxylic(1a~1v) with SOCl2and cinnamyl alcohol, respectively. The structures were characterized by1H NMR,13C NMR, IR and MS(EI). Effects of the dosage of crotonic acid(1i), cinnamic alcohol, SOCl2and DMAc on yield of cinnamyl crotonate(2i) and the addition protocol of alcohol on products composition were investigated, using the synthsis of 2i as example. The results indicated that under the optimized reaction conditions(1i 1.0 eq., cinnamic alcohol 1.3 eq., SOCl21.3 eq., DMAc 2 mL, CH2Cl26 mL, adding benzyl alcohol after acylation), the yield of 2i was 82.2%. The co-promoted mechanism of DMAc was confirmed by1H NMR tracing results.
dimethylacetamide; cinnamyl alcohol; cinnamyl carboxylate; synthesis; co-promoted mechanism
2016-09-26
江西省教育厅科技项目(GJJ151062); 信江英才866工程领军人才培养计划项目(2013-37)
祝显虹(1983-),男,汉族,江西玉山人,硕士,实验师,主要从事有机合成的研究。 E-mail: xianhongzhu@163.com
郑大贵,教授, E-mail: daguizheng@163.com
O625.52
A
10.15952/j.cnki.cjsc.1005-1511.2017.02.16229
