2型糖尿病患者血清同型半胱氨酸与凝血指标致动脉粥样硬化的作用
2017-02-22曲歌乐钱玉英周英智
曲歌乐, 钱玉英, 周英智, 朱 红
(首都医科大学宣武医院综合科,北京 100053;*通讯作者,E-mail:252802817@qq.com)
2型糖尿病患者血清同型半胱氨酸与凝血指标致动脉粥样硬化的作用
曲歌乐, 钱玉英*, 周英智, 朱 红
(首都医科大学宣武医院综合科,北京 100053;*通讯作者,E-mail:252802817@qq.com)
目的 分析2型糖尿病(T2DM)患者同型半胱氨酸(HCY)、凝血指标的关系及其对颈动脉粥样硬化斑块形成的影响。 方法 60例2型糖尿病患者以颈动脉超声结果为标准,分为无斑块组(n=21)及有斑块组(n=39),同时,选取24例健康体检者为正常对照组,比较各组间空腹血糖(FPG)、糖化血红蛋白(HbA1c)、同型半胱氨酸(HCY)及凝血指标(PT,APTT,TT,INR,FIB,D-Dimer)的差异,分析各指标之间的相关性,并对影响颈动脉粥样硬化斑块形成的危险因素进行Logistic回归分析。 结果 与正常对照组比较,T2DM无斑块组HCY、FPG、HbA1c均显著增高(P<0.05),凝血指标的变化不具有统计学意义;T2DM有斑块组HCY、FPG、HbA1c、FIB、D-Dimer均显著增高(P<0.05),其余凝血指标PT、INR、TT、APTT的变化不具有统计学意义。与T2DM无斑块组比较,T2DM有斑块组HCY、FPG、HbA1c及凝血指标的变化不具有统计学意义。未发现HCY与各凝血指标及FPG、HbA1c之间有显著相关性,HbA1c与PT、APTT、INR之间呈显著负相关(均P<0.05),Logistic回归分析表明HCY(OR=1.187,95%CI 1.009-1.398,P=0.039)及D-Dimer(OR=1.074,95%CI 1.021-1.130,P=0.006)水平升高是2型糖尿病患者颈动脉粥样硬化斑块形成的独立危险因素。 结论 血浆HCY水平及纤溶功能异常可能是导致糖尿病颈动脉粥样硬化斑块形成的危险因素,及早发现并干预高HCY血症及纤溶异常可能对预防动脉粥样硬化有重要意义。
同型半胱氨酸; 糖尿病; 凝血功能; 颈动脉粥样硬化
近年来,糖尿病在我国及全球范围内的发病率都在不断升高,已成为严重影响人们生活质量及身体健康的最大慢性病之一,英国糖尿病前瞻性研究(UKPDS)证实,2 型糖尿病患者在发病9年后大血管并发症的发病率为20%,大血管并发症是2型糖尿病患者的主要死因[1],其病理特征是大、中动脉粥样硬化。糖尿病患者普遍存在高凝状态和纤溶功能异常,这是导致其发生大血管并发症的原因之一。高同型半胱氨酸血症是近年来新发现的动脉粥样硬化的独立危险因素[2],其可以破坏机体凝血和纤溶系统之间的平衡。因此,本研究通过分析2型糖尿病患者的血浆同型半胱氨酸水平和凝血功能,以探讨在2型糖尿病患者中两者之间的关系及其对颈动脉粥样硬化性斑块形成的影响,为2型糖尿病患者防治大血管病变提供依据。
1 资料与方法
1.1 研究对象
T2DM组研究对象为2013-01~2016-06在首都医科大学宣武医院综合科住院的2型糖尿病患者,均符合2016年ADA糖尿病诊断标准。纳入60例患者,其中男42例,女18例,年龄34-65岁。根据患者颈动脉超声结果分为T2DM无斑块组21例及T2DM有斑块组39例。所有患者均排除引起凝血因子异常的疾病,排除其他类型糖尿病及合并糖尿病急性并发症,排除近期服用维生素B族史,排除各种感染、肿瘤病史、其他内分泌疾病、血液疾病、自身免疫性疾病、激素、免疫抑制剂服药史及近期手术史。正常对照组研究对象来自同期本院健康体检者,共24例,其中男12例,女12例,年龄35-62岁,无心血管、血液及内分泌代谢方面疾病,血脂、肝、肾功能正常,且颈动脉超声提示无颈动脉斑块形成。各组性别、年龄等差异无统计学意义(P>0.05,见表1)。
表1 三组患者的基线资料比较
Table 1 Comparison of baseline characteristics among three groups
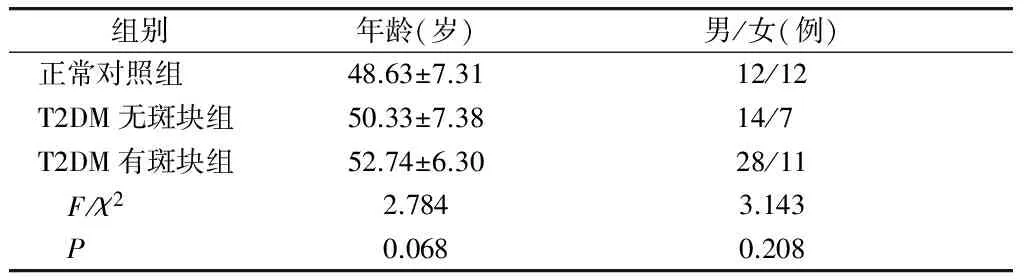
组别 年龄(岁)男/女(例)正常对照组48.63±7.3112/12T2DM无斑块组50.33±7.3814/7 T2DM有斑块组52.74±6.3028/11 F/χ22.7843.143 P0.0680.208
1.2 研究方法
1.2.1 生化及凝血指标测定 所有入组的患者均于入院后第2天清晨空腹时经肘静脉采血测空腹血糖(FPG)、糖化血红蛋白(HbA1c)、血清同型半胱氨酸(HCY)、相关凝血指标,包括凝血酶原时间(PT)、国际标准化比值(INR)、凝血酶时间(TT)、活化部分凝血活酶时间(APTT)、纤维蛋白原(FIB)、D-二聚体(D-Dimer)。生化指标的测定采用日立7170A全自动生化分析仪,以葡萄糖氧化酶法测定FBG,以循环酶法测定血浆HCY浓度,凝血指标的测定采用全自动血凝分析仪,HbAlC的测定采用VariantⅡ全自动糖化血红蛋白监测仪,以比色法测定。
1.2.2 颈动脉斑块检测 采用Philips iu22型超声诊断仪,使用3-9 MHz超宽频线阵探头及2-5 MHz凸阵探头,选取颈动脉内膜中层厚度(CIMT)最厚处测量,并计算双侧CIMT平均值作为CIMT值,由本院超声室高年资医师专门检测。斑块形成的诊断标准[3]:CIMT≥1.5 mm,或局限性CIMT增厚凸入动脉管腔至少0.5 mm,或较周围CIMT增加超过50%。
2 结果
2.1 所有研究对象各观察指标的比较
正常对照组、T2DM无斑块组、T2DM有斑块组之间进行各观察指标的比较,结果显示,PT、TT、APTT值的差异无统计学意义(P>0.05);FIB及D-Dimer值呈逐渐升高趋势,即正常对照组
HCY、FPG、HbA1c值呈逐渐升高趋势,即正常对照组
表2 三组患者观察指标的比较
Table 2 Comparison of observation index among three groups
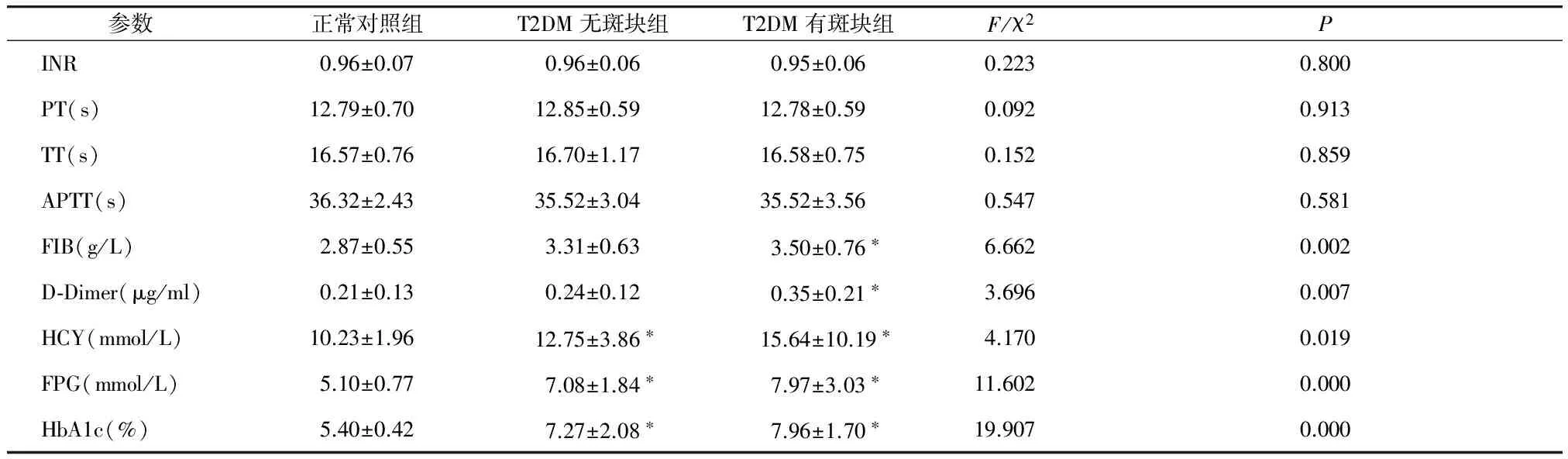
参数正常对照组T2DM无斑块组T2DM有斑块组F/χ2PINR0.96±0.070.96±0.060.95±0.060.2230.800PT(s)12.79±0.7012.85±0.5912.78±0.590.0920.913TT(s)16.57±0.7616.70±1.1716.58±0.750.1520.859APTT(s)36.32±2.4335.52±3.0435.52±3.560.5470.581FIB(g/L)2.87±0.553.31±0.633.50±0.76∗6.6620.002D⁃Dimer(μg/ml)0.21±0.130.24±0.120.35±0.21∗3.6960.007HCY(mmol/L)10.23±1.9612.75±3.86∗15.64±10.19∗4.1700.019FPG(mmol/L)5.10±0.777.08±1.84∗7.97±3.03∗11.6020.000HbA1c(%)5.40±0.427.27±2.08∗7.96±1.70∗19.9070.000
与正常对照组比较,*P<0.05
2.2 糖尿病患者各观察指标间的相关性分析
在所有糖尿病患者中,包括无斑块组有斑块组,将各观察指标进行相关性分析,发现HCY水平与PT、TT、APTT、INR、FIB、D-Dimer、HbA1c、FPG均无明显相关性(P>0.05),FPG与各凝血指标均无明显相关性(P>0.05),HbA1c与PT、APTT、INR呈显著负相关(r=-0.329,P=0.010;r=-0.258,P=0.047;r=-0.279,P=0.031),与TT、FIB、D-Dimer均无明显相关性(P>0.05,见表3)。
表3 各观察指标之间的相关性分析
Table 3 Pearson correlation analysis between the various index
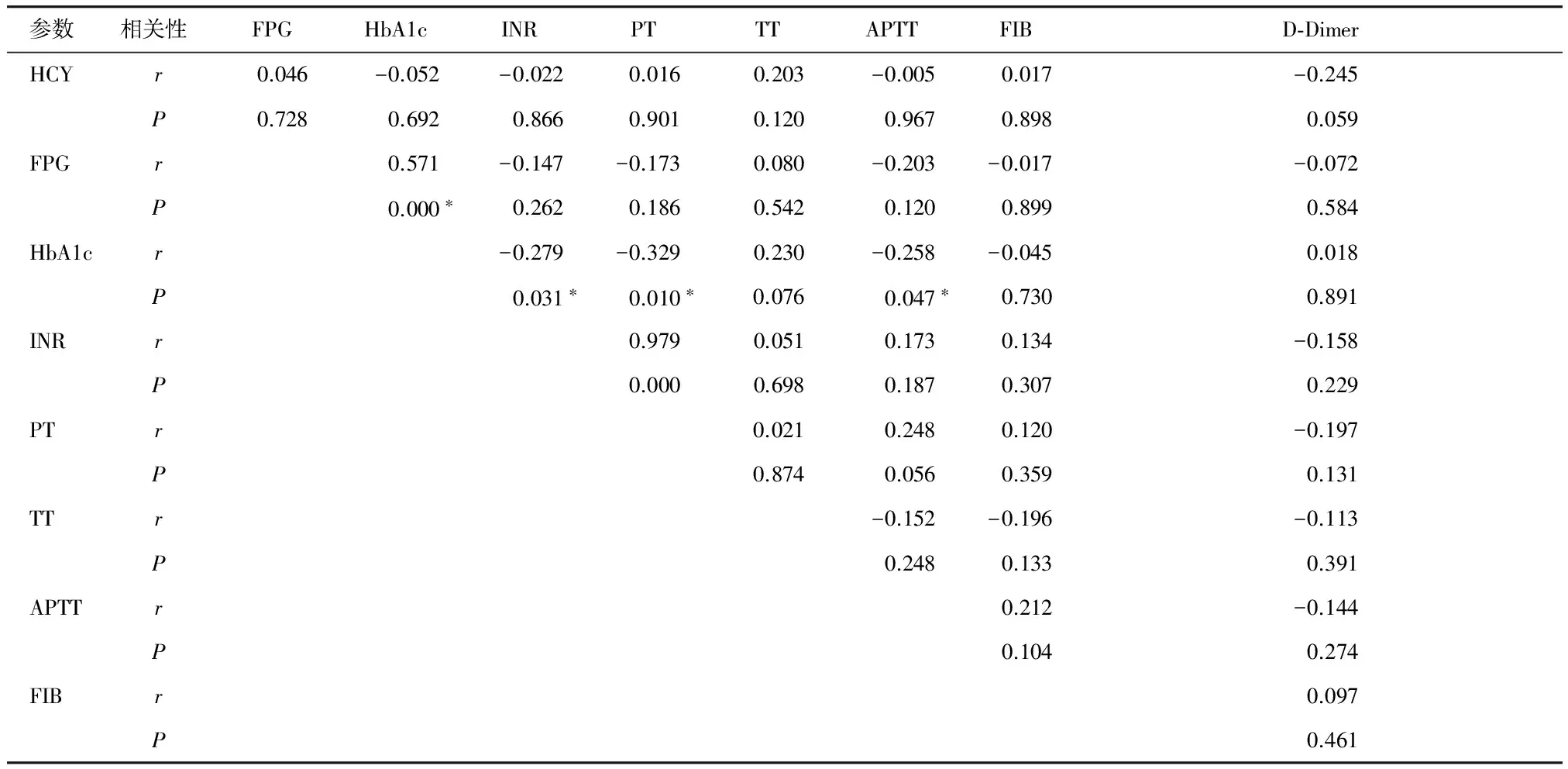
参数相关性FPGHbA1cINRPTTTAPTTFIBD⁃DimerHCYr0.046-0.052-0.0220.0160.203-0.0050.017-0.245P0.7280.6920.8660.9010.1200.9670.8980.059FPGr0.571-0.147-0.1730.080-0.203-0.017-0.072P0.000∗0.2620.1860.5420.1200.8990.584HbA1cr-0.279-0.3290.230-0.258-0.0450.018P0.031∗0.010∗0.0760.047∗0.7300.891INRr0.9790.0510.1730.134-0.158P0.0000.6980.1870.3070.229PTr0.0210.2480.120-0.197P0.8740.0560.3590.131TTr-0.152-0.196-0.113P0.2480.1330.391APTTr0.212-0.144P0.1040.274FIBr0.097P0.461
*P<0.05
2.3 糖尿病患者形成颈动脉粥样硬化斑块的Logistic回归分析
以2型糖尿病患者有无颈动脉斑块作为因变量,纳入HCY、FPG、HbA1c、PT、INR、TT、APTT、FIB、D-Dimer作为自变量进行Logistic回归分析,发现在2型糖尿病患者中HCY与D-Dimer是颈动脉粥样硬化性斑块形成的独立危险因素(见表4)。
3 讨论
全世界的糖尿病患者(主要是2型糖尿病)患病率迅速增加,发展中国家尤为明显,2013年最新《美国医学会杂志》数据显示全球约三分之一的糖尿病患者来自中国,随之而来的各种糖尿病并发症相应增加,已成为糖尿病患者致残和致死的主要原因,给社会发展带来了沉重的经济负担。大血管并发症是2型糖尿病患者的主要慢性并发症,与非糖尿病患者群相比,糖尿病患者群的动脉粥样硬化性疾病患病率高,发病年龄轻,病情进展快,多脏器同时受累多,因此积极预防和控制糖尿病患者动脉粥样硬化性疾病的发生发展可以显著提高患者的生活质量,延长生存寿命。
表4 2型糖尿病患者发生颈动脉病变的Logistic回归分析
Table 4 Logistic regression analysis for risk factors of carotid atherosclerotic plaque formation in patients with type 2 diabetes
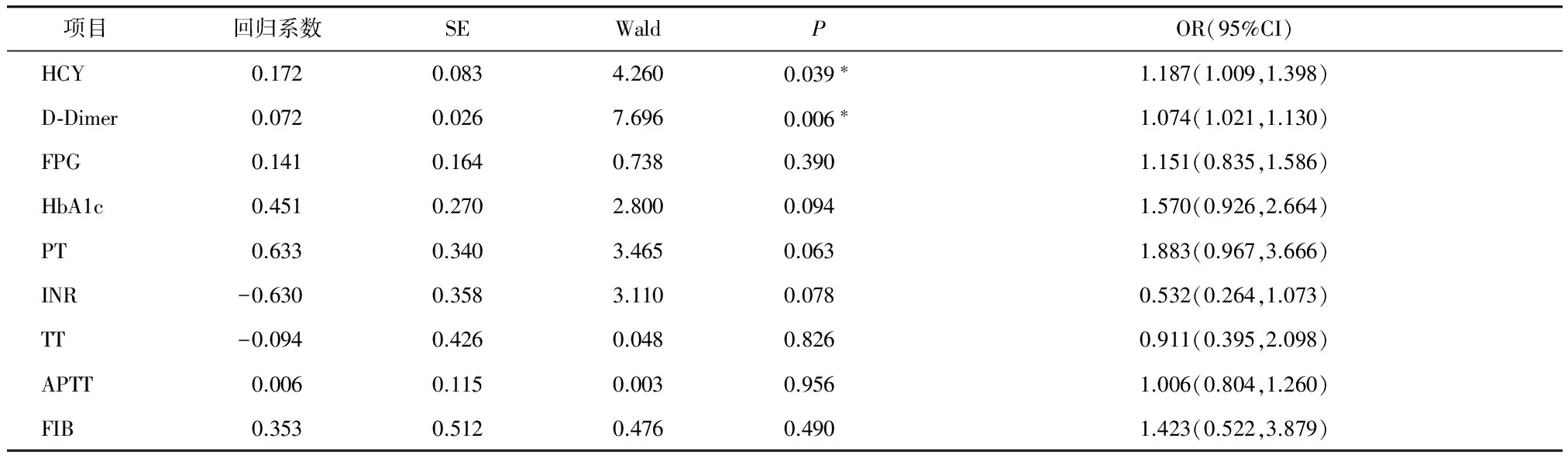
项目 回归系数SEWaldPOR(95%CI)HCY0.1720.0834.2600.039∗1.187(1.009,1.398)D⁃Dimer0.0720.0267.6960.006∗1.074(1.021,1.130)FPG0.1410.1640.7380.3901.151(0.835,1.586)HbA1c0.4510.2702.8000.0941.570(0.926,2.664)PT0.6330.3403.4650.0631.883(0.967,3.666)INR-0.6300.3583.1100.0780.532(0.264,1.073)TT-0.0940.4260.0480.8260.911(0.395,2.098)APTT0.0060.1150.0030.9561.006(0.804,1.260)FIB0.3530.5120.4760.4901.423(0.522,3.879)
*P<0.05
有研究[4,5]报道,炎症反应[6]、血液黏滞度增高、凝血机制及纤溶系统异常[7]与动脉粥样硬化性疾病的发生有关,其中炎症反应参与了动脉粥样硬化发生发展的全过程[8]。颈动脉粥样硬化(CAS)可间接反映全身动脉粥样硬化[9,10],且颈动脉粥样硬化斑块积分与心、脑血管病的严重程度呈正相关,是心脑血管疾病的早期临床指标[11],因此研究影响颈动脉粥样硬化的危险因素对防治糖尿病患者的慢性并发症具有重要意义。通常以颈动脉内膜-中层厚度(CIMT)作为CAS的征象指标,CIMT增厚是CAS的早期标志。
国外流行病学研究发现,血浆同型半胱氨酸升高可能导致颈动脉内中膜增厚,并参与颈动脉斑块形成[12,13],是动脉粥样硬化的危险因素。HCY是一种含硫氨基酸,主要来自饮食吸收的蛋氨酸,是体内蛋氨酸和半胱氨酸代谢中一个重要的中间产物,一般而言,它在健康人身体内很少。本研究提示HCY水平在三组对象之间呈逐渐升高趋势,即正常对照组 在糖尿病患者体内,升高的HCY与高糖毒性相辅相成,二者协同作用参与了糖尿病血管内皮细胞的损伤[20,21],因此在糖尿病患者中,高HCY血症进一步加重了大血管病变发生的危险性[22],在本研究中Logistic回归分析表明,HCY升高是2型糖尿病患者颈动脉粥样硬化斑块形成的独立危险因素,这与既往的研究结果是相符的[23]。但在本研究中对同型半胱氨酸与各凝血指标及HbA1c、FPG进行相关性分析,并未发现HCY与各指标之间存在显著相关性,说明HCY对糖尿病患者的致动脉粥样硬化作用可能与糖尿病患者本身的凝血状态及血糖控制情况关系不大,并进一步说明血浆同型半胱氨酸升高是导致动脉粥样硬化的独立危险因素。HCY水平和血管病变之间呈剂量依赖关系,即血清HCY水平每升高5 mmol/L,动脉粥样硬化的发生率增加60%-80%,HCY水平越高,颈动脉病变程度越重[24]。提示在糖尿病大血管病变的预防中,除将降糖作为基本治疗外,也应对HCY水平给予较多的关注。 正常情况下,外周血中凝血、抗凝血、纤溶、血小板等系统的功能处于相互制约的动态平衡。糖尿病患者的高糖毒性可引起血管内皮的损伤[25-27],且波动性高血糖比持续性高血糖危害更大,从而释放出各种血管活性物质,促进凝血功能的紊乱[28,29]。PT、INR、APTT是常见的评价凝血功能的指标,其值越低,越容易出现高凝状态。本研究发现糖尿病患者的HbA1c与PT、INR、APTT均呈显著负相关,这说明血糖控制程度越差,患者的高凝状态越明显,在本研究中,T2DM有斑块组和无斑块组的FBG及HbA1c水平均与正常对照组之间有显著差异,但T2DM有斑块组与无斑块组之间无显著差异,而FIB及D-Dimer在2型糖尿病有斑块患者中均显著高于正常对照组,但在2型糖尿病无斑块组与正常对照组以及T2DM有斑块组之间并无显著差异,从侧面说明糖尿病患者凝血功能的变化与血糖控制情况相关,同时在本研究中并未发现PT、TT、INR、APTT等在糖尿病病人与正常对照组之间有显著差异,提示糖尿病患者的凝血功能异常可能是由于高糖毒性对血管内皮的损伤造成的,而且在这个过程中纤溶系统比凝血系统更加敏感。可能是由于D-Dimer及FIB不仅参与了体内的凝血及止血过程,还是体内重要的炎症因子[30]。 许多研究表明动脉粥样硬化与凝血状态密切相关,纤溶系统失衡对动脉粥样硬化斑块的影响已逐渐受到临床重视,D-Dimer和FIB是监测机体凝血和纤溶系统十分有意义的实验标记物[31,32]。既往多个研究提示FIB及D-Dimer与颈动脉粥样硬化的严重程度密切相关[33]。本研究提示,FIB值和D-Dimer值在三组之间均有逐渐升高趋势,即正常对照组 综上所述,2型糖尿病患者的高HCY水平及纤溶系统异常是其颈动脉粥样硬化性斑块形成的重要因素,因此我们认为检测高HCY水平的T2DM患者的凝血功能,对糖尿病大血管并发症的预防和治疗具有一定的临床意义,早期降低血浆HCY浓度,控制凝血因子在正常水平可能是预防和延缓糖尿病大血管病变的有效方法之一。 [1] 刘金霞,项洁,卜瑞芳,等.2型糖尿病患者血清25-羟维生素D浓度与颈动脉内膜中层厚度的关系[J].中华心血管病杂志,2012,40(2):115-119. [2] 陈涛,王应良,王一萍,等.胱抑素C、同型半胱氨酸、超敏C反应蛋白和D-二聚体联合检测动脉粥样硬化性脑梗死的临床意义[J].中国临床神经科学,2013,21(5):562-565. [3] Touboul PJ,Hennerici MG,Meairs S,etal.Mannheim intima-media thickness consensus[J].Cerebrovasc Dis,2004,18(4):346-349. [4] Chuang SY,Bai CH,Chen WH,etal.Fibrinogen independently predicts the development of ischemic stroke in a Taiwanese population[J].Stroke,2009,40(5):1578-1584. [5] 马丽丽,梁辉任,金岩.银丹心脑通软胶囊治疗腔隙性脑梗死的临床疗效评价[J].中华神经医学杂志,2011,10(6):626-629. [6] Jain S,Khera R,Chirinos JA,etal.Inflammation and arterial stiffness in humans[J].Atherosclerosis,2014,237(2):381-390. [7] De Luca G,Verdoia M,Cassetti E,etal.High fibrinogen level is an independent predictor of presence and extent of coronary artery disease among Italian population[J].J Thromb Thrombolysis,2011,31(4):458-463. [8] 刘俊田.动脉粥样硬化发病的炎症机制的研究进展[J].西安交通大学学报,2015,36(2):141-152. [9] Aragón-Sánchez J,Lázaro-Martínez JL.Factors associated with calcification in the pedal arteries in patients with diabetes and neuropathy admitted for foot disease and its clinical significance[J].Int J Low Extrem Wounds,2013,12(4):252-255. [10] Lee YH,Shin MH,Kweon SS,etal.Normative and mean carotid intima-media thickness values according to metabolic syndrome in Koreans:the Namwon study[J].Atherosclerosis, 2014,234(1):230-236. [11] Ohira T,Diez Roux AV,Polak JF,etal.Associations of anger, anxiety, and depressive symptoms with carotid arterial wall thickness: the multi-ethnic study of atherosclerosis[J].Psychosom Med,2012,74(5):517-525. [12] Li Y,Yin HJ.Study on the relationship between plasma homocysteine,high-sensitivity creactive protein level and carotid atherosclerotic plaque[J].Med Inf,2014,27(8):197-198. [13] Dietrich M,Jacques PF,Polak JF,etal.Segment-specific association between plasma homocysteine level and carotid artery intima-media thickness in the Framingham Offspring Study[J].J Stroke Cerebrovasc Dis,2011,20(2):155-161. [14] Shi CH,Zhao HH,Hou N,etal.Identifying metabolite and protein biomarkers in unstable angina in-patients by feature selection based data mining method[J].Chem Res Chin Univ,2011,27(1):87-93. [15] 甄卓丽,陈晓铭,周飞,等.2型糖尿病肾病患者血清同型半胱氨酸水平的变化及意义[J].广东医学,2015,36(16):2556-2557. [16] Vayá A,Rivera L,Hernández-Mijares A,etal.Homocysteine levels in morbidly obese patients: its association with waist circumference and insulin resistance[J].Clin Hemorheol Microcirc,2012,52(1):49-56. [17] Chiang EP,Wang YC,Chen WW,etal.Effects of insulin and glucose on cellular metabolic fluxes in homocysteine transsulfuration, remethylation, S-adenosylmethionine synthesis, and global deoxyribonucleic acid methylation[J].J Clin Endocrinol,2009,94(3):1017-1025. [18] Noll C,Lacraz G,Ehses J,etal.Early reduction of circulating homocysteine levels in Goto-Kakizaki rat,a spontaneous nonobese model of type 2 diabetes[J].Biochim Biophys Acta,2011,1812(6):699-702. [19] de Jager J,Kooy A,Lehert P,etal.Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial[J].BMJ,2010,340:c2181. [20] Sharma S,Singh M,Sharma PL.Mechanism of hyperhomocysteinemia-induced vascular endothelium dysfunction-possible dysregulation of phosphatidylinositol-3-kinase and its downstream phosphoinositide dependent kinase and protein kinase B[J].Eur J Pharmacol,2013,721(1-3):365-372. [21] 邱石,魏衡,赵静,等.缺血性脑卒中进展的相关危险因素分析及血浆Hcy检测联合ESSEN评分对其预测作用[J].山东医药,2014,54(28):3-6. [22] Sonkar SK,Sonkar GK,Soni D,etal.Plasma homocysteine level and its clinical correlation with type 2 diabetes mellitus and its complications[J].Int J Diabetes Dev Countries,2014,34(1):3-6. [23] Li Y,Yin HJ.Study on the relationship between plasma homocysteine,high-sensitivity creactive protein level and carotid atherosclerotic plaque[J].Med Inf,2014,27(8):197-198. [25] Torimoto K,Okada Y,Mori H,etal.Relationship between fluctuations in glucose levels measured by continuous glucose monitoring and vascular endothelial dysfunction in type 2 diabetes mellitus[J].Cardiovasc Diabetol,2013,12:1. [26] Chang CM,Hsieh CJ,Huang JC,etal.Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus[J].Acta Diabetol,2012,49:S171-S177. [27] Wang JS,Yin HJ,Guo CY,etal.Influence of high blood glucose fluctuation on endothelial function of type 2 diabetes mellitus rats and effects of Panax Quinquefolius Saponin of stem and leaf[J].Chin J Integr,2013,19(3):217-222. [28] Mendivil CO,Robles-Osorio L,Horton ES,etal.Young Hispanics at risk of type 2 diabetes display endothelial activation, subclinical inflammation and alterations of coagulation and fibrinolysis[J].Diabetol Metab Syndr,2013,5(1):371-378. [29] Kim HK,Kim JE,Park SH,etal.High coagulation factor leels and low protein C levels contribute to enhanced thrombin generation in patients with diabetes who do not have macrovascular complications[J].J Diebetes Complications,2014,28:365-369. [30] Paramo JA,Rodriguez JA,Orbe J. Fibrinogen.An old hemostatic protein with a new function:non-invasive marker of subclinical atherosclerosis[J].Med Clin(Barc),2005,124(20):790-794. [31] Hou HC,Ge ZJ,Ying P,etal.Biomarkers of deep venous thrombosis[J].J Thromb Thrombolysis,2012,34(3):335-346. [32] Wada H,Matsumoto T,Yamashita Y.Diagnosis of thrombosis by hemostatic markers[J].Nihon Rinsho,2014,72(7):1232-1236. [33] Alpsoy S,Akyuz A,Erfan G,etal.Atherosclerosis,some serum inflammatory markers in psoriasis[J].G Ital Dermatol,2014,149(2):167-175. [34] Papageorgiou N,Tousoulis D,Miliou A,etal.Combined effects of fibrinogen genetic variability on atherosclerosis in patients with or without stable angina pectoris:focus on the coagulation cascade and endothelial function[J].Int J Cardiol,2013,168(5):4602-4607. [35] Zhou B,Pan Y,Yu Q,etal.Fibrinogen facilitates atherosclerotic formation in Sprague-Dawley rats:A rodent model of atherosclerosis[J].Exp Ther Med,2013,5(3):730-734. [36] Stępień E,Kabak-Ziembicka A,Musiaek P,etal.Fibrinogen and carotid intima media thickness determine fibrin density in different atherosclerosis extents[J].Int J Cardiol,2012,157(3):411-413. [37] 李晓彤,吕祥兄,张玲娣.脑梗死患者颈动脉斑块性质与D-二聚体的临床研究[J].中国临床神经科学,20l2,20(6):678-679. [38] 郑丽丽,许琳琳.血小板参数联合D-二聚体检测在2型糖尿病中的意义[J].上海预防医学,2012,24(4):223-224. Influences of homocysteine and coagulation index on carotid artery atherosclerosis in patients with type 2 diabetes mellitus QU Geyue, QIAN Yuying*, ZHOU Yingzhi,ZHU Hong (DepartmentofGeneralMedicine,XuanwuHospital,CapitalMedicalUniversity,Beijing100053,China;*Correspondingauthor,E-mail:252802817@qq.com) ObjectiveTo analyze the relationship between plasma homocysteine(HCY) and coagulation index in patients with type 2 diabetes mellitus(T2DM) and their influences on carotid atherosclerotic plaque formation.MethodsSixty patients with type 2 diabetes were divided into plaque group(n=39) and non-plaque group(n=21) according to the results of the bilateral carotid ultrasonography examination.Twenty-four normal controls were chosen as control group.Fasting blood glucose(FPG), plasma glycated hemoglobin(HbA1c), homocysteine(HCY) and coagulation indexes(PT,APTT,TT,INR,FIB,D-Dimer) were measured and compared among three groups.The correlations between the indicators and the influential factors of carotid atherosclerotic plaque formation were analyzed by Logistic regression analysis.ResultsLevels of HCY,FPG,HbA1c in non-plaque group were higher than in normal control group(P<0.05),but the differences of PT,APTT,TT,INR,FIB D-Dimer were not significant between normal control group and non-plaque group. Levels of HCY,FPG,HbA1c,FIB,D-Dimer in plaque group were higher than in normal control group(P<0.05),but the differences of PT, INR, TT, APTT were not significant between normal control group and plaque group.Compared with non-plaque group, HCY, FPG, HbA1c and coagulation index showed no significant changes in plaque group. Significant correlations between HCY and PT,APTT,TT,INR,FIB,D-Dimer,FPG,HbA1c were not found. HbA1c was negatively correlated with PT, APTT, INR(P<0.05).Logistic regression analysis revealed that increase of HCY(OR=1.187,95%CI 1.009-1.398,P=0.039) and D-Dimer(OR=1.074,95%CI 1.021-1.130,P=0.006) were independent risk factors for carotid atherosclerotic plaque formation in patients with type 2 diabetes mellitus.ConclusionPlasma HCY level and abnormal fibrinolytic function may be the risk factors of carotid atherosclerotic plaque formation in patients with type 2 diabetes mellitus. Early detection and intervention of hyperhomocystinemia and abnormal fibrinolytic function may have important significance in the prevention of atherosclerosis. homocysteine; type 2 diabetes mellitus; coagulation function; carotid artery atherosclerosis 曲歌乐,女,1987-05生,博士,住院医师,E-mail:z1206z1206@126.com 2016-08-12 R587.1 A 1007-6611(2017)01-0034-06 10.13753/j.issn.1007-6611.2017.01.008
