黄芩素和LY294002对人肝癌细胞系SMMC-7721细胞增殖和凋亡的影响研究
2017-02-17喻小兰夏纪毅王晓燕唐小平夏国栋
汪 枫,喻小兰,夏纪毅,王晓燕,唐 利,曹 勇,唐小平,夏国栋
·论著·
黄芩素和LY294002对人肝癌细胞系SMMC-7721细胞增殖和凋亡的影响研究
汪 枫1,喻小兰2,夏纪毅3,5,王晓燕3,唐 利3,曹 勇3,唐小平3,夏国栋4*
目的 探讨黄芩素和LY294002对人肝癌细胞系SMMC-7721细胞增殖和凋亡的影响。方法 2015年3月—2016年1月,取人肝癌细胞系SMMC-7721,制备细胞悬液。加入黄芩素(1、2、5、10、20、50、100、200、300 μmol/L,分别命名为A1、A2、A3、A4、A5、A6、A7、A8、A9组)或LY294002(1、2、5、10、20、30 μmol/L,分别命名为B1、B2、B3、B4、B5、B6组),设定空白对照组和二甲基亚砜(DMSO)对照组,采用CCK8试剂盒检测各组细胞增殖水平;采用20 μmol/L黄芩素(C1组)单独处理,20 μmol/L黄芩素联合10 μmol/L LY294002(C2组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,采用流式细胞术检测各组细胞周期;采用2、5、10、20 μmol/L黄芩素(分别命名为D1、D2、D3、D4组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,采用显微摄影检测各组细胞数量;采用20 μmol/L黄芩素(E1组)单独处理,20 μmol/L黄芩素联合10 μmol/L LY294002(E2组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,采用流式细胞术检测各组早期凋亡和晚期凋亡情况;采用20 μmol/L黄芩素(F1组)、10 μmol/L LY294002(F2组)或20 μmol/L黄芩素联合10 μmol/L LY294002(F3组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,采用实时荧光定量PCR(Real-time PCR)法检测细胞外调节蛋白激酶(ERK)1/2、周期素D1(CyclinD1)、糖原合成酶激酶-3β(GSK-3β)、丝氨酸/苏氨酸蛋白激酶(AKT) mRNA表达水平;采用20 μmol/L黄芩素(G1组)、10 μmol/L LY294002(G2组)或20 μmol/L黄芩素联合10 μmol/L LY294002(G3组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,采用Western blotting法检测磷酸化ERK1/2(P-ERK1/2)、CyclinD1、磷酸化GSK-3β(P-GSK-3β)、磷酸化AKT(P-AKT)表达水平。结果 A8组、A9组、B6组细胞增殖水平低于空白对照组、DMSO对照组(P<0.05)。C2组G0/G1期、G2/M期细胞比例高于空白对照组、DMSO组,S期细胞比例低于空白对照组、DMSO组(P<0.05)。D4组细胞数量少于空白对照组、DMSO组(P<0.05)。空白对照组、DMSO组、E1组、E2组早期凋亡和晚期凋亡比较,差异无统计学意义(P>0.05)。F3组ERK1/2、CyclinD1、GSK-3β、AKT mRNA表达水平均低于空白对照组、DMSO组(P<0.05)。G3组P-ERK1/2、CyclinD1、P-GSK-3β、P-AKT表达水平低于空白对照组、DMSO组(P<0.05)。结论 黄芩素和LY294002可抑制人肝癌细胞系SMMC-7721细胞增殖,但不影响其凋亡。
肝肿瘤;细胞增殖;细胞凋亡;黄芩素;LY294002;人肝癌细胞系SMMC-7721
汪枫,喻小兰,夏纪毅,等.黄芩素和LY294002对人肝癌细胞系SMMC-7721细胞增殖和凋亡的影响研究[J].中国全科医学,2017,20(3):323-330.[www.chinagp.net]
WANG F,YU X L,XIA J Y,et al.Effects of baicalein and LY294002 on proliferation and apoptosis of human hepatocellular carcinoma cell line SMMC-7721[J].Chinese General Practice,2017,20(3):323-330.
肝癌(liver cancer)是指发生于肝脏的恶性肿瘤,病死率仅次于胃癌、食管癌,其包括原发性肝癌和转移性肝癌两种,人们日常说的肝癌多指原发性肝癌,其发病率高、转移早、预后差,目前尚无有效的治疗方法[1-3]。细胞外调节蛋白激酶(extracellular regulated protein kinases,ERK)1/2、磷酸肌醇3-激酶(PI-3K)/丝氨酸/苏氨酸蛋白激酶(AKT)和糖原合成酶激酶-3β(GSK-3β)信号通路的失调与肝癌的发生发展密切相关,其调控癌细胞的增殖和凋亡:ERK1/2是丝裂原活化蛋白激酶(MAPK)家族的成员之一,与癌细胞恶性增殖、分化和侵袭转移息息相关,细胞受刺激后,通过G蛋白偶联受体、生长因子受体或酪氨酸蛋白激酶受体,经大鼠肉瘤蛋白(Ras)/快速反应肉瘤蛋白(Raf)/ERK激酶(MEK)/ERK级联信号激活ERK,ERK1/2通过磷酸化调节某些转录因子的活性如Elk1、EtsI、c-myc、Tal、信号转导和转录激活因子(STATS)等,抑制Myb,间接激活原癌基因c-fos和c-jun,促进转录因子激活蛋白1(activator protein-1,AP-1)活性,调节癌细胞的恶性增殖和侵袭转移;PI-3K/AKT信号通路是调节癌细胞恶性增殖、分化、凋亡和侵袭转移的关键信号通路,LY294002是PI-3K的特异性抑制剂,能完全抑制p110亚基的催化活性;GSK-3β是调控Wnt/β-catenin信号通路的重要激酶,在胞质中与结肠腺瘤性息肉病蛋白(adenomatous polyposis coli,APC)、轴抑制蛋白(Axin)形成降解复合物后,使β-catenin磷酸化并降解,在细胞质内保持低水平,当GSK-3β磷酸化后会失活,β-catenin会在细胞质内聚集然后转运至细胞核,刺激与癌细胞恶性增殖、分化、凋亡和侵袭转移等相关基因的转录和表达[4-9]。黄芩素和LY294002均可抑制癌细胞增殖,促进癌细胞凋亡,但二者在肝癌中的作用机制还未得到完全阐明。本研究探讨了黄芩素和LY294002对癌细胞的作用机制,报道如下。
1 材料与方法
1.1 研究材料 人肝癌细胞系SMMC-7721购自中国科学院上海生命科学研究院生物化学与细胞生物学研究所;黄芩素购自Sigma公司,纯度为99%,充分溶解于二甲基亚砜(dimethyl sulfoxide,DMSO)中,储存液浓度为5 000 μg/L(DMSO的终浓度≤0.2%),4 ℃避光保存,使用前用细胞培养基稀释成实验所需的浓度;LY294002购自Alexis公司;Trizol试剂购于Invitrogen公司;细胞周期试剂盒购自南京凯基生物科技发展有限公司;反转录试剂盒购自Fermentas公司;GAPDH、辣根过氧化物酶山羊抗兔IgG(H+L)及辣根过氧化物酶山羊抗小鼠IgG(H+L)购自碧云天生物科技有限公司;ERK1/2、周期素D1(CyclinD1)、GSK-3β、AKT、磷酸化ERK1/2(P-ERK1/2)、磷酸化GSK-3β(P-GSK-3β)、磷酸化AKT(P-AKT)引物由生工生物工程(上海)股份有限公司合成,对应一抗购自CST公司;CCK8(Cell Counting Kit-8)试剂盒购自上海拓然生物科技有限公司。
1.2 方法
1.2.1 CCK8试剂盒检测细胞增殖水平 2015年3月—2016年1月,取人肝癌细胞系SMMC-7721,制备细胞悬液;将细胞悬液接种至96孔板中,100 μl/孔,加入黄芩素(1、2、5、10、20、50、100、200、300 μmol/L,分别命名为A1、A2、A3、A4、A5、A6、A7、A8、A9组)或LY294002(1、2、5、10、20、30 μmol/L,分别命名为B1、B2、B3、B4、B5、B6组);37 ℃培养箱培养4 h;加入10 μl CCK8混匀;培养4 h,生成结晶甲瓒(Formazan);测定450 nm处吸光度(OD值)即细胞增殖水平:采用双波长进行测定,检测波长450~490 nm,参比波长600~650 nm。设定空白对照组:在不含细胞悬液的培养基中加入CCK8,测定450 nm处OD值;DMSO对照组:在不含细胞悬液且加入DMSO的培养基中加入CCK8,测定450 nm处OD值。实验独立重复3次。
1.2.2 流式细胞术检测细胞周期 将人肝癌细胞系SMMC-7721接种于12孔板,分别采用20 μmol/L黄芩素(C1组)单独处理,20 μmol/L黄芩素联合10 μmol/L LY294002(C2组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组(加入用于溶解黄芩素所需要的等体积DMSO),24 h后收集细胞,室温磷酸盐缓冲液(PBS)洗涤2次,加入1 ml 70%预冷乙醇溶液固定过夜,12 000 r/min离心5 min(离心半径10 cm),去除乙醇溶液,PBS重悬,加入核糖核酸酶(ribonuclease,RNase)至终浓度为100 mg/L,37 ℃水浴30 min,加入碘化丙啶(propidium iodide,PI)至终浓度为50 mg/L,4 ℃避光染色30 min,利用细胞周期试剂盒、采用激发波长为488 nm的红色荧光检测细胞周期。结果采用细胞周期拟合软件进行分析,记录亚二倍体峰,即G0/G1期、S期、G2/M期细胞比例。实验独立重复3次。
1.2.3 显微摄影检测细胞数量 采用2、5、10、20 μmol/L黄芩素(分别命名为D1、D2、D3、D4组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,24 h后低倍镜(×100)下选取9个视野计数细胞数量。
1.2.4 流式细胞术检测早期凋亡和晚期凋亡 分别采用20 μmol/L黄芩素(E1组)单独处理,20 μmol/L黄芩素联合10 μmol/L LY294002(E2组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,24 h后按照Annexin V/PI试剂盒说明书操作即细胞处理后,收集悬浮细胞,PBS洗2次,加入400 μl 1×Binding Buffer重悬细胞,加入5 μl Annexin V-异硫氰酸荧光素(fluorescein isothiocyanate,FITC)混匀,室温避光孵育15 min,加入10 μl PI染色液至终浓度为50 μg/ml混匀,避光孵育5 min,采用流式细胞仪检测细胞早期、晚期凋亡数目。结果采用Cell Quest软件进行分析。实验独立重复3次。1.2.5 实时荧光定量PCR(Real-time PCR)法检测ERK1/2、CyclinD1、GSK-3β、AKT mRNA表达水平 严格按照实验所用试剂盒说明书操作,采用20 μmol/L黄芩素(F1组)、10 μmol/L LY294002(F2组)、20 μmol/L黄芩素联合10 μmol/L LY294002(F3组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,去掉培养基,PBS冲洗2次,采用Trizol试剂提取总RNA,采用反转录试剂盒反转录成cDNA第一链,采用Real-time PCR法检测凋亡相关因子(ERK1/2、CyclinD1、GSK-3β、AKT)mRNA表达水平。ERK1/2上游引物为5′-AATCACACGGTAGACACTGAAATGCC-3′,下游引物为5′-CATCATCCCATCTAAAATGTCCCCTG-3′;CyclinD1上游引物为5′-TACGGUCAUGUCCAAAGUTAT-3′,下游引物为5′-CAGACGCACGGCTTTGACCTTCTT-3′;GSK-3β上游引物为5′- GCCCTGAGGGCCCGAACTGTTACT-3′,下游引物为5′- ATUGUUGGACAUGACCGGAAC-3′;AKT上游引物为5′-AGCGACGTGGCTATTGTGAAG-3′,下游引物为5′-GCCATCATTCTTGAGGAGGAAGT-3′;内参GAPDH上游引物为5′-GCACCGTCAAGGCTGAGAAC-3′,下游引物为5′-TGGTGAAGACGCCAGTGGA-3′。1.2.6 Western blotting法检测P-ERK1/2、CyclinD1、P-GSK-3β、P-AKT表达水平 严格按照实验所用试剂盒说明书操作,采用20 μmol/L黄芩素(G1组)、10 μmol/L LY294002(G2组)或20 μmol/L黄芩素联合10 μmol/L LY294002(G3组)处理人肝癌细胞系SMMC-7721,设定空白对照组和DMSO组,24 h后用预冷的PBS冲洗细胞2~3次,加入蛋白酶抑制剂和裂解液,冰浴20 min,使细胞全部裂解,12 000 r/min离心5 min(离心半径10 cm),取上清液,收集细胞总蛋白。取5 μl变性的蛋白样本进行10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE),用电转仪将蛋白质转入聚偏氟乙烯(PVDF)膜上,5%脱脂奶粉封闭2 h,一抗(P-ERK1/2、CyclinD1、P-GSK-3β稀释比例为1∶1 000,P-AKT稀释比例为1∶500,内参GAPDH稀释比例为1∶2 000)4 ℃孵育过夜。1×TBST漂洗2次,20 min/次,HRP辣根过氧化物酶山羊抗兔IgG(H+L)及HRP辣根过氧化物酶山羊抗小鼠IgG(H+L)(二抗稀释比例均为1∶2 000)室温孵育1 h,1×TBST漂洗2次,5 min/次,凝胶成像仪成像后进行灰度分析,计算相关蛋白的表达水平。实验独立重复3次。

2 结果
2.1 黄芩素或LY294002对人肝癌细胞系SMMC-7721细胞增殖的影响 空白对照组、DMSO对照组及A1~A9组细胞增殖水平比较,差异有统计学意义(P<0.05);其中A8组、A9组细胞增殖水平低于空白对照组、DMSO对照组,差异有统计学意义(P<0.05,见表1)。空白对照组、DMSO对照组及B1~B6组细胞增殖水平比较,差异有统计学意义(P<0.05);其中B6组细胞增殖水平低于空白对照组、DMSO对照组,差异有统计学意义(P<0.05,见表2)。
2.2 黄芩素或LY294002对人肝癌细胞系SMMC-7721细胞周期的影响 空白对照组、DMSO组、C1组、C2组G0/G1期、S期、G2/M期细胞比例比较,差异均有统计学意义(P<0.05);其中C2组G0/G1期、G2/M期细胞比例高于空白对照组、DMSO组,S期细胞比例低于空白对照组、DMSO组,差异有统计学意义(P<0.05,见表3、图1)。
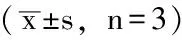
Table 1 Effect of baicalin on the proliferation of human hepatocellular carcinoma cell line SMMC-7721
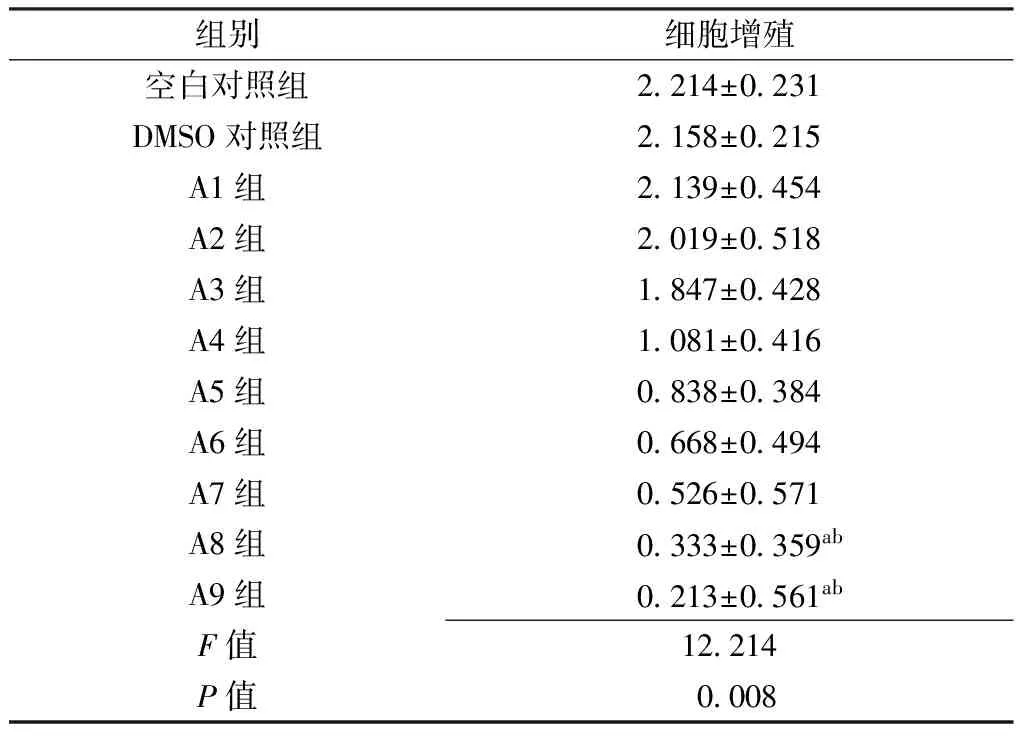
组别细胞增殖空白对照组2214±0231DMSO对照组2158±0215A1组2139±0454A2组2019±0518A3组1847±0428A4组1081±0416A5组0838±0384A6组0668±0494A7组0526±0571A8组0333±0359abA9组0213±0561abF值12214P值0008
注:DMSO=二甲基亚砜;与空白对照组比较,aP<0.05;与DMSO对照组比较,bP<0.05
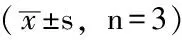
Table 2 Effect of LY294002 on the proliferation of human hepatocellular carcinoma cell line SMMC-7721

组别细胞增殖空白对照组4234±0042DMSO对照组3634±0031B1组3881±0011B2组3578±0015B3组3321±0143B4组2554±0011B5组2178±0121B6组1697±0074abF值14234P值0004
注:与空白对照组比较,aP<0.05;与DMSO对照组比较,bP<0.05

Table 3 Effect of baicalin on cell cycle of human hepatocellular carcinoma cell line SMMC-7721

组别G0/G1期S期G2/M期空白对照组5546±2143771±101683±215DMSO组5616±1873725±132658±321C1组7568±1541870±151561±101C2组8128±221ab1049±162ab822±141abF值9454806311372P值002600380001
注:与空白对照组比较,aP<0.05;与DMSO组比较,bP<0.05
2.3 黄芩素对人肝癌细胞系SMMC-7721细胞数量的影响 空白对照组、DMSO组、D1~D4组细胞数量比较,差异有统计学意义(P<0.05);其中D4组细胞数量少于空白对照组、DMSO组,差异有统计学意义(P<0.05,见表4、图2)。
2.4 黄芩素或LY294002对人肝癌细胞系SMMC-7721早期凋亡和晚期凋亡的影响 空白对照组、DMSO组、E1组、E2组早期凋亡和晚期凋亡比较,差异无统计学意义(P>0.05,见表5、图3)。

Table 4 Effect of baicalin on the cell amount of human hepatocellular carcinoma cell line SMMC-7721
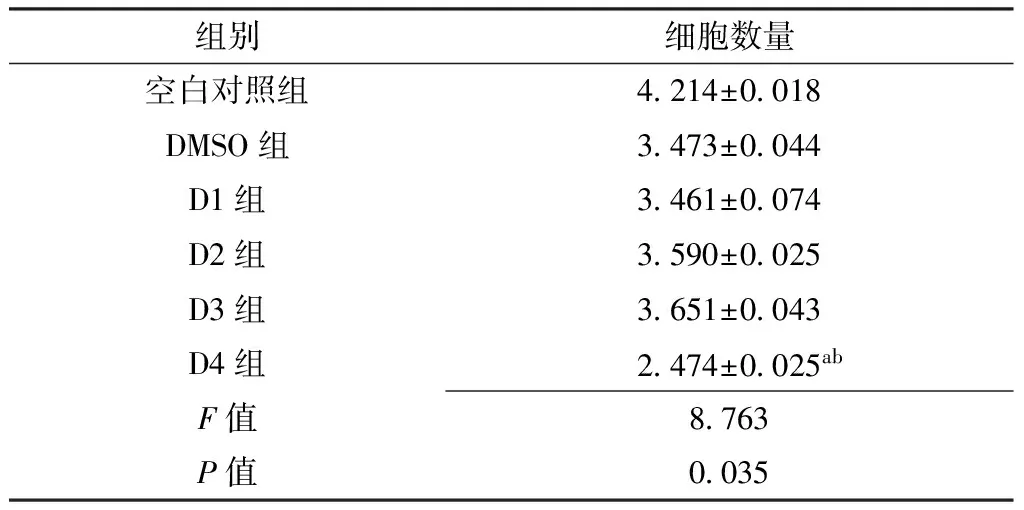
组别细胞数量空白对照组4214±0018DMSO组3473±0044D1组3461±0074D2组3590±0025D3组3651±0043D4组2474±0025abF值8763P值0035
注:与空白对照组比较,aP<0.05;与DMSO组比较,bP<0.05

Table 5 Effects of baicalin or LY294002 on early apoptosis and late apoptosis of human hepatocellular carcinoma cell line SMMC-7721
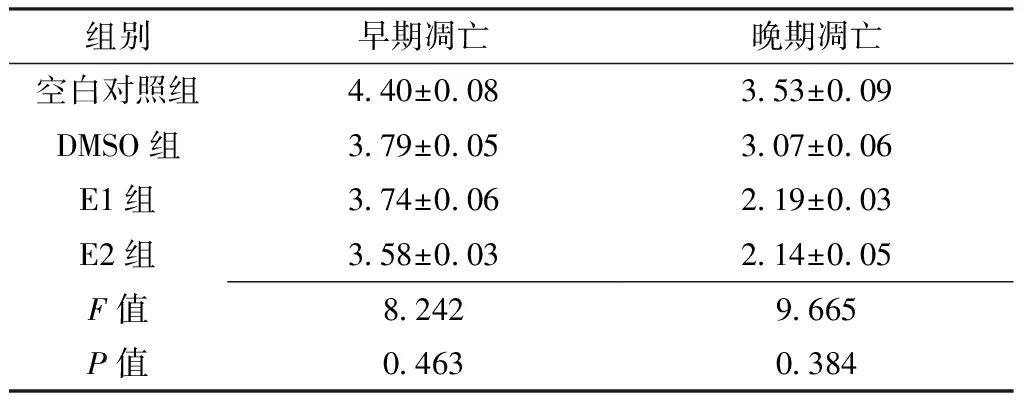
组别早期凋亡晚期凋亡空白对照组440±008353±009DMSO组379±005307±006E1组374±006219±003E2组358±003214±005F值82429665P值04630384
2.5 黄芩素或LY294002对人肝癌细胞系SMMC-7721细胞增殖相关因子ERK1/2、CyclinD1、GSK-3β、AKT mRNA表达水平的影响 空白对照组、DMSO组、F1~F3组ERK1/2、CyclinD1、GSK-3β、AKT mRNA表达水平比较,差异均有统计学意义(P<0.05);其中F3组ERK1/2、CyclinD1、GSK-3β、AKT mRNA表达水平均低于空白对照组、DMSO组,差异有统计学意义(P<0.05,见表6)。
2.6 黄芩素或LY294002对人肝癌细胞系SMMC-7721细胞增殖相关蛋白P-ERK1/2、CyclinD1、P-GSK-3β、P-AKT表达水平的影响 空白对照组、DMSO组、G1~G3组P-ERK1/2、CyclinD1、P-GSK-3β、P-AKT表达水平比较,差异均有统计学意义(P<0.05);其中G3组P-ERK1/2、CyclinD1、P-GSK-3β、P-AKT表达水平低于空白对照组、DMSO组,差异有统计学意义(P<0.05,见表7)。
Table 6 Effects of baicalin or LY294002 on the expression levels of the correlation factors of cell proliferation as ERK1/2,CyclinD1,GSK-3β and AKT mRNA in human hepatocellular carcinoma cell line SMMC-7721
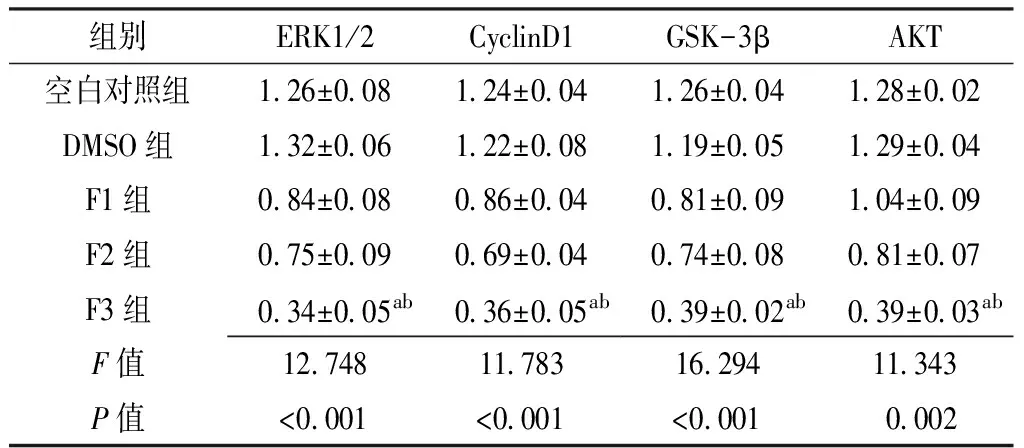
组别ERK1/2CyclinD1GSK-3βAKT空白对照组126±008124±004126±004128±002DMSO组132±006122±008119±005129±004F1组084±008086±004081±009104±009F2组075±009069±004074±008081±007F3组034±005ab036±005ab039±002ab039±003abF值12748117831629411343P值<0001<0001<00010002
注:与空白对照组比较,aP<0.05;与DMSO组比较,bP<0.05;ERK=细胞外调节蛋白激酶,CyclinD1=周期素D1,GSK-3β=糖原合成酶激酶-3β,AKT=丝氨酸/苏氨酸蛋白激酶
Table 7 Effects of baicalin or LY294002 on the expression of P-ERK1/2,CyclinD1,P-GSK-3β,P-AKT and in human hepatocellular carcinoma cell line SMMC-7721

组别P-ERK1/2CyclinD1P⁃GSK-3βP-AKT空白对照组118±008115±004119±004114±012DMSO组121±006118±009123±005121±021G1组085±009079±005083±009101±022G2组066±009068±004067±009082±028G3组034±004ab036±005ab044±004ab043±001abF值13475126551334812212P值<0001<0001<0001<0001
注:与空白对照组比较,aP<0.05;与DMSO组比较,bP<0.05;P-ERK1/2=磷酸化ERK1/2,P-GSK-3β=磷酸化GSK-3β,P-AKT=磷酸化AKT

注:DMSO=二甲基亚砜
图1 流式细胞术检测黄芩素或LY294002对人肝癌细胞系SMMC-7721细胞周期的影响
Figure 1 Effect of baicalein or LY294002 on cell cycle of human hepatocellular carcinoma cell line SMMC-7721 detected by flow cytometry

图2 显微摄影检测黄芩素对人肝癌细胞系SMMC-7721细胞数量的影响
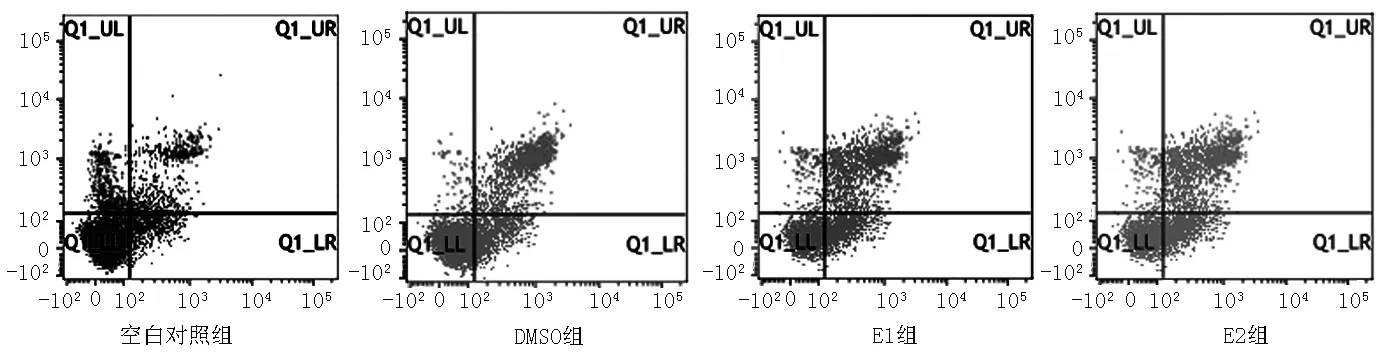
图3 流式细胞术检测黄芩素或LY294002对人肝癌细胞系SMMC-7721早期凋亡和晚期凋亡的影响
Figure 3 Effect of baicalein or LY294002 on early apoptosis and late apoptosis of human hepatocellular carcinoma cell line SMMC-7721 by flow cytometry detection
3 讨论
原发性肝癌是临床上常见的恶性肿瘤之一,据统计,全世界每年新发肝癌患者约60万,居恶性肿瘤的第五位[10]。针对肝癌的治疗方式众多,但疗效欠佳,严重影响患者的生存质量[11]。中药对肝癌等肿瘤的治疗作用逐渐被人们认可,并取得较好的治疗效果[12]。黄芩素提取于唇形科植物高黄芩,具有抗感染、抗肿瘤、抗氧化、抗微生物和解热等作用,主要用于降低脑血管阻力,改善脑血循环以及抑制乳腺癌等肿瘤增殖、侵袭、生长等[13]。而LY294002是PI-3K信号通路抑制剂,在肿瘤增殖、凋亡中发挥重要作用[14]。但黄芩素和LY294002在肝癌中的具体作用和机制尚未阐明。
本研究结果显示,A8组、A9组细胞增殖水平低于空白对照组、DMSO对照组,D4组细胞数量少于空白对照组、DMSO组,B6组细胞增殖水平低于空白对照组、DMSO对照组,说明黄芩素与LY294002均可显著抑制人肝癌细胞系SMMC-7721细胞增殖。C2组G0/G1期、G2/M期细胞比例高于空白对照组、DMSO组,S期细胞比例低于空白对照组、DMSO组,说明黄芩素与LY294002联合作用可抑制人肝癌细胞系SMMC-7721细胞周期。空白对照组、DMSO组、E1组、E2组早期凋亡和晚期凋亡无差异,说明黄芩素与LY294002不影响人肝癌细胞系SMMC-7721细胞凋亡。细胞周期的超常快速转换或检查点的破坏将导致失控的肝癌细胞生长,引起肝癌的发生发展。细胞周期中发挥关键作用的是CyclinD1,当CyclinD1表达水平异常升高可导致G1期至S期转换速度增加,进而促进肿瘤的进展[15-16]。而本研究结果显示,F3组CyclinD1 mRNA表达水平均低于空白对照组、DMSO组,G3组CyclinD1表达水平低于空白对照组、DMSO组,说明黄芩素和LY294002联合作用肝癌细胞后,可通过抑制CyclinD1表达进而导致细胞周期G1/S期发生改变,抑制肝癌细胞增殖。ERK通过磷酸化反应可调节某些转录因子的活性,促进P-ERK入核增多,使其下游因子c-fos表达水平增加,进而促进c-fos和c-jun二聚化形成AP-1,引起致癌作用[17-19]。本研究结果显示,F3组ERK1/2 mRNA表达水平均低于空白对照组、DMSO组,G3组P-ERK1/2表达水平低于空白对照组、DMSO组,证实黄芩素和LY294002联合作用肝癌细胞后可通过下调ERK表达水平抑制P-ERK1/2,进而进一步抑制肝癌细胞增殖。PI-3K/AKT作为重要的信号通路,AKT磷酸化后,可增加肿瘤细胞的运动能力,增强对生长因子受体的调节,降低细胞间的黏附力,参与细胞增殖和凋亡,可抑制肿瘤细胞凋亡,发挥抗凋亡作用。LY294002是PI-3K的特异性抑制剂,能完全抑制PI-3K的活性,进而发挥抗肿瘤作用[14,20]。既往研究证实,黄芩素可通过调控PI-3K以及ERK信号通路发挥抗癌作用[13]。本研究结果显示,F3组AKT mRNA表达水平均低于空白对照组、DMSO组,G3组P-AKT表达水平低于空白对照组、DMSO组,说明黄芩素和LY294002联合作用肝癌细胞后可调控PI-3K/AKT信号通路。P-GSK-3β可导致核因子(NF)-κB活化,进入细胞核,进而促进肿瘤细胞DNA转录,导致肿瘤细胞增殖[21]。本研究结果显示,F3组GSK-3β mRNA表达水平均低于空白对照组、DMSO组,G3组P-GSK-3β表达水平低于空白对照组、DMSO组,说明黄芩素和LY294002联合作用于肝癌细胞后可抑制P-GSK-3β。综上,黄芩素和LY294002可抑制肝癌细胞的增殖,黄芩素和LY294002联合作用可调控细胞周期关键蛋白CyclinD1表达,抑制P-ERK1/2、P-GSK-3β、P-AKT,进而抑制肝癌细胞周期G1/S期的转变。
本研究尚存在一定的局限性,目前仅在细胞水平证实了黄芩素和LY294002对肝癌的作用,但尚未在动物模型中深入研究其作用和相关机制,且具体治疗效果还有待于临床评估。
综上所述,黄芩素和LY294002可抑制人肝癌细胞系SMMC-7721细胞增殖,但不影响其凋亡。本研究为肝癌治疗方式的选择提供新的选择和理论依据。
作者贡献:夏国栋进行实验设计与实施、资料收集整理、撰写论文、成文并对文章负责;夏纪毅负责对具体细节进行修正,汪枫、喻小兰、王晓燕、唐利、曹勇、唐小平进行实验实施、评估。
本文无利益冲突。
[1]DUFOUR J F,JOHNSON P.Liver cancer:from molecular pathogenesis to new therapies:summary of the EASL single topic conference[J].J Hepatol,2010,52(2):296-304.
[2]HOLCZBAUER A,FACTOR V M,ANDERSEN J B,et al.Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types[J].Gastroenterology,2013,145(1):221-231.[3]TANAKA S.Liver cancer:progress in diagnosis and treatments.Topics:Ⅲ.Whole genome analysis to reveal the pathogenesis of hepatocellular carcinoma[J].Nihon Naika Gakkai Zasshi,2014,103(1):19-26.
[4]YANG H,NIE Y,LI Y,et al.ERK1/2 deactivation enhances cytoplasmic Nur77 expression level and improves the apoptotic effect of fenretinide in human liver cancer cells[J].Biochem Pharmacol,2011,81(7):910-916.
[5]WANG C,CIGLIANO A,DELOGU S,et al.Functional crosstalk between AKT/mTOR and Ras/MAPK pathways in hepatocarcinogenesis:implications for the treatment of human liver cancer[J].Cell Cycle,2013,12(13):1999-2010.
[6]ZHANG C Z,WANG X D,WANG H W,et al.Sorafenib inhibits liver cancer growth by decreasing mTOR,AKT,and PI-3K expression[J].J BUON,2015,20(1):218-222.
[7]LI T W,PENG H,YANG H,et al.S-Adenosylmethionine and methylthioadenosine inhibit β-catenin signaling by multiple mechanisms in liver and colon cancer[J].Mol Pharmacol,2015,87(1):77-86.
[8]SON H S,KWON H Y,SOHN E J,et al.Activation of AMP-activated protein kinase and phosphorylation of glycogen synthase kinase3 β mediate ursolic acid induced apoptosis in HepG2 liver cancer cells[J].Phytother Res,2013,27(11):1714-1722.
[9]SPRINGER J,TSCHIRNER A,HAGHIKIA A,et al.Prevention of liver cancer cachexia-induced cardiac wasting and heart failure[J].Eur Heart J,2014,35(14):932-941.
[10]NGUYEN L H,ROBINTON D A,SELIGSON M T,et al.Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murine models[J].Cancer Cell,2014,26(2):248-261.
[11]STEFFENSEN K R.Are synthetic compounds that silence the liver-X-receptor the next generation of anti-cancer drugs?[J].Cancer Cell,2015,28(1):3-4.
[12]XING X,SU L,ASARE P F,et al.Danzhi Qing′e(DZQE) activates AMPK pathway and regulates lipid metabolism in a rat model of perimenopausal hyperlipidaemia[J].Exp Physiol,2016,101(11):1406-1417.
[13]LIU H,DONG Y,GAO Y,et al.The fascinating effects of baicalein on cancer:a review[J].Int J Mol Sci,2016,17(10):E1681.
[14]GALUPPO R,MAYNARD E,SHAH M,et al.Synergistic inhibition of HCC and liver cancer stem cell proliferation by targeting RAS/RAF/MAPK and WNT/β-catenin pathways[J].Anticancer Res,2014,34(4):1709-1713.
[15]LI M,ZHOU W,YUAN R,et al.ROCK2 promotes HCC proliferation by CEBPD inhibition through phospho-GSK3β/β-catenin signaling[J].FEBS Lett,2015,589(9):1018-1025.
[16]MA Y,SHE X G,MING Y Z,et al.miR-24 promotes the proliferation and invasion of HCC cells by targeting SOX7[J].Tumour Biol,2014,35(11):10731-10736.
[17]CHEN Y,LIN C,LIU Y,et al.HMGB1 promotes HCC progression partly by downregulating p21 via ERK/c-Myc pathway and upregulating MMP-2[J].Tumour Biol,2016,37(4):4399-4408.
[18]CALVISI D F,PINNA F,LADU S,et al.Forkhead box M1B is a determinant of rat susceptibility to hepatocarcinogenesis and sustains ERK activity in human HCC[J].Gut,2009,58(5):679-687.
[19]YU X,LU K,XIA J,et al.Baicalein induces HeLa cell growth inhibition by down-regulation of matrix metalloproteinases and activating extracellular signal-regulated kinase[J].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi,2014,30(8):798-801.
[20]LIN G Y,CHEN Z L,LU C M,et al.Immunohistochemical study on p53,H-rasp21,c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients[J].World J Gastroenterol,2000,6(2):234-238.
[21]焦志军,张蓓,徐翀,等.GSK-3β在结直肠癌细胞凋亡中的作用[J].上海第二医科大学学报,2005,25(2):161-163,166. JIAO Z J,ZHANG B,XU C,et al.Study on the glycogen synthase Kinase-3β in 5-fluorouracil induced apoptosis in colorectal carcinoma cell line colo 320[J].Academic Journal of Shanghai Second Medicai University,2005,25(2):161-163,166.
(本文编辑:崔丽红)
Effects of Baicalein and LY294002 on Proliferation and Apoptosis of Human Hepatocellular Carcinoma Cell Line SMMC-7721
WANGFeng1,YUXiao-lan2,XIAJi-yi3,5,WANGXiao-yan3,TANGLi3,CAOYong3,TANGXiao-ping3,XIAGuo-dong4*
1.DepartmentofEducationalAdministration,theAffiliatedHospitalofSouthwestMedicalUniversity,Luzhou646000,China2.DepartmentofObstetricsandGynecology,theAffiliatedTCMHospitalofSouthWestMedicalUniversity,Luzhou646000,China3.DepartmentofExperimentalMedicineCenter,theAffiliatedHospitalofSouthwestMedicalUniversity,Luzhou646000,China4.DepartmentofGastroenterology,theAffiliatedHospitalofSouthwestMedicalUniversity,Luzhou646000,China5.DepartmentoftheScienceandTechnology,SouthwestMedicalUniversity,Luzhou646000,China
*Correspondingauthor:XIAGuo-dong,Associateprofessor;E-mail:xjyi0615@163.com
Objective To study the effects of baicalein and LY294002 on proliferation and apoptosis of human hepatocellular carcinoma cell line SMMC-7721.Methods From March 2015 to January 2016,human hepatocellular carcinoma cell line SMMC-7721 was cultured in vitro to produce cell suspension.The cells were treated by baicalein(1,2,5,10,20,50,100,200,300 μmol/L,and were respectively named A1,A2,A3,A4,A5,A6,A7,A8,and A9 group),or LY294002(1,2,5,10,20,30 μmol/L,and were respectively named B1,B2,B3,B4,B5,and B6 group).The blank control group and DMSO control group were set up,and the cell proliferation level of SMMC-7721 was measured by CCK8 kit;20 μmol/L baicalein alone(C1 group) or combined with 10 μmol/L LY294002(C2 group) was added to SMMC-7721,the blank control group and DMSO group were set up,and the cell cycle was detected by flow cytometry;2,5,10,20 μmol/L baicalein(D1,D2,D3,D4 group were named respectively) was added to SMMC-772,the blank control group and DMSO group were set up,and the cell amount in each group was analyzed by microscopy;20 μmol/L baicalein alone(E1 group) or combined with 10 μmol/L LY294002(E2 group) was added to SMMC-7721,the blank control group and DMSO group were set up,and the early and late apoptosis in each group were measured by flow cytometry;20 μmol/L baicalein alone(F1 group),10 μmol/L LY294002 alone(F2 group) or combination of these two(F3 group) was added to SMMC-7721,the blank control group and DMSO group were set up,and the expression level of ERK1/2,CyclinD1,GSK-3β,AKT mRNA was detected by real-time fluorescence quantification PCR(Real-time PCR);20 μmol/L baicalein alone(G1 group),10 μmol/L LY294002 alone(G2 group) or combination of these two(G3 group) was added to SMMC-7721,the blank control group and DMSO control group were set up,and the expression level of P-ERK1/2,CyclinD1,P-GSK-3β,and P-AKT was analyzed by Western blotting method.Results The cell proliferation level in A8 group,A9 group and B6 group was lower than that in blank control group and DMSO control group(P<0.05).The cell ratio at G0/G1and G2/M period in C2 group was higher than that in blank control group and DMSO group,while the ratio at S period was lower than that in blank control group and DMSO group(P<0.05).The cell amount in D4 group was fewer than that in blank control group and DMSO group(P<0.05).There was no significant difference in early and late apoptosis among blank control group,DMSO group,E1 group and E2 group(P>0.05).The expression level of ERK1/2,CyclinD1,GSK-3β,AKT mRNA in F3 group was lower than that in blank control group and DMSO group(P<0.05).The expression level of P-ERK1/2,CyclinD1,P-GSK-3β and P-AKT in G3 group was lower than that in blank control group and DMSO group(P<0.05).Conclusion Baicalein and LY294002 can inhibit the cell proliferation of human hepatocellular carcinoma cell line SMMC-7721,but they cannot influence its apoptosis.
Liver neoplasms;Cell proliferation;Apoptosis;Baicalein;LY294002;Human liver cancer cell line SMMC-7721
四川省科技厅-泸州市人民政府-泸州医学院2014年联合科研项目(14JC01383-LH53)
R 735.7
A
10.3969/j.issn.1007-9572.2017.03.012
2016-08-19;
2016-12-01)
1.646000 四川省泸州市,西南医科大学附属医院教务部
2.646000 四川省泸州市,西南医科大学附属中医医院妇产科
3.646000 四川省泸州市,西南医科大学附属医院医学实验中心
4.646000 四川省泸州市,西南医科大学附属医院消化内科
5.646000 四川省泸州市,西南医科大学科技处
*通信作者:夏国栋,副教授;E-mail:xjyi0615@163.com
