两例基于相同的含氮及联苯二羧基混合配体构筑的Zn/Cd配位聚合物:不同的穿插结构和荧光性质
2017-02-16卢久富许宇航靳玲侠郭小华赵蔡斌郑楠姜敏葛红光
卢久富许宇航靳玲侠郭小华赵蔡斌郑楠姜敏葛红光
(陕西理工大学化学与环境科学学院,陕西省催化基础与应用重点实验室,汉中723001)
卢久富*许宇航靳玲侠郭小华赵蔡斌郑楠姜敏葛红光*
(陕西理工大学化学与环境科学学院,陕西省催化基础与应用重点实验室,汉中723001)
在溶剂热条件下合成了2个锌/镉配位聚合物:{[Zn(1,3-bip)(bpdc)]·0.5H2bpdc}n(1),{[Cd2(1,3-bip)2(bpdc)2]·DMF}n(2),H2bpdc=4,4′-联苯二甲酸,1,3-Bip=1,3-二(咪唑基)丙烷。并通过X射线单晶衍射,粉末XRD、红外光谱、元素分析以及热重分析对其结构进行表征。单晶解析结果表明:配位聚合物1是一个五重穿插3D→3D三维空间网络结构,配位聚合物2是一个二重穿插的2D→2D二维的(4,4)网格层状结构。另外,研究了2个配位聚合物在室温下的热稳定和荧光性能。
溶剂热合成;配位聚合物;双咪唑;拓扑;荧光性能
Construction of new metal coordination polymers (MCPs)has attracted attention due to their diverse structural topologies and potential applications as functional materials such as gas storage,magnetism, catalysis,and luminescence[1-5].Metal-directed selfassembly to construct rigid and robust metal-organic polymers(MCPs)has provided an extensive class of solid materials with high stability and desired physical properties[6-8].Within this context,interpenetration and polycatenation networks with optimal functionalities have attracted particular attention.Interpenetrating net can be described as a series of independent nets that penetrate mutually and polycatenation nets can be described molecules with loops through loops.The structuresofinterpenetrationandpolycatenation arraysnotonlyprovideinterestingtopological structures but also exhibit promising applications as superhardmaterialsfortheirpeculiarmagnetic, optical,and catalytic properties,etc[9-12].
In recent years,the direct use of two types of organic ligands have been found to be an effective method for the synthesis of MCPs,because of their richcoordinationmodes,includingmonodentate, bridging and chelating[13-14].To date,many MCPs have been prepared on the basis of carboxylate-type O-donors and amine-or N-donors.Thereinto,Diphenic acid as O-donor ligand has received much attention in the designed synthesis of coordination polymers[15-17], for example,biphenyl-4,4′-dicarboxylic acid(H2bpdc) is a good candidate for construction of coordination polymers.Meanwhile,flexible bis(imidazole)ligands (such as 1,2-bis(imidazole)ethane(1,2-bie),1,3-bis (imidazole)propane(1,3-bip),1,4-bis(imidazole)butane (1,4-bib),1,5-bis(imidazole)pentane(1,5-bip),1,6-bis (imidazole)hexane(1,6-bih)and 1,4-bis(imidazol-1-ylmethyl)benzene(bix)[18-21])bearing alkyl spacers are good choice of N-donor ligands,in which the flexible nature of spacers allows the ligands to bend and rotate when it coordinates to metal centers,and this often causes the structural diversity and different capacities of spatial extension.
In this paper,we report the metal ions induced synthesis of two new complexes,{[Zn(1,3-bip)(bpdc)]· 0.5H2bpdc}n(1)and{[Cd2(1,3-bip)2(bpdc)2]·DMF}n(2), constructedbyusingthesamebiphenyl-4,4′-dicarboxylic acid and 1,3-bis(imidazole)propane as the mixed ligands.
1 Experimental
1.1 Materials and measurements
All reagents used in the syntheses were of analyticalgrade.Elementalanalysesforcarbon, hydrogen,and nitrogen atoms were performed on a Vario ELⅢelemental analyzer.The infrared spectra (4000~400cm-1)were recorded by using KBr pellet on an Avatar 360E.S.P.IR spectrometer.Thermogravimetric analysis(TGA)was performed on a TASDT Q600thermal analyzer under N2atmosphere with a heating rate of 10℃·min-1in the range of 30~1000℃.The fluorescence spectra of samples were measured with a Hitachi F-4500fluorescence spectrophotometer at room temperature using powder crystal samples.
1.2 Synthesis of complex
1.2.1 Synthesis of{[Zn(1,3-bip)(bpdc)]·0.5H2bpdc}n(1)
A mixture of Zn(NO3)2·6H2O(0.2mmol,58.2mg), 1,3-bip(0.20mmol,35.2mg),NaOH(0.2mmol,8mg) and H2bpdc(0.20mmol,58.2mg)in DMF-H2O(6mL, 1∶1,V/V)binary solvent was placed in a 25mL Teflonlined stainless steel container,which was heated at 150℃for 3d,and then cooled to room temperature over 24h.Colourless block crystals of 1were collected. Yield:75%based on Zinc.Elemental analysis Calcd. for C30H25ZnN4O6(%):C 59.81,H 4.15,N 9.30;Found (%):C 59.36,H 3.96,N 9.81.IR(cm-1):3442(w),3125(m),2919(w),1693(s),1612(s),1532(m),1382(s), 1252(s),1105(m),852(m)and 763(m).
1.2.2 Synthesis of{[Cd2(1,3-bip)2(bpdc)2]·DMF}n(2)
The preparation of 2was similar to that of 1except that Zn(NO3)2·6H2O was replaced by Cd(NO3)2· 4H2O(0.20mmol,61.6mg).Colourless block crystals of 2were obtained.Yield:75%based on Cadmium. Elemental analysis Calcd.for C49H47Cd2N9O9(%):C 52.00,H 4.16,N 11.14;Found(%):C 52.36,H 4.02, N 11.41.IR(cm-1):3444(w),3111(m),2919(w),2865(w),1671(m),1585(m),1524(s),1393(s),1238(w),1088(m),846(m)and 771(m).
1.3 Determination of crystal structures
Crystals of 1(0.22mm×0.18mm×0.14mm)and 2(0.23mm×0.18mm×0.14mm)were carefully selected under an optical microscope,and data collection were performed on a CCD automatic diffractometer with graphite-monochromatized Mo Kα radiation(λ= 0.071073nm)by using the ω-scan mode at room temperature.An empirical absorption correction was applied using the Semi-empirical from equivalents program[22].The structures were both solved by direct methods using SHELXS-97[23].Crystallographic data for 1and 2are presented in Table 1.The main bond lengths and angles are listed in Table 2.
CCDC:1011050,1;1013480,2.

Table 1Crystallographic data and structure refinement for 1and 2

Table 2Selected bond lengths(nm)and angles(°)for 1and 2
2 Results and discussion
2.1 Description of the structures
2.1.1 Crystal structural description of 1
SingleX-raydiffractionstudyrevealedthat complex 1was isostructural with{[Co(1,3-bip)(bpdc)]· 0.5H2bpdc}n,which has been previous reported by our group[17].The asymmetric unit of 1is composed of a four-coordinate Zncenter,a 1,3-bip ligand,a bpdc2-ligand and a half uncoordinated H2bpdc molecule (Fig.1a).Zn1ionadoptsadistortedtetrahedral geometry,coordinating to two nitrogen donors of two 1,3-bip ligands and two oxygen donors of two bpdc2-ligands(Zn1-O10.1960(3)nm,Zn1-O30.1967(4)nm, Z1-N10.2022nm,Z1-N40.1991nm).Both Zn-N and Zn-O bond lengths are well-matched to those observed in similar complexes[24-26].Then,1,3-bip ligand adopts a bis-monodentate bridging mode connecting two Zncenters to form a[Zn(1,3-bip)]24+macrocyclic unit(Fig.1b).Neighboring[Zn(1,3-bip)]24+macrocyclic unit as a 4-connected secondary building unit are further linked by two conformations of bpdc2-ligands in which the two phenyl rings about the central bond have a twist with a dihedral angle of 0°and 41.02°to form a 12-connected oblong building unit{[Zn2(1,3-bip)2]6(bpdc)6},and the uncoordinated H2bpdc molecules are bound inside the pores with intermolecular O-H…O hydrogen bonding interactions(OH2bpdc-H…Obpdc) between O atom of the coordinated bpdc2-ligand and O atom of uncoordinated H2bpdc molecule with O…O distances of 0.2626nm(Fig.1c).The bpdc2-acts as a bridging ligand with bis-monodentatecoordination mode connecting above-mentioned 12-connected oblong building unit to form the porous 3D architecture(Fig. 1d).From the topological perspective,the[Zn(1,3-bip)]24+macrocyclic unit can be considered as a 4-connected uninodal node with a Schlfli symbol of (65,8)cds type topology.By mutual interpenetration of five independent equivalent frameworks,it leads tothe formation of 5-fold 3D→3D parallel interpenetrating architecture(Fig.1e).

Fig.1(a)Coordination environment of Znion in 1;(b)View of a 4-connected second building unit of[Zn(1,3-bip)(c)View of uncoordinated H2bpdc molecules encapsulated in the pore of the 3D framework of 1;(d)View of the 3D network along the c axis in 1;(e)5-fold 3D→3D parallel interpenetrating structure of 1
2.1.2 Crystal structural description of 2
The complex 2crystallizes in the monoclinic system with P21/c space group.Each asymmetric unit of 2contains two Cdion,two bpdc2-anions,two 1,3-bip ligands and one DMF molecule.As shown in Fig.2a,the two Cdion exhibit same coordination geometry and are coordinated by four carboxylate O atoms(Cd-O 0.2282(6)~0.2524(6)nm)from two bpdc2-anions as the chelate form and two N atoms (Cd-N 0.2245(9)~0.2284(6)nm)from two 1,3-bip ligands,showing a distorted octahedral geometry.Each 1,3-bip acts as a bridging ligand coordinating the adjacent Cdion to form a one dimensional{[Cd (1,3-bip)]chain,where the 1,3-bip ligands show the cis-conformation with the same Cd…Cd distance of 1.1094(9)nm.These chains are further linked by bridging bpdc2-ligands to generate a 2D infinite undulatedrectangular(4,4)gridincorporatinga [Cd4(1,3-bip)2(bpdc)2]window of 1.109nm×1.524nm based on the Cd…Cd distances(Fig.2b).Then a pair of identical 2D single nets are interlocked with each other to form a 2-fold parallel interpenetrated 2D architecture by(Cbip-H…Obpdc0.2348,0.2470,0.2555, 0.2582,0.2719nm)hydrogen bonds(Fig.2c and d), in which the DMF molecules are resided.Although thetwoMCPswereconstructedbythesame carboxylate and N-donor ligand,coordination modes are quite different,resulting in variable framework of the structures,respectively.
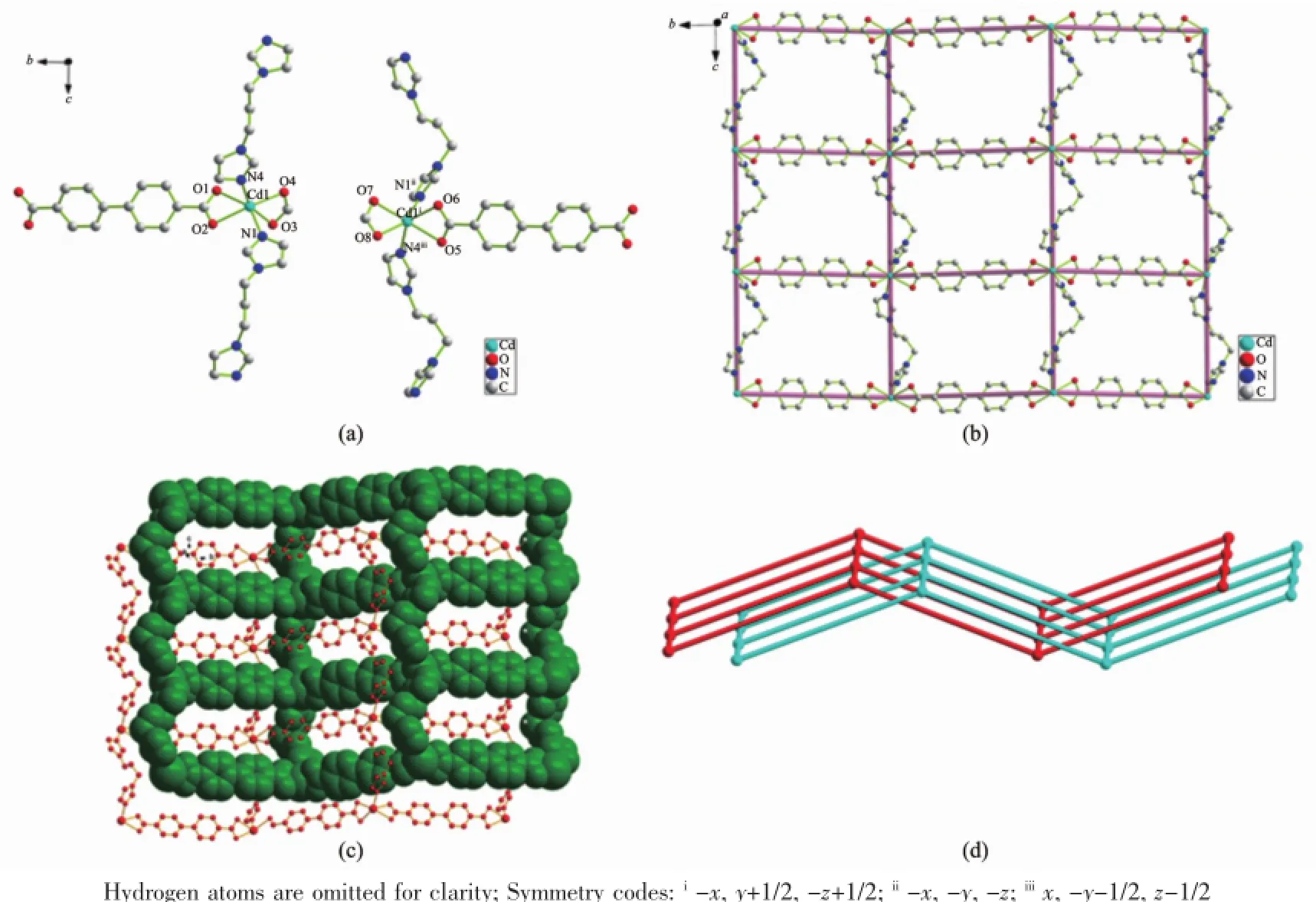
Fig.2(a)Coordination environment of Cdion in 2;(b)View of 2D infinite undulated rectangular(4,4)grid in 2; (c)Perspective view of the 2D coordination network along the a axis in 2;(d)Schematic view of the 2-fold interpenetrating along the b axis in 2
2.2 FTIR spectra,X-ray powder diffraction patterns and thermal properties
As shown in Fig.3,the middle band at 3442cm-1for 1further indicates the presence of hydron band betweenuncoordinateddicarboxylateligandand molecular skeleton.The weak bands at 3444cm-1for 2can be assigned to N-H stretching frequency of DMF molecule.The middle band at 3111cm-1and strong band at 1693cm-1belong to O-H and C=O stretchingvibrationbandofuncoordinateddicarboxylate ligand,respectively.The strong peak at 1382cm-1for 1and 1393cm-1for 2indicate the presence of deprotonated-COO-groups.The peaks at 1585and 1393cm-1for 2correspond to the asymmetric and symmetric vibration of the carboxylate group (COO-),and the difference of the value between them is less than 200cm-1,which indicates that the carboxylate groups behave as the chelate coordination modes[27].
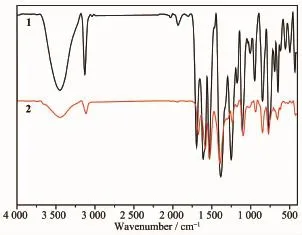
Fig.3FTIR spectra of complexes 1and 2
Fig.4and 5show the powder XRD patterns of as-synthesized compounds and the simulated patterns on the basis of single-crystal structures of 1and 2, respectively.Thediffractionpeaksonpatterns correspond well in position,indicating the phase purity of the as-synthesized samples.
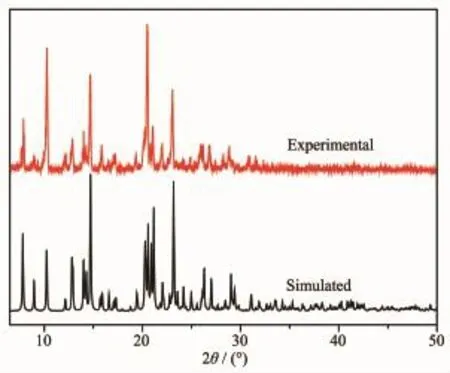
Fig.4XRD pattern of complex 1
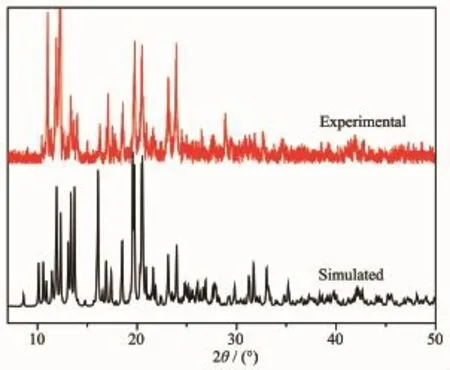
Fig.5XRD pattern of complex 2
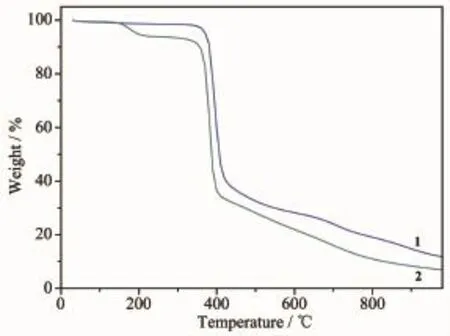
Fig.6TG curves of complexes 1and 2
Thermogravimetric(TG)measurementswere carried out from 30to 1000℃under N2atmosphere flow with a heating rate of 10℃·min-1.As shown in Fig.6,the framework of complex 1is stable up to 350℃and then the framework begins to collapse.A subsequent weight loss of 88.2%in the temperature range of 350~980℃corresponds to the release of coordinated 1,3-bip,bpdc2-ligands and a half uncoordinated H2bpdc molecule(Calcd.89.3%).Complex 2exhibits a weight loss of 6.07%from 100to 230℃, corresponding to the loss of one free DMF molecule (Calcd.6.45%).It is stable up to about 350℃and then the framework starts to collapse after that temperature.The ultimate frameworks of 1and 2can be stabilized up to 350℃,which are larger than the usual non-interpenetration and polycatenation structures[28].
2.3 Fluorescence properties
Thesolid-stateluminescencepropertiesof complexes 1and 2as well as the free 1,3-bip ligand were investigated at room temperature.Free 1,3-bip shows emission with a maximum at 462nm upon excitation at 330nm in solid state,matching with the previous reports[19].As shown in Fig.7,the maximum emission peaks for complexes 1and 2are observed at458and 460nm upon excitation at 330nm,respectively, which show slight blue shift in wavelength compared with 1,3-bip ligand.The blue shift of the emission maximum between the complex and the ligand may originate from the coordination of the ligand to the metal centers.The Zn/Cdion are difficult to oxidize or reduce because of the d10configuration.So the emissions of these MCPs are neither metal-toligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT)in nature[29-30].Thus,they may be assigned to a mixture characteristics of intraligand and ligand-to-ligand charge transition(LLCT),as reported for other ZnMCPs constructed from mixed N-donor and O-donor ligands[31].The enhancement of luminescence in d10complexes may be attributed to ligand chelation to the metal center which effectively increases the rigidity of the ligand and reduces the loss of energy by radiationless decay.The difference of the emission behaviors for 1and 2probably derives from the differences in the rigidity of solid-state crystal packing.
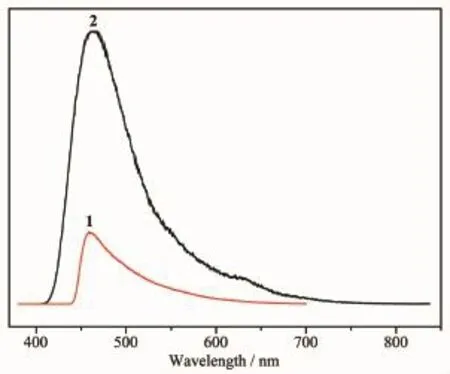
Fig.7Solid-state emission spectra of the complexes 1and 2
3 Conclusions
In conclusion,two coordination polymers based on H2bpdc and 1,3-bip mixed ligands have been prepared under solvothermal conditions.By changing the central ion,two new complexes with different structures were constructed.Complex 1features a 3D→3Dfivefoldinterpenetratingframeworkviathe connectivityamongcationic[Zn(1,3-bip)]24+macrocycles and bpdc2-ligands.Complex 2features a 2D→2D (4,4)net.In addition,the photoluminescence investigation shows that two coordination polymers appear potentially applied as luminescent materials.Further experiments exploring the structural effects of the spacer length of bis(imidazole)ligands on coordination polymers,and any resulting changes in physicochemical properties,are underway in our laboratory.
[1]Eddaoudi M,Moler D B,Li H L,et al.Acc.Chem.Res., 2001,34:319-330
[2]Halder G J,Kepert C J,Moubaraki B,et al.Science,2002, 98:1762-1765
[3]Dybtsev D N,Chun H,Yoon S H,et al.J.Am.Chem.Soc., 2004,126:32-33
[4]Kesanli B,Cui Y,M.Smith R,et al.Angew.Chem.Int.Ed., 2005,44:72-75
[5]Ghosh A K,Jana A D,Ghoshal D,et al.Cryst.Growth Des., 2006,6:701-707
[6]Gong Y,Li J,Qin J B,et al.Cryst.Growth Des.,2011,11: 1662-1674
[7]Guo Q Q,Xu C Y,Zhao B,et al.Cryst.Growth Des.,2012, 12:5439-5446
[8]Mu Y Y,Han G,Li Z,et al.Cryst.Growth Des.,2012,12: 1193-1200
[9]Zuo Y,Fang M,Xiong G,et al.Cryst.Growth Des.,2012,12: 3917-3926
[10]Reger D L,Leitner A,Smith M D.Inorg.Chem.,2013,52: 10041-10051
[11]Xi X B,Liu Y,Cui Y.Inorg.Chem.,2014,53:2352-2354
[12]Xie Y B,Gan L,Saudo E C,et al.CrystEngComm,2015,17: 4136-4142
[13]Anokhina E V,Vougo-Zanda M,Wang X Q,et al.J.Am. Chem.Soc.,2005,127:15000-15001
[14]Wang R H,Han L,Jiang P L,et al.Cryst.Growth Des., 2005,5:129-135
[15]Xu X X,Lu Y,Wang E B,et al.Cryst.Growth Des.,2006, 6:2029-2035
[16]Wang Y B,Zheng X J,Zhuang W J,et al.Eur.J.Inorg. Chem.,2003,12:3572-3578
[17]Yang Y,Du P,Ma J F,et al.Cryst.Growth Des.,2011,11: 5540-5547
[18]Zhu S R.,Zhang H,Zhao Y M,et al.J.Mol.Struct.,2008, 892:420-426
[19]Qua H,Qiu L,Leng X K,et al.Inorg.Chem.Commun.,2011,14:1347-1352
[20]Wu Y P,Li D S,Zhao J,et al.CrystEngComm,2012,14: 4745-4750
[21]Han M L,Wang J G,Ma L F,et al.CrystEngComm,2012,14: 2691-2698
[22]Sheldrick G M.SHELXS-97,A Program for the Siemens Area Detector Absorption Correction,University of Göttingen, Germany,1997.
[23]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of of Göttingen,Germany, 1997.
[24]Zhao X,He H,Hu T,et al.Inorg.Chem.,2009,48:8057-8061
[25]He H,Dai F,Sun D.Dalton Trans.,2009,763:365-371
[26]Zhao X,He H,Dai F,et al.Inorg.Chem.,2010,49:8650-8656
[27]Nakamoto K.Infrared and Raman Spectra of Inorganic and Coordination Compound.5th Ed.New York:Wiley,1997.
[28]Li X Y,Liu X X,Yue K F,et al.RSC Adv.,2015,5:81689-81695
[29]Wen L L,Li Y Z,Lu Z D,et al.Cryst.Growth Des.,2006,6: 530-537
[30]Zhang L P,Ma J F,Yang J,et al.Inorg.Chem.,2010,49: 1535-1550
[31]Su Z,Fan J,Okamura T,et al.Cryst.Growth Des.,2010,10: 1911-1922
LU Jiu-Fu*XU Yu-HangJIN Ling-XiaGUO Xiao-Hua
ZHAO Cai-BinZHENG NanJIANG MinGE Hong-Guang*
(Shaanxi Province Key Laboratory of Catalytic Foundation and Application,College of Chemical& Environment Science,Shaanxi Sci-Tech University,Hanzhong,Shaanxi 723001,China)
Two metal coordination polymers,namely{[Zn(1,3-bip)(bpdc)]·0.5H2bpdc}n(1),{[Cd2(1,3-bip)2(bpdc)2]· DMF}n(2),where 1,3-bip=1,3-bis(imidazol)propane,H2bpdc=biphenyl-4,4′-dicarboxylic acid,were synthesized by changing the central ion with the same mixed ligands under solvothermal conditions.The complexes were further characterized by single crystal X-ray diffraction,powder XRD,FTIR,TGA and elemental analysis techniques. Single crystal X-ray analysis revealed that complex 1exhibits a 5-fold 3D→3D parallel interpenetrating frameworks.Complex 2features a 2D→2D(4,4)net,which are interlocked with each other to form a 2-fold parallel interpenetrating 2D architecture.The varieties in coordination numbers of the central metals are the key reasons for the structural differences.In addition,the photoluminescence properties of 1and 2in the solid state at room temperature were also investigated.CCDC:1011050,1;1013480,2.
solvothermal synthesis;coordination polymers;interpenetrating frameworks;photoluminescence properties
O614.24+1;O614.24+2
A
1001-4861(2017)01-0115-08
10.11862/CJIC.2017.001
2016-02-14。收修改稿日期:2016-09-22。
国家青年自然科学基金(No.21603133)、陕西理工大学博士启动经费(No.SLGKYQD2-13,SLGKYQD14-10)和陕西理工大学校级项目(No.SLGKY15-36)资助。
*通信联系人。E-mail:jiufulu@163.com,gehg@snut.edu.cn
