三个镧系金属氮氧自由基配合物的合成、结构及磁性
2017-02-16胡鹏高媛媛肖凤仪邓肖娟黄国洪张淼苏芬王莉娜
胡鹏高媛媛肖凤仪邓肖娟黄国洪张淼苏芬王莉娜
(1内蒙古工业大学化工学院,呼和浩特010000)
(2肇庆学院化学化工学院,肇庆526061)
(3复旦大学附属妇产科医院,上海200011)
三个镧系金属氮氧自由基配合物的合成、结构及磁性
胡鹏2高媛媛*,1肖凤仪*,3邓肖娟2黄国洪2张淼2苏芬2王莉娜2
(1内蒙古工业大学化工学院,呼和浩特010000)
(2肇庆学院化学化工学院,肇庆526061)
(3复旦大学附属妇产科医院,上海200011)
以氮氧自由基为配体,合成了3例氮氧自由基-稀土三自旋单核配合物[Ln(hfac)3(NIT-Ph-4-Br)2](Ln=Gd(1),Tb(2),Dy(3), hfac=六氟乙酰丙酮,NIT-Ph-4-Br=4,4,5,5-四甲基-2-(4′-溴)-咪唑啉-3-氧化-1-氧基自由基。单晶结构分析表明3个配合物均属单斜晶系P21/c空间群,配合物中的Ln离子为八配位模式,并且拥有相似的自由基-稀土-自由基单核结构。对配合物的磁性测试结果表明,配合物1中自由基与Gd离子之间存在着铁磁相互作用,自由基与自由基之间存在着反铁磁相互作用;配合物2,3中,稀土离子与自由基之间存在弱的反铁磁相互作用
氮氧自由基;稀土;晶体结构;磁性
0 Introduction
Molecular mag netic materials have attracted scientists attention in the past two decades due to their potential applications in high-density magnetic memories,quantum computing devices and molecular spintronics[1-6].Among the different approaches to preparemolecularmagneticmaterialsthemetalradical strategy that consists of matching paramagnetic organic molecules with transition metal complex gives rise to a variety of compounds with different structural and magnetic dimensionalities.Up to now various organic paramagnetic molecules such as verdazyl, semiquinone and nitronyl nitroxide(NIT)radicals have beenwidelystudiedinthefieldofmolecular magnetism[7-14].Among them,nitronyl nitroxide radicals have received noteworthy attention because this type of radicals can act as bidentate ligands with identical N-O coordination groups.Besides,nitronyl nitroxide family of radicals are relatively stable and easy to obtain derivatives with substituents containing donor atoms.However,NIT radicals are poorly donating ligands,thus utilization of strong electron withdrawing coligands such as hexafluoroacetylacetonate(hfac)[15-17]and trifluoroacetylacetonate(tfac)[18-20]in the metal to improve weak coordination ability is necessary.The stericdemandofhfacortfacrestrictthe dimensionality of the resulting metal-radical complex. Thus numbers of zero-and one-dimensional complexes were prepared by this strategy.Recently lanthanide ions based molecular magnetic materials have been extensively studied because lanthanide ions such as terbiumand dysprosiumare good candidates for the construction of SMMs due to their significant magneticanisotropyarisingfromthelarge, unquenched orbital angular momentum[21-24].
To further study the magnetic properties of NIT radical-lanthanide compounds,in this paper we report a nitronyl nitroxide radical(Scheme 1)and its corresponding Ln-nitronyl nitroxide compounds[Ln(hfac)3(NIT -Ph-4-Br)2](Ln=Gd(1),Tb(2),Dy(3),hfac=hexafluoroacetylacetonate,NIT-Ph-4-Br=2-(4′-bromine phenyl) -4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide),their crystalstructuresandmagneticpropertieswere described in detail.

Scheme 1Molecular structure of NIT-Ph-4-Br
1 Experimental
1.1 Materials and measurements
All reagents and solvents were purchased from Aladdin and used without further purification.The radical ligand NIT-Ph-4-Br was synthesized according to literature[25].Elemental analyses(C,H and N)were performedonaPerkin-Elmer240Celemental analyzer.IR spectra were recorded on a Nicolet IS10IR spectrometer using KBr pellets in the range of 4000~500cm-1.The magnetic measurements were carried out with MPMSXL-7SQUID magnetometer. DiamagneticcorrectionsweremadewithPascals constants for all the constituent atoms.
1.2 Synthesis of complex 1
A suspension of Gd(hfac)3·2H2O(0.05mmol)in 15mL dry boiling heptane was heated to reflux for about 1h.Then the solution was cooled to 65℃,a solution of NIT-Ph-4-Br 0.1mmol)in 2mL of CHCl3was added.The resulting solution was stirred for about 2min and then cooled to room temperature.The filtrate was allowed to stand at room temperature for slowevaporation.Slowevaporationofthefinal solution for about four days yielded dark-blue block crystals suitable for single-crystal X-rayanalysis. Yield:43.5%based on rare-earth.Elemental analysis calculated for C41H35Br2GdF18N4O10(%):C:35.10;H:2.51;N:3.99.Found(%):C:33.88;H:2.79;N:4.12. FTIR(KBr,cm-1):1656(s),1527(w),1385(w),1350(w),1255(s),1198(s),1096(s),796(w),660(w).
1.3 Synthesis of Complex 2
Complex 2was synthesizedusingthesame procedure for complex 1with Tb(hfac)3·2H2O instead of Gd(hfac)3·2H2O.Yield:47.9%.Elemental analysis calculated for C41H35Br2TbF18N4O10(%):C:35.06;H: 2.51;N:3.99.Found(%):C:35.69;H:2.57;N:4.11. FTIR(KBr,cm-1):1655(s),1599(w),1528(w),1399(w), 1352(w),1255(s),1199(s),1096(w),797(w),660(w).
1.4 Synthesis of Complex 3
Complex 3was synthesizedusingthesame procedure for complex 1with Dy(hfac)3·2H2O instead of Gd(hfac)3·2H2O.Yield:42.9%.Elemental analysis calculated for C41H35Br2DyF18N4O10(%):C:34.97;H: 2.51;N:3.98.Found(%):C:34.91;H:2.77;N:3.87(%).FTIR(KBr,cm-1):1655(s),1600(w),1527(w), 1399(w),1352(w),1255(s),1199(s),1096(w),798(w),662(w).
1.5 X-ray Crystallographic Study
The crystal structure data of complexes 1,2and 3werecollectedusingaRigakuSaturnCCD diffractometer equipped with graphite-monochromated Mo Kα radiation(λ=0.071073nm).Crystal size of complexes 1,2and 3are 0.15mm×0.12mm× 0.11mm,0.17mm×0.11mm×0.10mm,0.12mm× 0.11mm×0.10mm respectively.The structures were solved by the direct methods with SHELXS-97[26]and refined by full-matrix least-squares methods on F2with SHELXL-97program package[27].Anisotropic thermal parameters were assigned to all non-hydrogen atoms.The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.The details of the crystal parameters,data collection,and refinements for these complexes were listed in Table 1and 2.
CCDC:1496095,1;1496096,2;1496097,3.

Table 1Crystal data and structure refinement for 1,2and 3

Table 2Selected bond lengths(nm)and angles(°)for 1,2and 3
2 Results and discussion
2.1 Crystal structures of complexes 1~3
AsisshowninFig.1,Compound1isa mononuclear coordination compound crystallizing in the monoclinic space group P21/c with Z=4.The central Gdion is eight coordinated in slightly distortedtriangulardodecahedralGdO8geometry completed by two non-bridged NO groups from two separate organic radicals and three bischelate hfacanions.The distances of Gd-O bonds range from 0.2356(4)to 0.2407(4)nm.The coordinated N(3)-O(2)andN(2)-O(3)bond lengths of nitronyl nitroxide radicals are 0.1307(5)nm and 0.1299(6)nm respectively and the uncoordinated N(4)-O(1)and N(1)-O(4) bond lengths are 0.1264(6)nm and 0.1260(7)nm respectively,which are comparable to those of reported tri-spin radical-Ln-radical complexes[28-33].The nearest Gd…Gd distancebetweenadjacentmoleculesis 1.0716(5)nm(Fig.1).

Fig.1Molecular structure(left)with thermal ellipsoids drawn at 30%probability and crystal packing diagram(right)of complex 1
Compound 2is isostructural to compound 1and the bond lengths of Tb-O are in the range of 0.2338(5)~0.2394(5)nm,which are a little bit shorter than the bond lengths of Gd-O.The nearest Tb…Tb distance between adjacent molecules is 1.0812(4)nm.
Compound 3is also isostructural to compound 1and the bond lengths of Dy-O are in the range of 0.2330(2)~0.2386(2)nm,which are a little bit shorter than the bond lengths of Gd-O and Tb-O.The nearest Dy…Dy distance between adjacent molecules is 1.0608(3)nm.
2.2 Magnetic property of complex 1
The temperature dependence of the magnetic susceptibilities 1,2,and 3were measured from 300to 2.0K in an applied field of 1kOe,and the magnetic behaviors of complex 1are shown in Fig.2.At 300K, the χMT value is 8.61cm3·K·mol-1.The values are in good agreement with the theoretical value of 8.63cm3· K·mol-1(uncoupled one Gdion(f7electron configuration,χMT=7.88cm3·K·mol-1)plus two organic radicals(S=1/2,χMT=0.375cm3·K·mol-1)).Upon cooling,the χMT value of complex 1increase steadily to a maximum of 10.03cm3·K·mol-1at 13.9K, afterward decreases to 9.13cm3·K·mol-1at 2.0K.

Fig.2Temperature dependence of χMT(left)and field dependence of magnetization at 2.0K(right)for complex 1
There are two kinds of magnetic interactions in this radical-Gd-radical complex at the same time. The first one is Gd-radical interaction and the second one is radical-radical interaction.
The magnetic interactions between Gdand the radicals can bedescribed by isotropicexchange interaction.Thereforetheexperimentaldatafor complex 1can be analyzed with an expression derived from a spin Hamiltonian.Considering the g value range of the radical and Gdion,we assume that the radical and Gdion have the same g value.Thus, the variable-temperature magnetic susceptibility data for complex 1can be analyzed by a theoretical expression(Eq.(2))deduced from a spin Hamiltonian (Eq.(1)).The JRad-Gdrepresent the magnetic coupling for the Gd-radical and JRad-Radfor radical-radical,and the zJ′in Eq.(2)representing the intermolecular interactions.
The best fitting results give coupling parameters g=1.99,JRad-Gd=2.81cm-1,JRad-Rad=-10.85cm-1,zJ′=-0.02cm-1,R=1.64×10-5,where R is defined as R=(χM,obsχM,calc)2/(χM,obs)2for complex 1.The positive value of JRad-Gdindicatesthatthereisaweakferromagnetic interaction between the Gdand the radicals in the molecule.ThenegativeJRad-Radindicatesthe antiferromagneticinteractionbetweenthetwo intramolecular radicals.It is worth noting that the value of zJ′is much smaller than that of JRad-Rad.The obtained J value is comparable with the previously reported Gd-radicals compounds[28-34].
Furthermore,thefielddependenceof magnetization of complex 1has been determined at 2K in the range of 0~70kOe(Fig.2).Upon increasing in the applied field,M increases up to 8.87Nβ at 70kOe,which corresponds well to the value expected for a ground state with a spin multiplicity of S=9/2in the case of one Gdand two radical ferromagnetically coupled.At the lower fields the value is smaller than themagnetizationcalculatedwiththeBrillouin function for noncoupled S=7/2and two S=1/2spin centers(g=2.0,T=2K),which suggests the dominant antiferromagnetic interaction between the Gdion and the coordinated NIT radical.
2.3 Magnetic properties ofcomplex2and complex 3
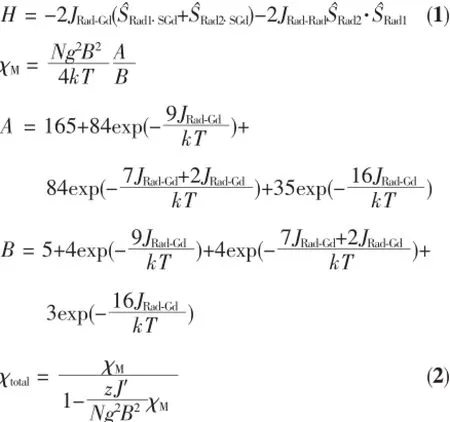
While for complex 2(Fig.3),at 300K,the χMT value is 12.46cm3·K·mol-1,and the values are in goodagreementwithtothetheoreticalvalue 12.57cm3·K·mol-1in uncoupled system of one Tbion(f9electron configuration,χMT=11.82cm3·K·mol-1) and two organic radical(S=1/2,χMT=0.375cm3·K· mol-1).Upon cooling,the χMT values of complex 2maintain a constant behavior down to about 50K then the value decrease gradually and reach a minimum of 10.37cm3·K·mol-1.
Complex 3shows similar magnetic properties with complex 2(Fig.3).At 300K,the χMT value is 14.31cm3· K·mol-1,close to the theoretical value of 14.92cm3·K· mol-1(one Dyion(f9electron configuration,χMT= 14.17cm3·K·mol-1)plus two organic radicals(S=1/2, χMT=0.375cm3·K·mol-1)).Upon cooling,the χMT values of complex 3maintain a constant behavior down to about 50K then the value decrease gradually and reach a minimum of 8.21cm3·K·mol-1.
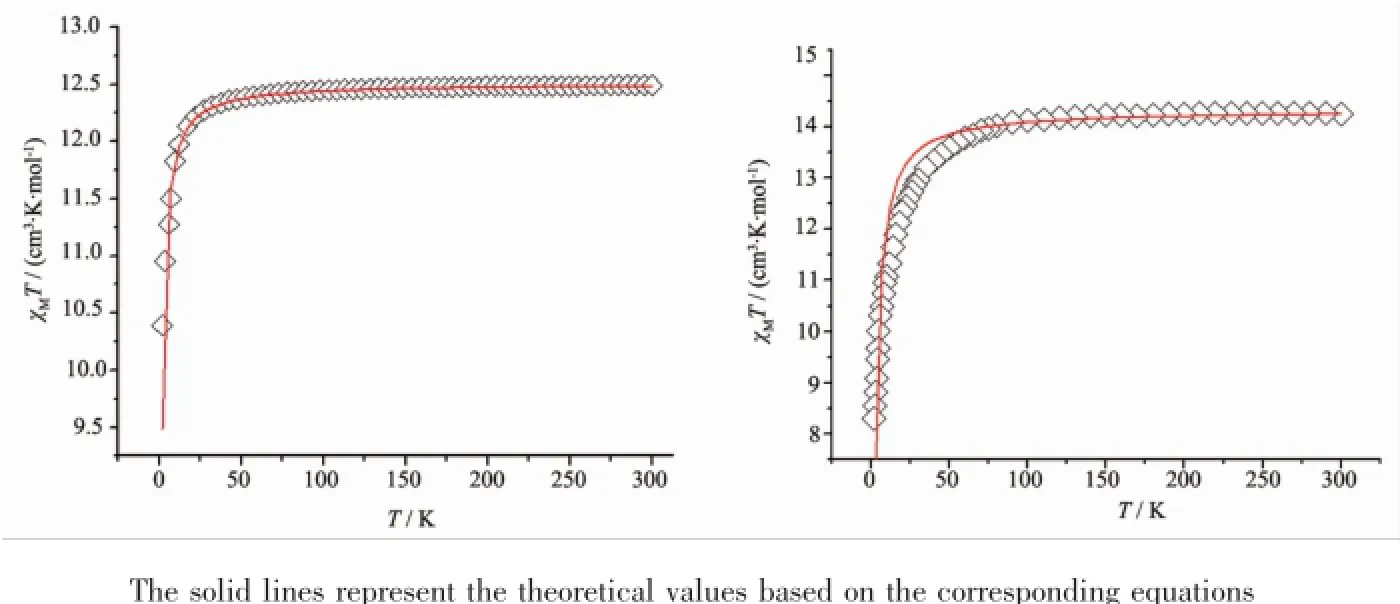
Fig.3Temperature dependence of χMT for complex 2(left)and complex 3(right)
There is no available expression to determine the magnetic susceptibilities of Lnsystems with large anisotropy.To obtain a rough quantitative estimation ofthemagneticinteractionbetweenLnand radicals,themagneticsusceptibilityχtotalofthe complex can be assumed as the sum of χLnof the isolated Dyor Tbion and χRadof the radical(Eq. (4)).The χTband χDycan be described as Eq.(5)and (6),respectively.
In the expression,Δ is the zero-field-splitting parameter,g is the Lande factor,k is the Boltzmann constant,β is the Bohr magneton constant and N is Avogadros constant.The zJ′parameter based on the molecular field approximation in Eq.(7)is introduced to simulate the magnetic interactions between all the paramagnetic species in the system.Thus the magneticdata can be analyzed by the following approximate treatment of Eq.(4)~(7)[29,31].Giving the best fitting parameters for complex 2are g=1.49,=3.89×10-2cm-1, zJ′=1.63×10-2cm-1,R=1.12×10-5and for complex 3are g=1.36,Δ=3.17×10-2cm-1,zJ′=2.14×10-2cm-1,R= 1.92×10-5,where R is defined as R=(χM,obs-χM,calc)2/(χM,obs)2. The very small positive zJ′values are indicative of very weak ferromagnetic interaction between Lnions and the coordinated nitronyl nitroxide,which is consistent with the reported heavy lanthanide-nitronyl nitroxide complexes.




Fig.4Field dependence of magnetization of 2and 3at 2.0K
Thefielddependencesofmagnetizationsfor complexes 2and 3have been determined at 2K in the range of 0~70kOe(Fig.4).Upon increasing in the applied field,M increases up to 5.87Nβ and 7.73Nβ at 70kOe for 2and 3,respectively,which is much lower than the saturation value of 11.0Nβ(one Tbion with g=3/2and J=6(9.0Nβ)plus two radicals with g=2.0and S=1/2)and 12.0Nβ(one Dyion with g=4/ 3and J=15/2(10.0Nβ)plus two radicals with g=2.0and S=1/2).Considering the strong spin-orbit coupling in Lnions,the large gaps between experimental data and theoretical saturation values for compounds 2and 3can be ascribed to the presence of a magnetic anisotropy and/orlow-lyingexcitedstatesinthe system.
2.4 Dynamic magnetic properties for 2and 3
Alternatingcurrent(ac)susceptibility measurements for 2and 3were carried out in the low temperatureregionunderazerodcfieldwith frequency of 111and 511Hz.The result(Fig.5)shows that there are no obvious frequency dependent in-phase(χ′)and out-of-phase(χ″)signals for both complex 2and 3,they do not express SMMs behavior at low temperature.

Fig.5Temperature dependence of the in-phase and out-of-phase components of ac susceptibility for 2(left)and 3(right)in zero dc field with an oscillation of 3.5Oe
3 Conclusions
In conclusion,we report three new complexes based on nitronyl nitroxide radicals and lanthanide ions.These three compounds have similar structures, in which two radical ligands are coordinated to the Lnions via the oxygen atoms of the nitroxide to form the three spin system.The magnetic studies reveal thatferromagneticinteractions(betweenthe intramolecular Ln and radical)and antiferromagnetic interactions(between the intramolecular radicals) coexist in complex 1.Complexes 2and 3show very weak ferromagnetic interaction between Lnions and the coordinated nitronyl nitroxide.Both complex 2and 3do not have SMMs behavior at low temperature,this may due to the small energy barrier which could not prevent the inversion of spin.
[1]Kahn O.Molecular Magnetism.New York:VCH Publishers Inc.,1993:1-23
[2]Moller S,Perlov C,Jackson W,et al.Nature,2003,426:166-169
[3]Kahn M L,Sutter J P,Golhen S,et al.J.Am.Chem.Soc., 2000,122:3413-3421
[4]Zhang P,Guo Y N,Tang J K.Coord.Chem.Rev.,2013,257: 1728-1737
[5]Graham M J,Zadrozny J M,Shiddiq M,et al.J.Am.Chem. Soc.,2014,136:7623-7626
[6]Bar A K,Pichon C,Gogoi N.Chem.Commun.,2015,51: 3616-3619
[7]Rinehart J D,Fang M,Evans W J,et al.J.Am.Chem.Soc., 2011,133:14236-14239
[8]Rinehart J D,Fang M,Evans W J,et al.Nat.Chem.,2011, 3:538-542
[9]Wang X F,Hu P,Li Y G,et al.Chem.-Asian J.,2015,10: 325-330
[10]Adugna S,Revunova K,Djukic B,et al.Inorg.Chem.2010, 49:10183-10190
[11]Chernick E T,Casillas R,Zirzlmeier J,et al.J.Am.Chem. Soc.,2015,137:857-863
[12]Mailman A,Winter S M,Wong J W L,et al.J.Am.Chem. Soc.,2015,137:1044-1049
[13]Brook D J R,Richardson C J,Haller B C,et al.Chem. Commun.,2010,46:6590-6592
[14]Norel L,Chamoreau L M,Journaux Y,et al.Chem.Commun., 2010,45:2381-2383
[15]Murakami R,Ishida T,Yoshii S,et al.Dalton Trans.2013, 42:13968-13973
[16]Bernot K,Bogani L,Caneschi A,et al.J.Am.Chem.Soc., 2006,128:7947-7956
[17]Zhu M,Hu P,Li Y,et al.Chem.Eur.J.,2014,20:13356-13364
[18]Hu P,Zhang C,Gao Y,et al.Inorg.Chim.Acta,2013,398: 136-140
[19]Mei X L,Ma Y,Li L C.Dalton Trans.,2012,41:505-510
[20]Mei X L,Liu R N,Wang C.Dalton Trans.,2012,41:2904-2909
[21]Bernot K,Luzon J,Bogani L,et al.J.Am.Chem.Soc.,2009, 131:5573-5579
[22]Liu J L,Wu J Y,Chen Y C,et al.Angew.Chem.Int.Ed., 2014,53:12966-12970
[23]Chatelain L,Walsh J P S,Pecaut J,et al.Angew.Chem. Int.Ed.,2014,53:13434-13439
[24]Zhang P,Zhang L,Wang C.J.Am.Chem.Soc.,2014,136: 4484-4489
[25]Ullman E F,Osiecki J H,Boocock D G B,et al.J.Am. Chem.Soc.,1972,94:7049-7059
[26]Sheldrick G M.SHELXS-97:Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[27]Sheldrick G M.SHELXL-97:Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[28]Wang C,Wang Y L,Qin Z X,et al.Inorg.Chem.Commun., 2012,20:112-117
[29]Zhang C X,Qiao X M,Kong Y K,et al.J.Mol.Struct., 2015,108:1348-1354
[30]Du F X,Hu P,Gao Y Y,et al.Inorg.Chem.Commun.,2014, 48:166-170
[31]Zhang C X,Chen H W,Wang W M,et al.Inorg.Chem. Commun.,2012,24:177-181
[32]Zhou N,Ma Y,Wang C,et al.Dalton Trans.,2009:8489-8492
[33]Li L L,Liu S,Zhang Y,et al.Dalton Trans.,2015,44:6118-6125
[34]Sutter J-P,Golhen S,Kahn O,et al.Chem.Eur.J.,1998,4: 571-576
Three Lanthanide Nitronyl Nitroxide Radical Compounds: Synthesises,Structures and Magnetic Properties
HU Peng2GAO Yuan-Yuan*,1XIAO Feng-Yi*,3DENG Xiao-Juan2
HUANG Guo-Hong2ZHANG Miao2SU Feng2WANG Li-Na2
(1College of Chemical Engineering,Inner Mongolia University of Technology,Hohhot 010051,China)
(2College of Chemistry and Chemical Engineering,Zhaoqing University,Zhaoqing,Guangdong 526061,China)
(3Obstetrics and Gynecology Hospital of Fudan University,Shanghai 200011,China)
Three lanthanide-nitronyl nitroxide radical compounds[Ln(hfac)3(NIT-Ph-4-Br)2](Ln=Gd(1),Tb(2),Dy (3),hfac=hexafluoroacetylacetonate,NIT-Ph-4-Br=2-(4′-bromide)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide) have been successfully prepared and characterized by single crystal X-ray diffraction,IR spectroscopy and elemental analyses.Single crystal X-ray crystallographic analyses reveal that all these three compounds are isostructural and crystallize in the P21/c space group,which are composed of one Ln(hfac)3unit and two NIT-Ph-4-Br radicals.Magnetic studies reveal that ferromagnetic interactions and antiferromagnetic interactions coexist in Gd complex and there are very weak ferromagnetic interactions between Lnions and the coordinated nitronyl nitroxide in Tb complex and Dy complex.CCDC:1496095,1;1496096,2;1496097,3.
nitronyl nitroxide radical;lanthanides;crystal structure;magnetic properties
O614.33+9;O614.341;O614.342
A
1001-4861(2017)01-0033-08
10.11862/CJIC.2017.019
2016-08-13。收修改稿日期:2016-11-09。
内蒙古自然科学基金(No.2016BS0206)、广东省教育厅青年创新人才项目(No.CQ2014064)和广东省大创项目(No.201510580046, 201610580046)资助。
*通信联系人。E-mail:gaoyuanyuan@imut.edu.cn,michellexiao2014@163.com
