天然产物中木栓烷型三萜核磁共振波谱特征
2017-02-08刘向前李小军金伦喆陆昌洙
刘向前,李小军,,金伦喆,陆昌洙(
1.湖南中医药大学药学院,湖南长沙410208;2.圆光大学药学院,韩国益山570-749;3.庆熙大学药学院,韩国首尔130-701)
天然产物中木栓烷型三萜核磁共振波谱特征
刘向前1,李小军1,2,金伦喆2,陆昌洙3(
1.湖南中医药大学药学院,湖南长沙410208;2.圆光大学药学院,韩国益山570-749;3.庆熙大学药学院,韩国首尔130-701)
对天然产物中发现的木栓烷型三萜化合物的13C-NMR、1H-NMR谱学特征进行综述,以期减少天然产物特别是木栓烷型三萜结构鉴定工作的盲目性和重复性,为进一步研究分析木栓烷型三萜提供经验借鉴。
木栓烷型三萜;核磁波谱特征;13C-NMR;1H-NMR
本文引用:刘向前,李小军,金伦喆,陆昌洙.天然产物中木栓烷型三萜核磁共振波谱特征[J].湖南中医药大学学报,2017,37(1):87-105.
木栓烷型(friedelane type)三萜及其皂苷主要分布于卫矛科(Celastraceae)、翅子藤科(Hippocrateaceae)、大戟科(Euphorbiaceae)、大风子科(Flacourtiaceae)和藤黄科(Guttiferae/Clusiaceae)等植物中,卫矛科(Celastraceae)和翅子藤科(Hippocrateaceae)中尤为常见。现代药理学研究表明,该类化合物具有抗肿瘤、抗炎、抗-HIV、抗菌、抗白血病和抗氧化等药理活性作用[1-6]。早在20世纪70年代,药物化学等领域的专家学者就对其进行了热门研究。近年来,越来越多结构复杂、新颖的木栓烷型三萜被发现,因其具有良好的药理活性而一直成为天然产物研究的热点。
与其它天然产物研究一样,木栓烷型三萜及其苷类化合物分离纯化得到单体化合物后,更为重要的一步是其结构和构型的鉴定(结构表征)。常用的波谱学鉴定方法主要有UV、IR、NMR、MS、X-Ray及CD等,其中一维和二维NMR在三萜的解析中起着至关重要的作用。通过FAB-MS、ESI-MS、HRMS和MSMS等质谱技术可准确测定木栓烷型三萜的分子量及相应的结构信息,一维和二维NMR综合分析可快速确定三萜苷元、糖和苷元的连接位置、糖链结构等信息,再综合UV、IR、CD等鉴定手段和该物质的理化性质,以及必要的文献查阅,可准确地推测出其平面和立体结构。
本文对1980-2015年已报道的246个木栓烷型三萜的13C-NMR和1H-NMR数据进行归纳总结,以期有助于相关研究者进行这类化合物的结构鉴定,为进一步分析研究木栓烷型三萜提供经验借鉴。
1 木栓烷型三萜的结构类型
天然产物中的木栓烷型三萜根据其结构特征主要分为5类:木栓烷型三萜(Intact friedelanes,TypeⅠ)、降碳类木栓烷型三萜(Norfriedelanes,TypeⅡ)、开环型木栓烷型三萜(Secofriedelanes,TypeⅢ)、环氧型木栓烷型三萜(Epoxyfriedelanes,TypeⅣ)和二聚体类木栓烷型三萜(Dimers,TypeⅤ)。从生物合成途径来看,木栓烷型三萜及其衍生物由角鲨烯-2,3-环氧化物的环化而得,在木栓烷型三萜的基本母核的结构基础上再进行碳环骨架的重排、转化、氧化和聚合,得降碳类、开环型、环氧型和二聚体类木栓烷型三萜及其衍生物。它们的基本结构类型和天然来源分别见图1和表1。
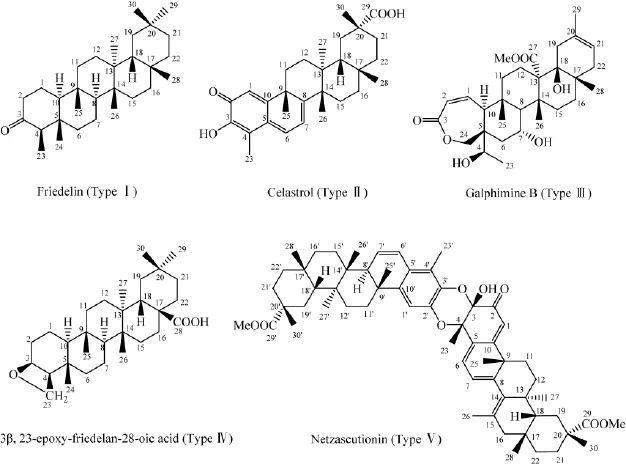
图1 木栓烷三萜I-V类型的代表性化合物
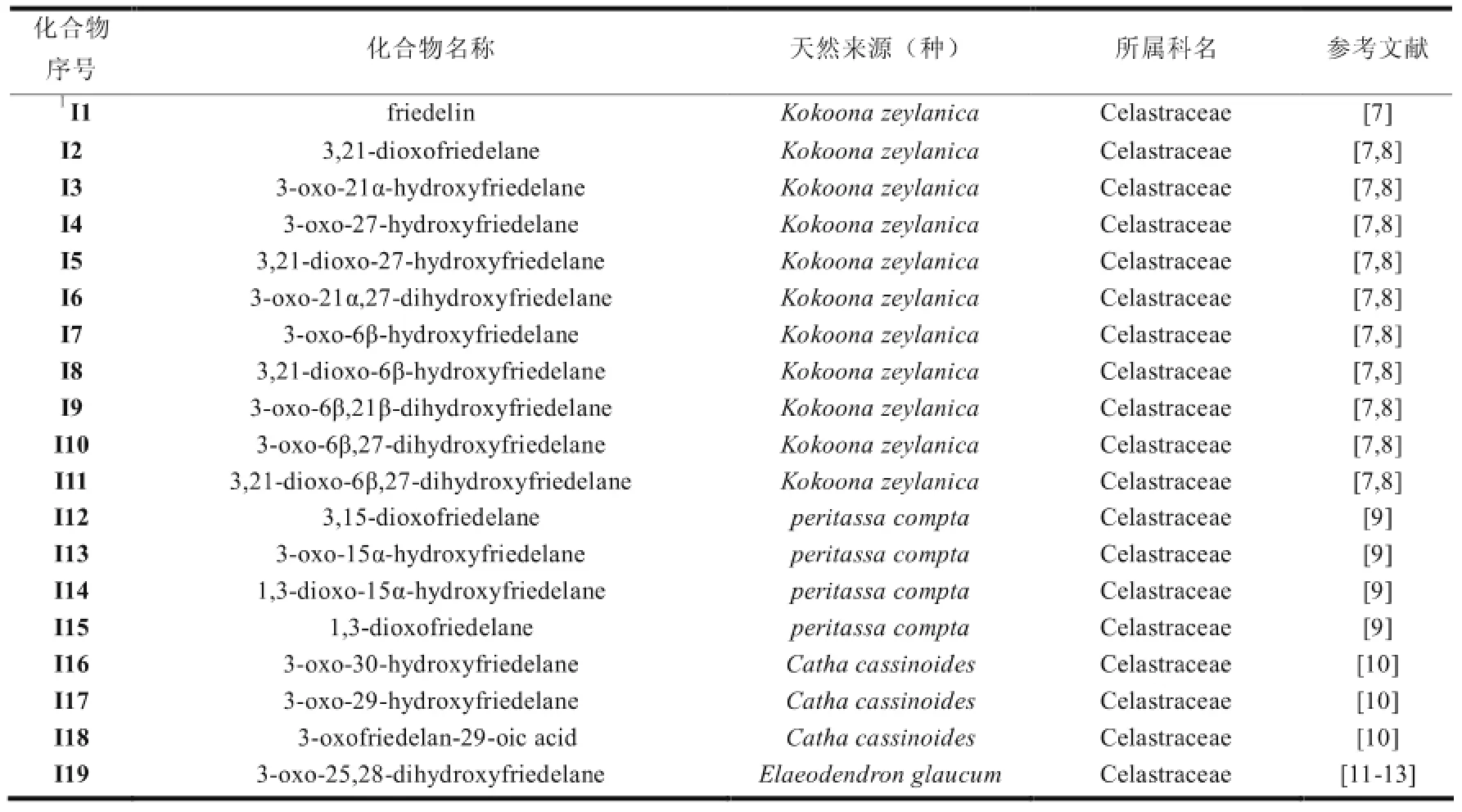
表1 木栓烷型三萜的天然来源
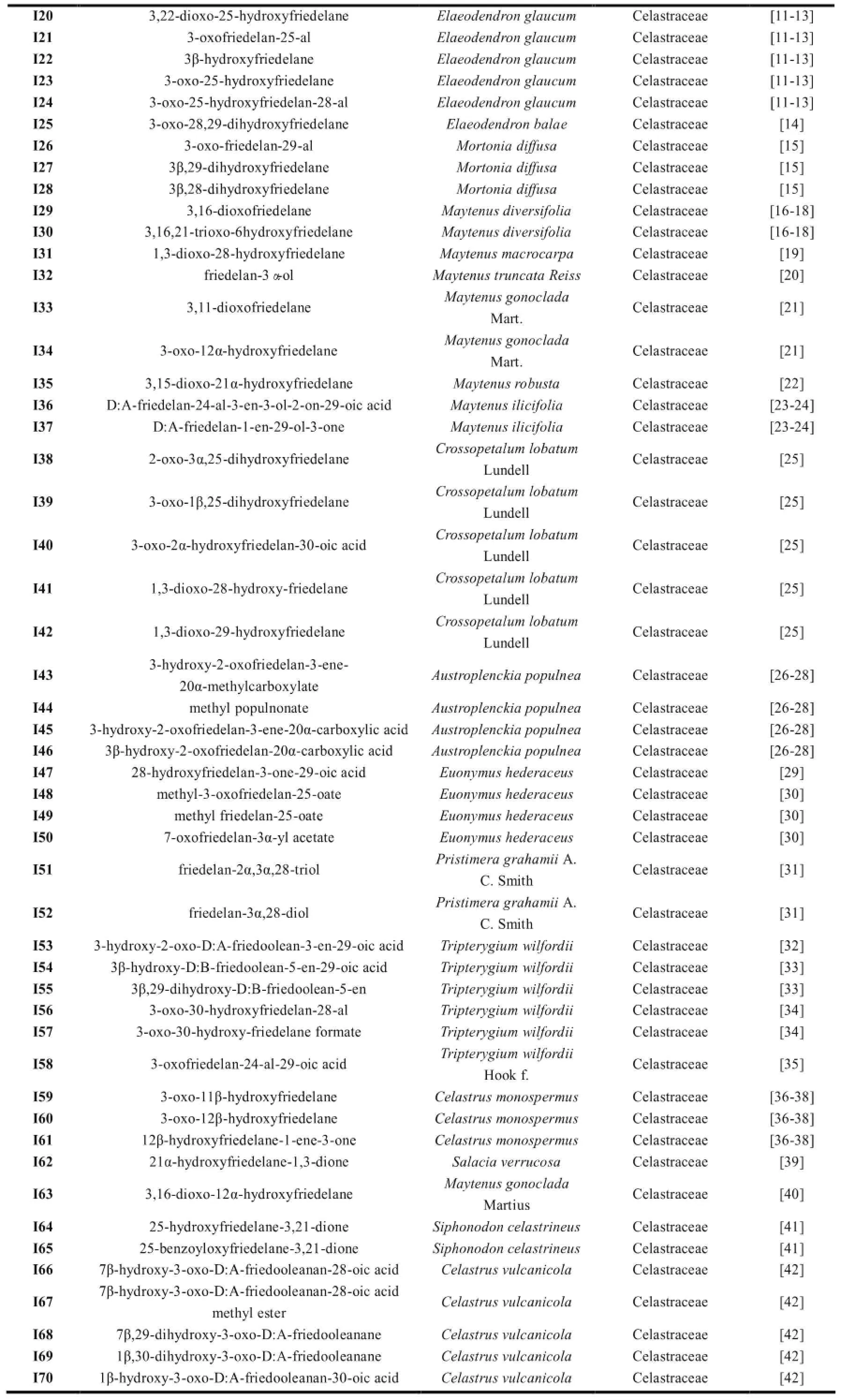
续表1
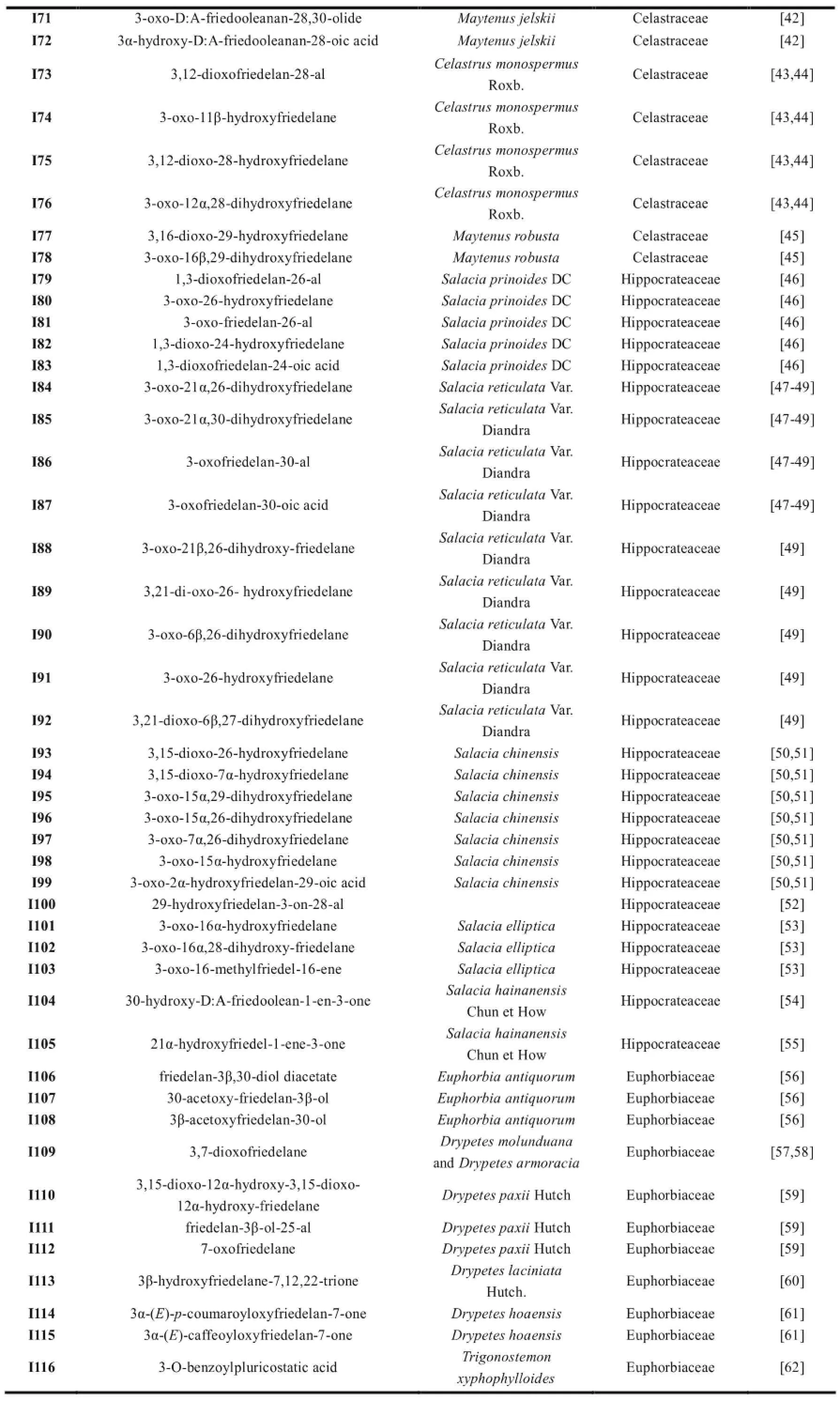
续表1
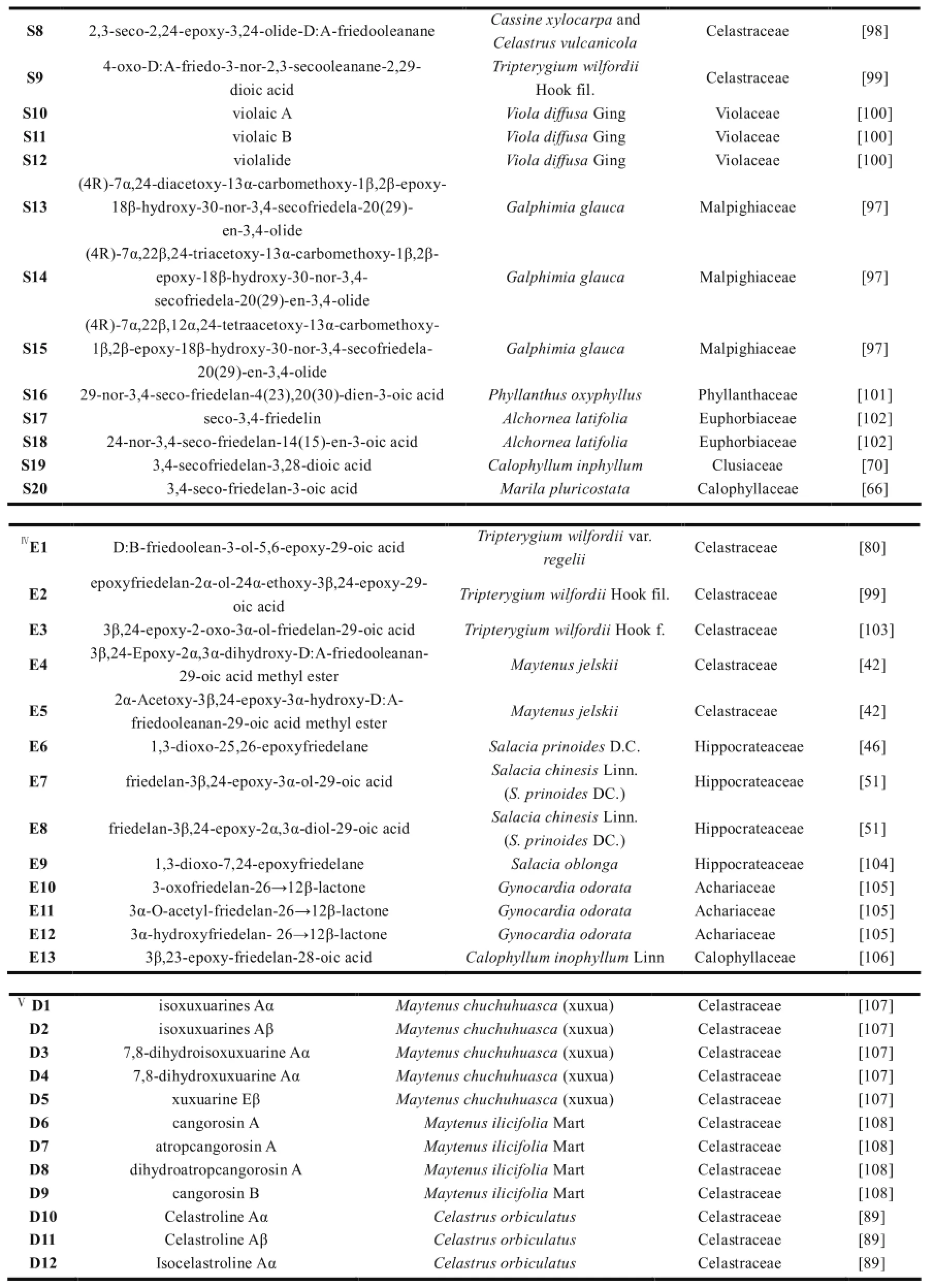
续表1
2 木栓烷型三萜的NMR特征
2.1 木栓烷型三萜的13C-NMR特征
木栓烷型三萜苷元中除了与氧相连的碳外,其余碳一般在δ60以下。在13C-NMR中,角甲基一般出现在δ6.2~35.5,其中23-CH3一般在6.2~13.5左右;23位为甲基时,24甲基的δ值为13.7~23.5;25-28位CH3的δ值一般出现在18~32;29-30位甲基δ值一般为31~35。无氧取代时,-CH2-的δ值一般分布于δ18-42左右,-CH-在δ37-60,而季碳的δ值则一般在δ33-57。木栓烷型三萜苷元和糖上与氧相连的碳δ值在60~90之间,具体而言,OH碳位δ61~82左右,乙酰基取代比相应的OH取代向低场位移2~3左右。当有单OH或多OH取代时,会因为取代基效应而引起α-C向低场位移34~50,β-C向低场位移2~10左右,γ-C效应与前面两种效应相反,向高场位移0~9。烯碳为δ109~160左右,羰基碳δ170~220,一般羰基碳在δ180左右,而形成酯键则稍向高场位移,醛基碳则一般在δ195~210左右。以下综述了具有典型代表性的五类木栓烷型三萜的13C-NMR数据。
2.1.1 基本结构木栓烷型三萜的13C-NMR特征
正常结构的木栓烷型三萜的13C-NMR特征一般与上述相似,在角甲基无氧取代的情况下一般会出现8个角甲基信号,最具特征的是23-CH3一般在δ6.2~13.5左右,当23-CH3为β型时,δ值一般在10以下;当23-CH3为α型时,由于空间效应的影响,其与24-CH3中的H的斥力作用减弱导致直接相连C的电子云密度减弱,从而减小了屏蔽效应,化学位移移向低场,一般出现在δ13.5附近。当相应位置出现氧代时由于氧的吸电子效应会使相应的C的δ值升高。见表2。
2.1.2 降碳木栓烷型三萜的13C-NMR特征
降碳木栓烷型三萜一般降碳的位置出现在取代甲基部位:如23-nor(N47和N48)、24-nor(N8和N29-N33)、29-nor(N8)、30-nor(N49)等。此类三萜13CNMR最大特征就是甲基信号的相应减少。见表3。
2.1.3 开环木栓烷型三萜的13C-NMR特征
该种类型的木栓烷型三萜最典型的结构特征是母环中A环的开环,且最常见的开环位置一般为3,4-seco(如S2-S5、S10-S20)或2,3-seco(如S1、S6-S9)。在开环部位一般都有-O-原子的介入,使得相应开环部位的化学位移向低场移动。另外,在开环的同时也常常伴随着降碳现象的出现(S9、S16)。见表4。
2.1.4 环氧木栓烷型三萜的13C-NMR特征
该类型的三萜在结构上保留了木栓烷母环的完整性,其结构特点是在母环外接有环外的环氧桥,这使得环氧桥上与-O-原子相连的-C-原子化学位移向低场移动。见表5。
2.1.5 二聚体类木栓烷型三萜的13C-NMR特征
该类三萜的结构特征是由2分子的木栓烷母环聚合而成,一般为一边含有1个醌环(quinoid),具有典型的醌类化合物碳信号;另一边带有一个芳香环(aromatic),具有芳香化合物碳信号。通常各相应位置的碳信号为成对出现的。见表6。
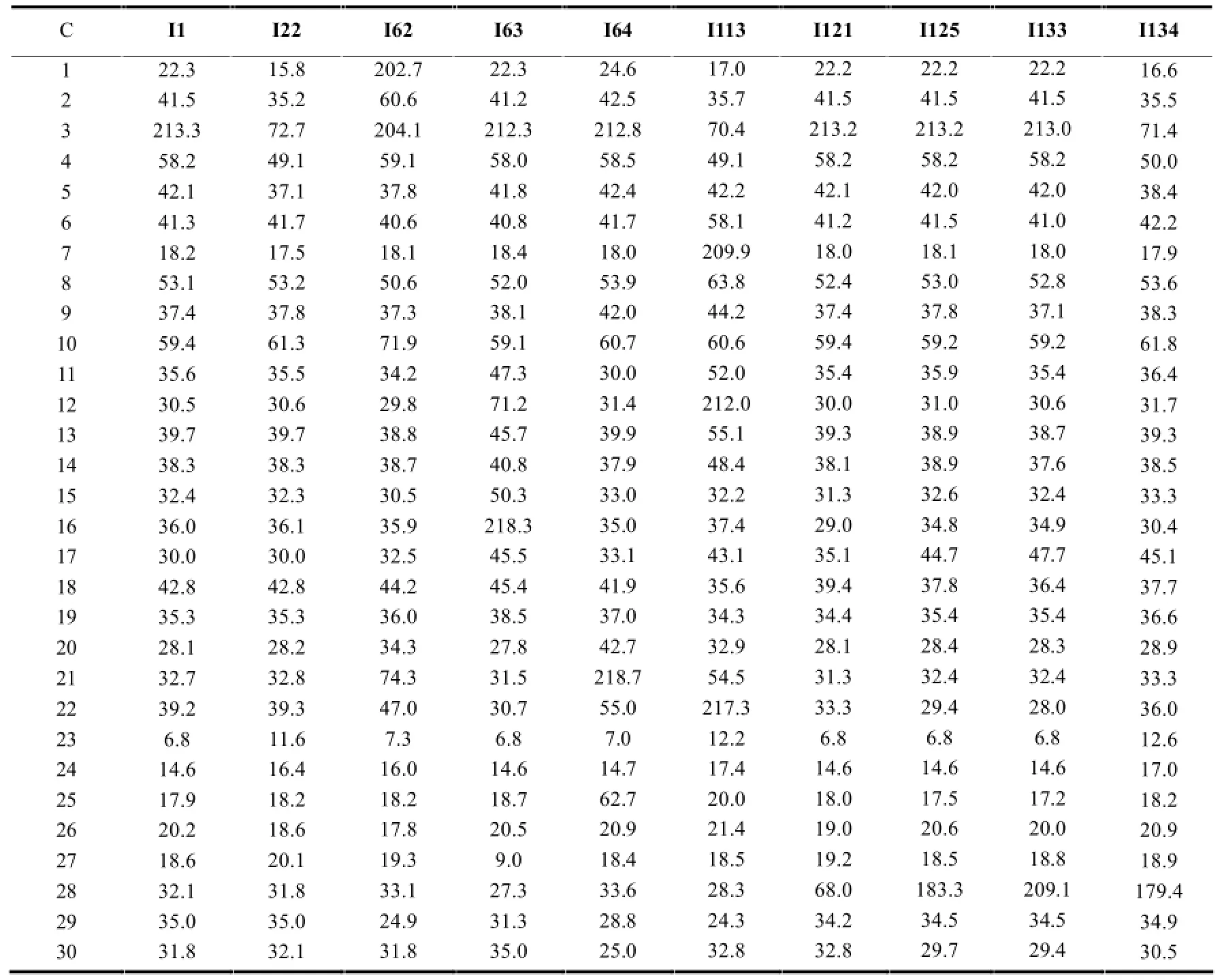
表2 正常结构木栓烷型三萜的13C-NMR数据
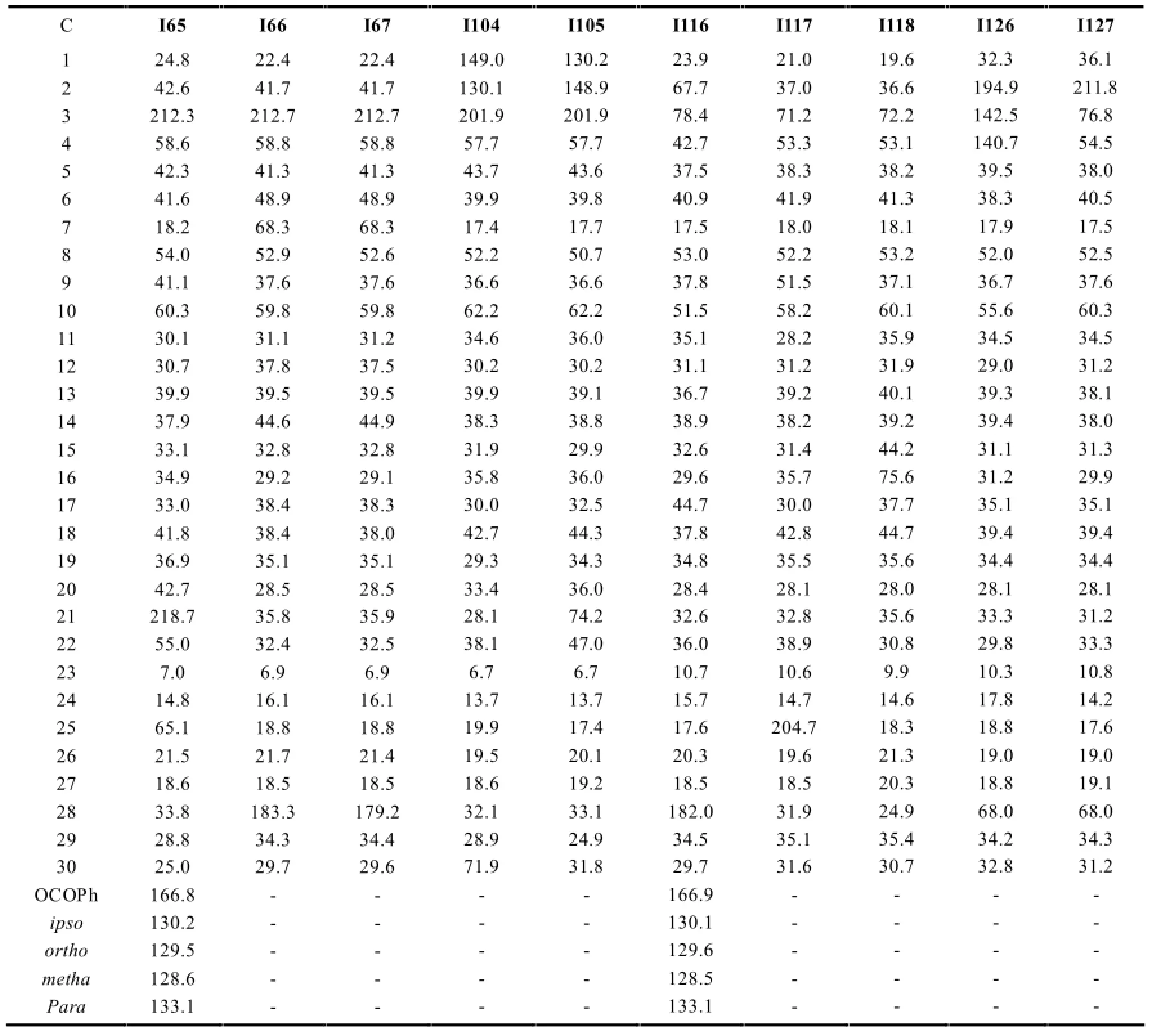
续表2
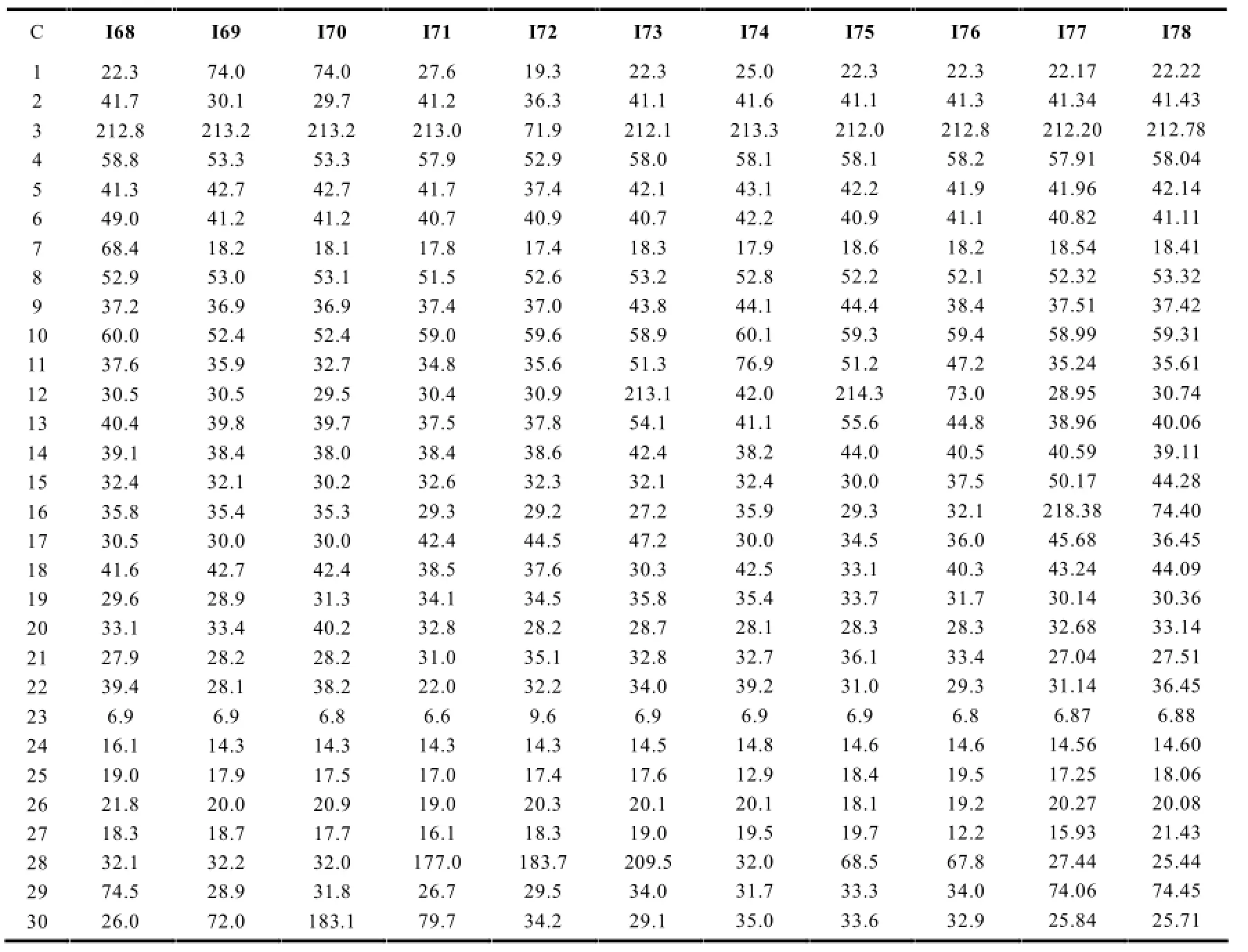
C I68 I69 I70 I71 I72 I73 I74 I75 I76 I77 I78 1 2 3 4 5 6 7 8 9 1 0 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 22.3 41.7 212.8 58.8 41.3 49.0 68.4 52.9 37.2 60.0 37.6 30.5 40.4 39.1 32.4 35.8 30.5 41.6 29.6 33.1 27.9 39.4 6.9 16.1 19.0 21.8 18.3 32.1 74.5 26.0 74.0 30.1 213.2 53.3 42.7 41.2 18.2 53.0 36.9 52.4 35.9 30.5 39.8 38.4 32.1 35.4 30.0 42.7 28.9 33.4 28.2 28.1 6.9 14.3 17.9 20.0 18.7 32.2 28.9 72.0 74.0 29.7 213.2 53.3 42.7 41.2 18.1 53.1 36.9 52.4 32.7 29.5 39.7 38.0 30.2 35.3 30.0 42.4 31.3 40.2 28.2 38.2 6.8 14.3 17.5 20.9 17.7 32.0 31.8 183.1 27.6 41.2 213.0 57.9 41.7 40.7 17.8 51.5 37.4 59.0 34.8 30.4 37.5 38.4 32.6 29.3 42.4 38.5 34.1 32.8 31.0 22.0 6.6 14.3 17.0 19.0 16.1 177.0 26.7 79.7 19.3 36.3 71.9 52.9 37.4 40.9 17.4 52.6 37.0 59.6 35.6 30.9 37.8 38.6 32.3 29.2 44.5 37.6 34.5 28.2 35.1 32.2 9.6 14.3 17.4 20.3 18.3 183.7 29.5 34.2 22.3 41.1 212.1 58.0 42.1 40.7 18.3 53.2 43.8 58.9 51.3 213.1 54.1 42.4 32.1 27.2 47.2 30.3 35.8 28.7 32.8 34.0 6.9 14.5 17.6 20.1 19.0 209.5 34.0 29.1 25.0 41.6 213.3 58.1 43.1 42.2 17.9 52.8 44.1 60.1 76.9 42.0 41.1 38.2 32.4 35.9 30.0 42.5 35.4 28.1 32.7 39.2 6.9 14.8 12.9 20.1 19.5 32.0 31.7 35.0 22.3 41.1 212.0 58.1 42.2 40.9 18.6 52.2 44.4 59.3 51.2 214.3 55.6 44.0 30.0 29.3 34.5 33.1 33.7 28.3 36.1 31.0 6.9 14.6 18.4 18.1 19.7 68.5 33.3 33.6 22.3 41.3 212.8 58.2 41.9 41.1 18.2 52.1 38.4 59.4 47.2 73.0 44.8 40.5 37.5 32.1 36.0 40.3 31.7 28.3 33.4 29.3 6.8 14.6 19.5 19.2 12.2 67.8 34.0 32.9 22.17 41.34 212.20 57.91 41.96 40.82 18.54 52.32 37.51 58.99 35.24 28.95 38.96 40.59 50.17 218.38 45.68 43.24 30.14 32.68 27.04 31.14 6.87 14.56 17.25 20.27 15.93 27.44 74.06 25.84 22.22 41.43 212.78 58.04 42.14 41.11 18.41 53.32 37.42 59.31 35.61 30.74 40.06 39.11 44.28 74.40 36.45 44.09 30.36 33.14 27.51 36.45 6.88 14.60 18.06 20.08 21.43 25.44 74.45 25.71
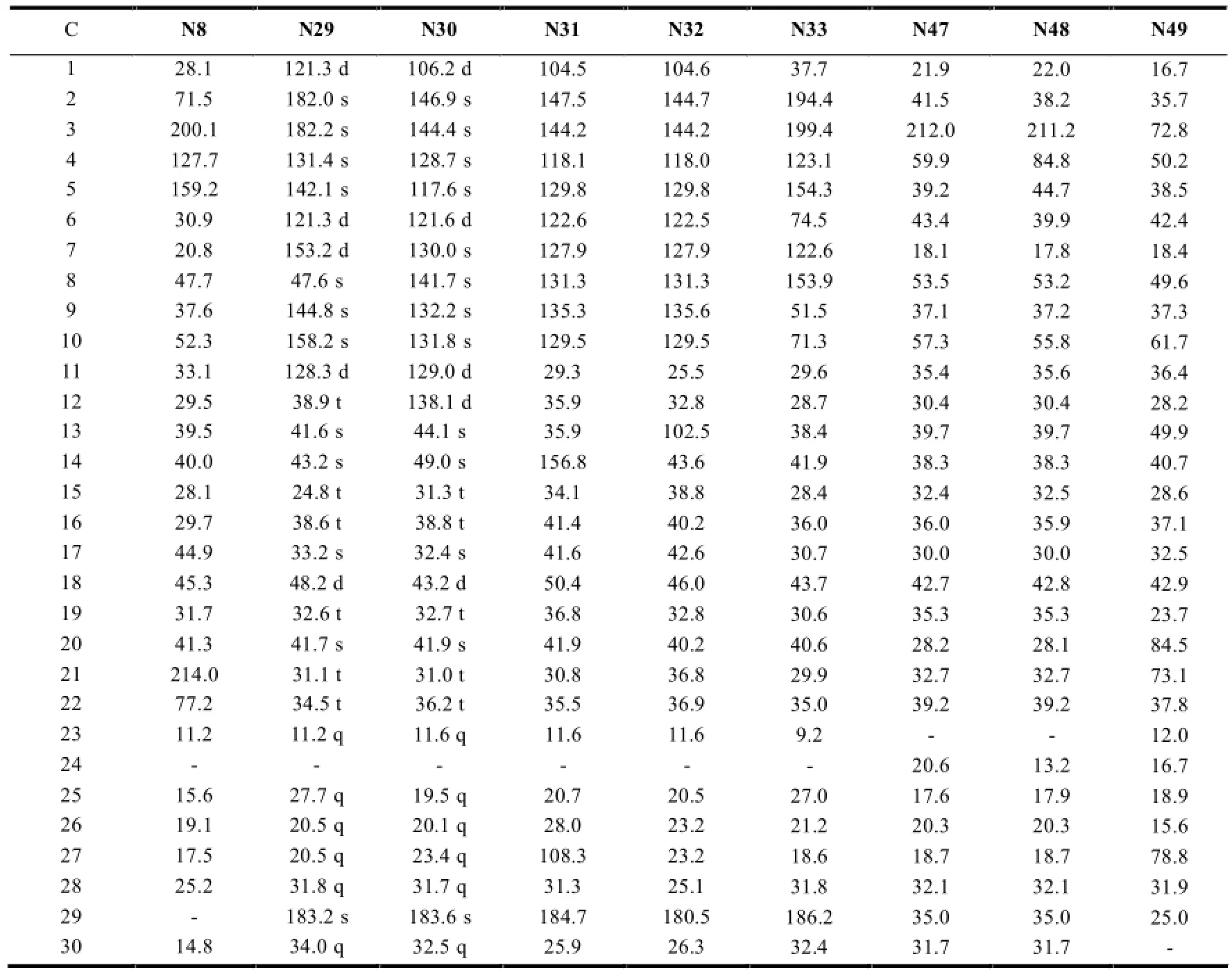
表3 降碳木栓烷型三萜的13C-NMR数据
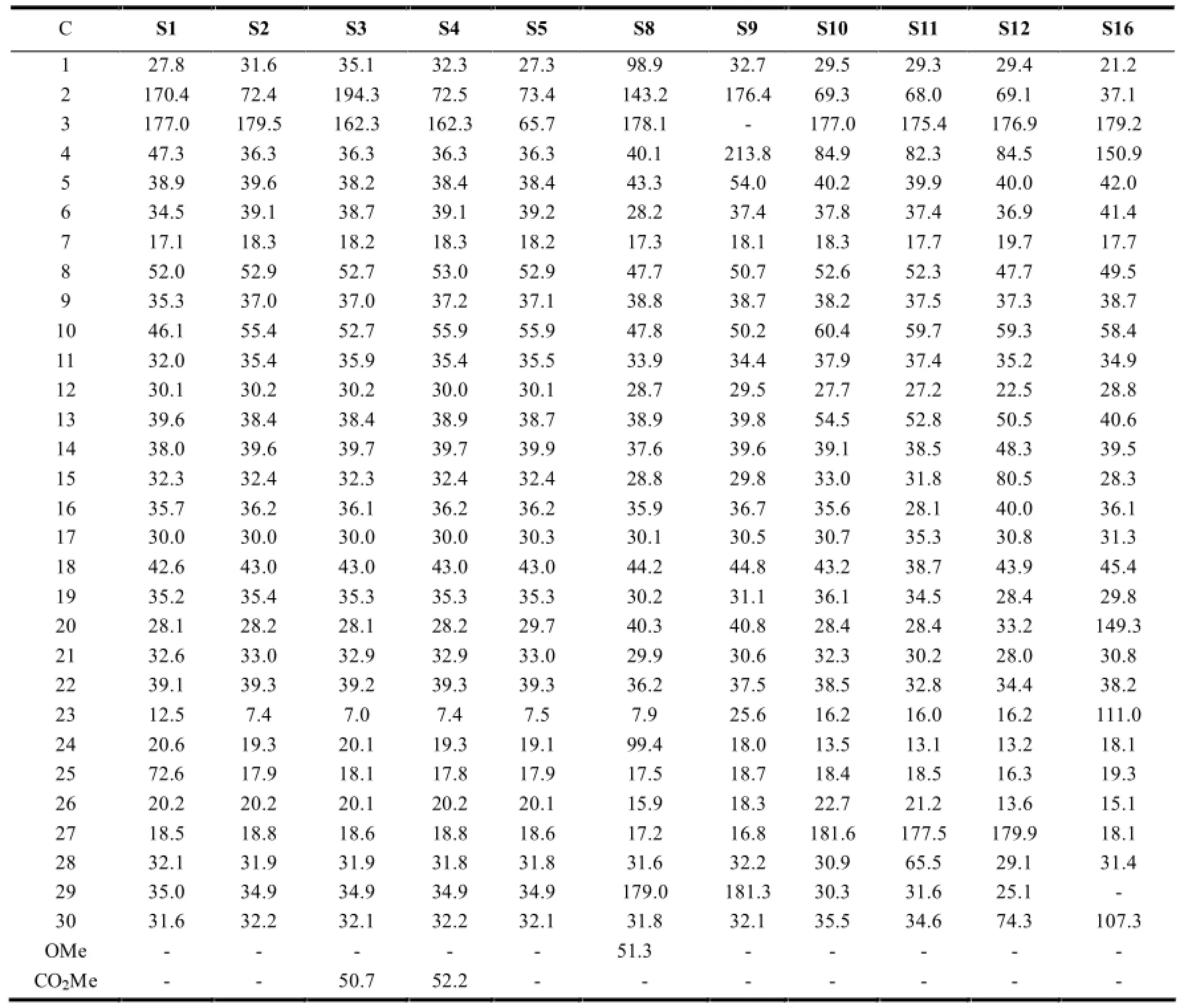
表4 开环木栓烷型三萜的13C-NMR数据
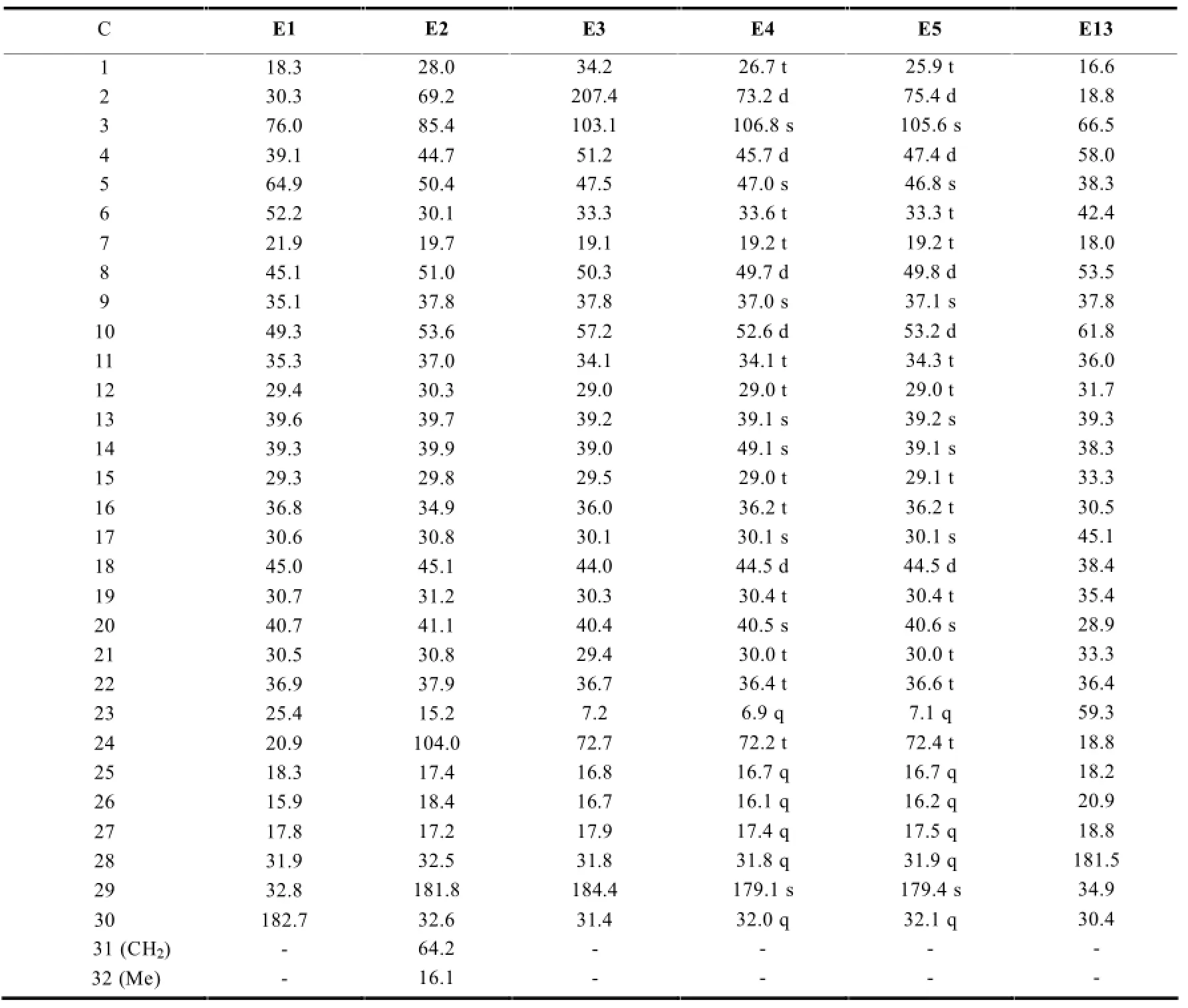
表5 环氧木栓烷型三萜的13C-NMR数据
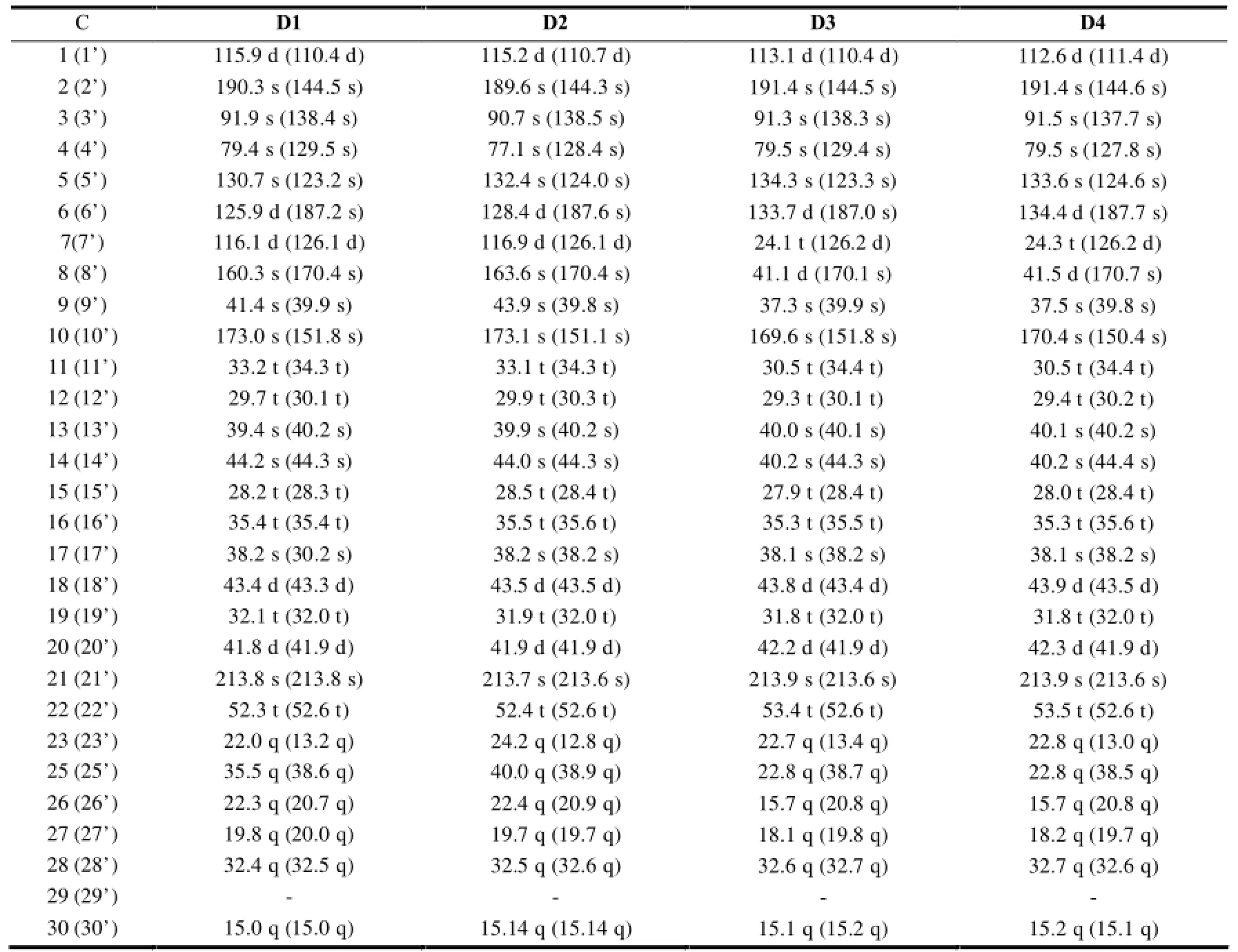
表6 二聚体类木栓烷型三萜的13C-NMR数据

续表6
2.2 木栓烷型三萜的1H-NMR特征
在木栓烷型三萜氢谱中,主要包括甲基(-CH3)信号、亚甲基(-CH2-)信号、次甲基(-CH-)信号、双键质子(CH=CH)信号、以及常见的羟基(-OH)氢信号和羧基(-COOH)醛基(-CHO)等官能团的活泼氢信号。
在木栓烷型三萜中,-CH3的氢化学位移在δ0.6-1.9之间,23-H一般以二重峰(d峰)出现,一般在δ1.0左右;非取代基直接连接的-CH2-一般在δ0.75-2.6之间,同一碳上的两个氢因空间位置不同,位移相差δ0-0.9左右;非取代基直接相连的-CH-氢信号一般在δ0.67-2.75左右,4-H因与23-H相互偶合,一般以四重峰(q峰)出现。10和18-H则列分为2个二重峰(dd峰)。有OH等取代时,同碳H的δ值明显向低场移动。表7~11综述了具代表性的五类木栓烷型三萜的1H-NMR数据特征。
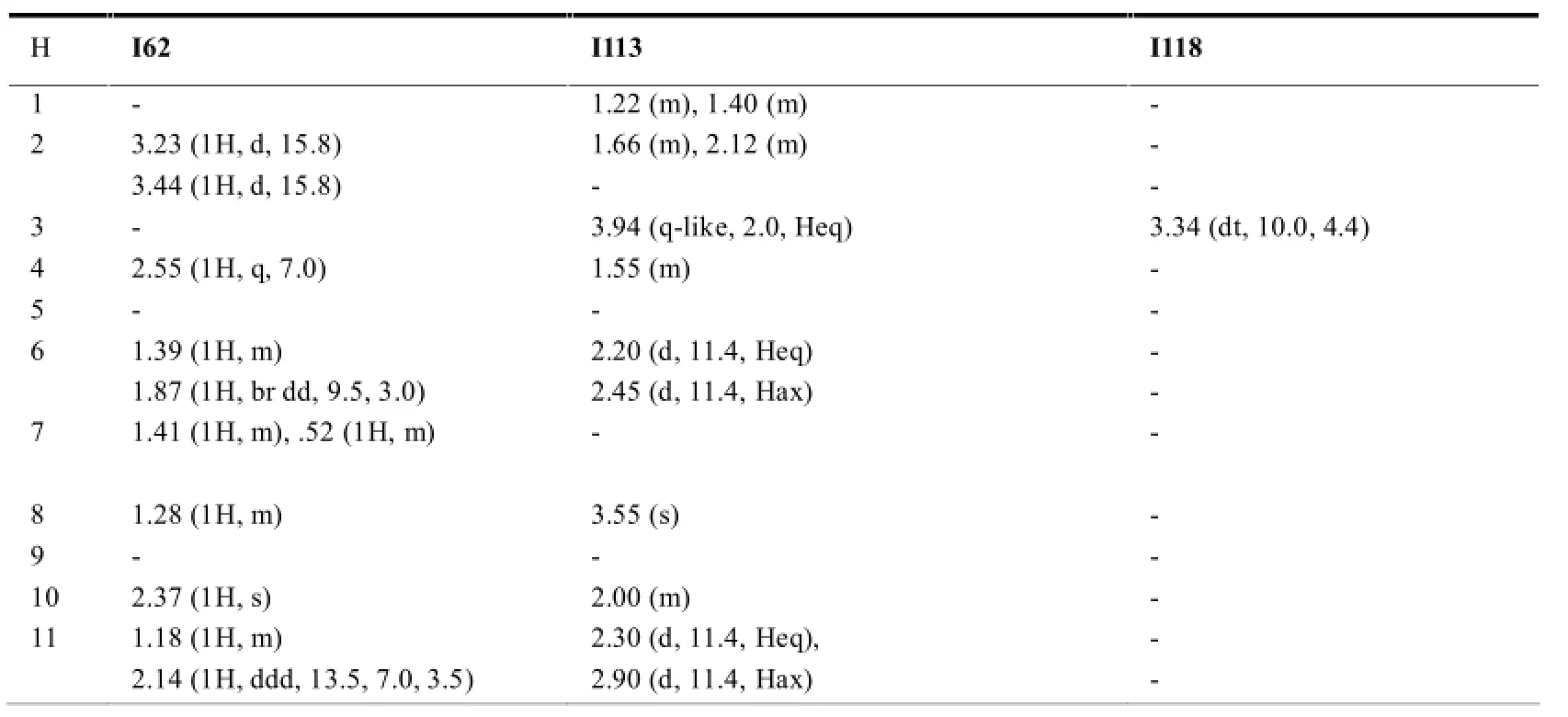
表7 正常结构木栓烷型三萜的1H-NMR数据(mult,J in Hz)
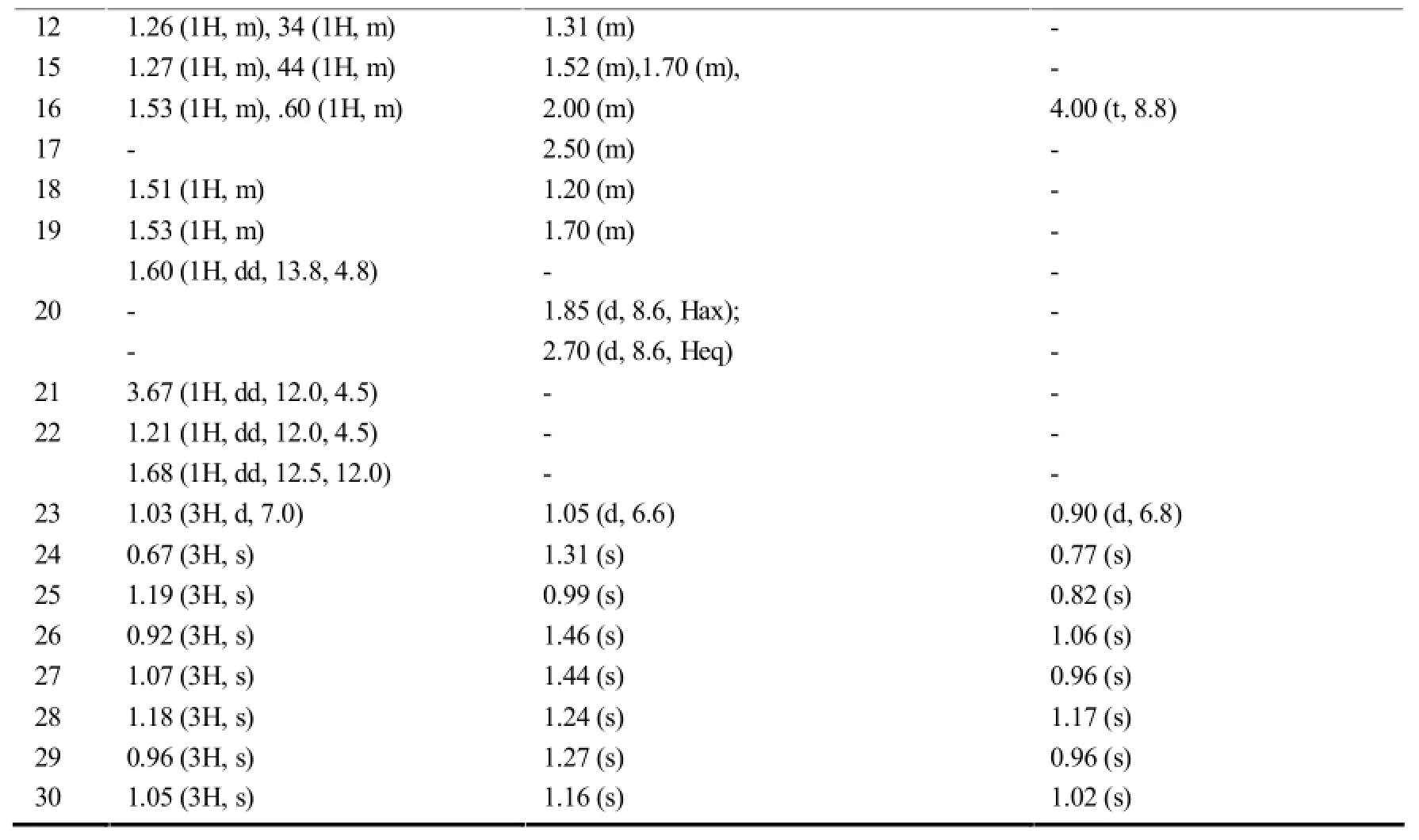
续表7
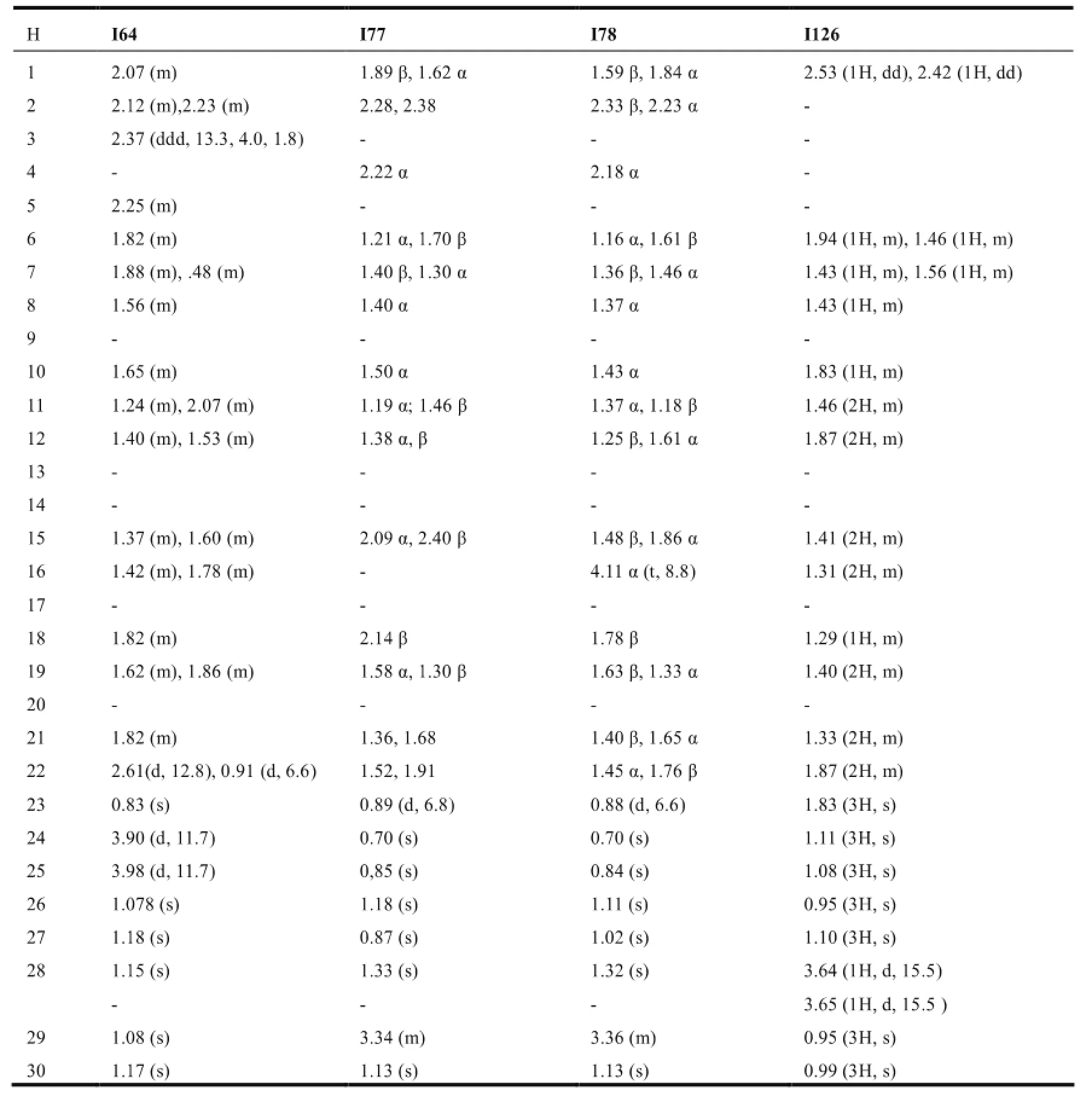
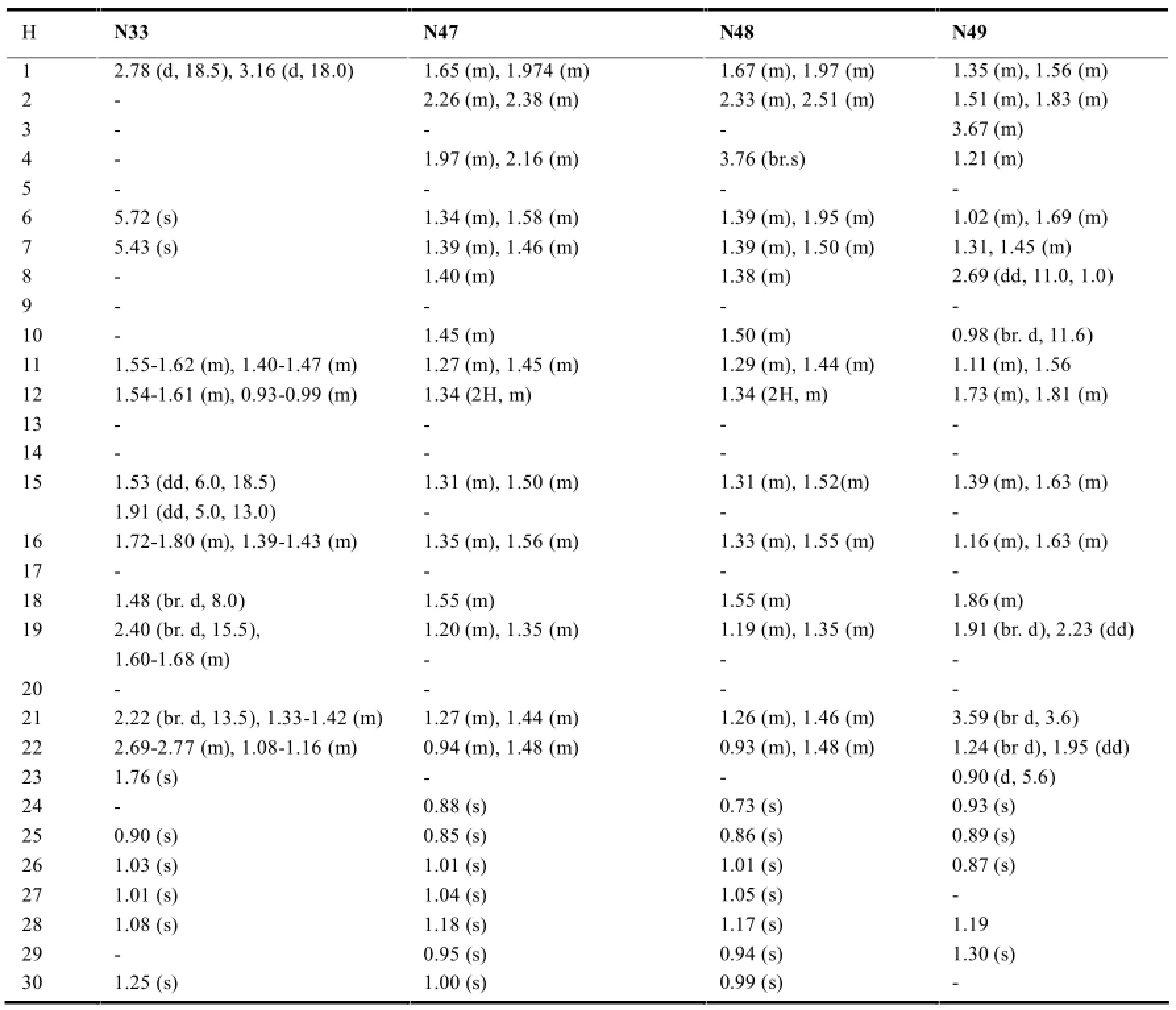
表8 降碳木栓烷型三萜的1H-NMR数据(mult,J in Hz)
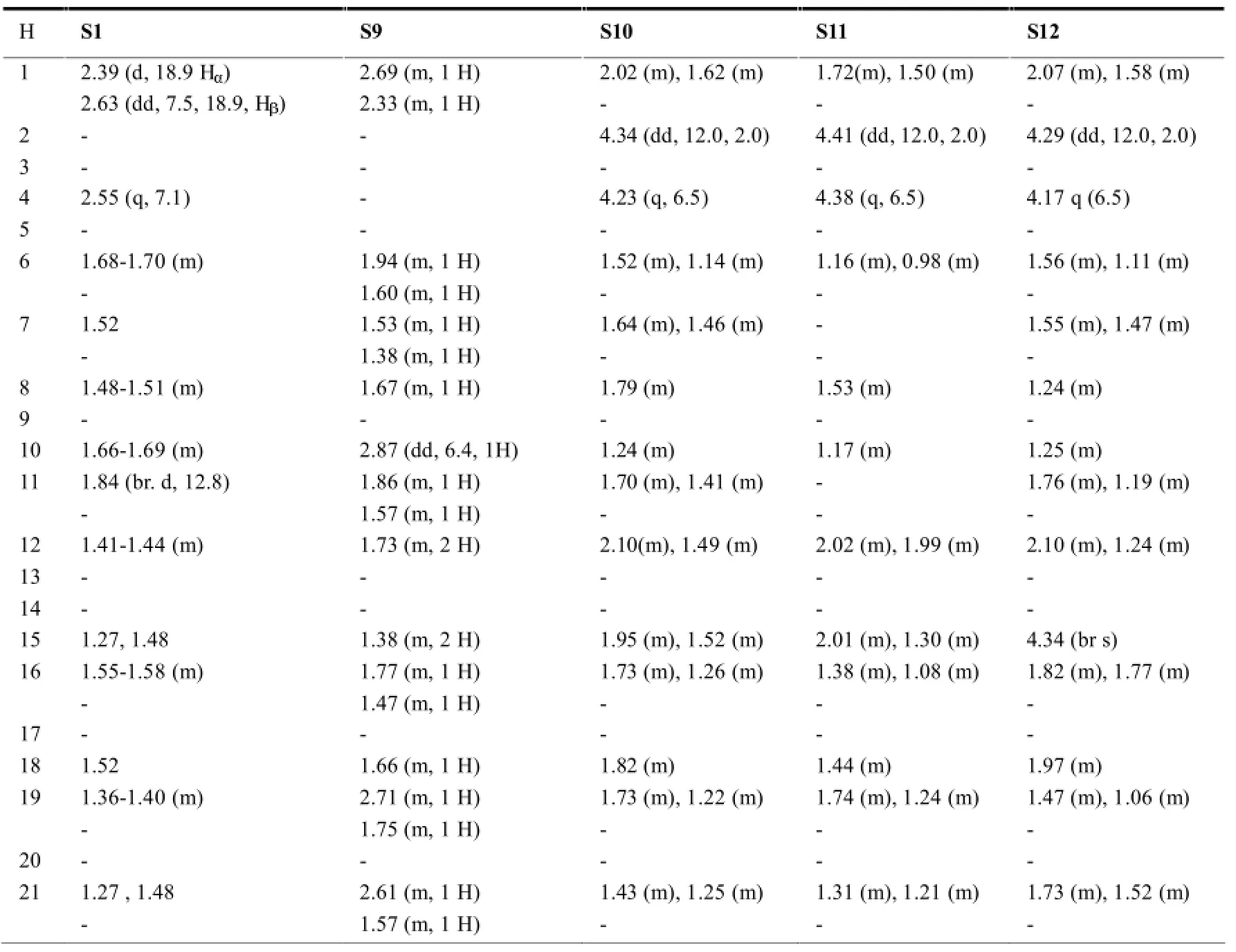
表9 开环木栓烷型三萜的1H-NMR数据(mult,J in Hz)
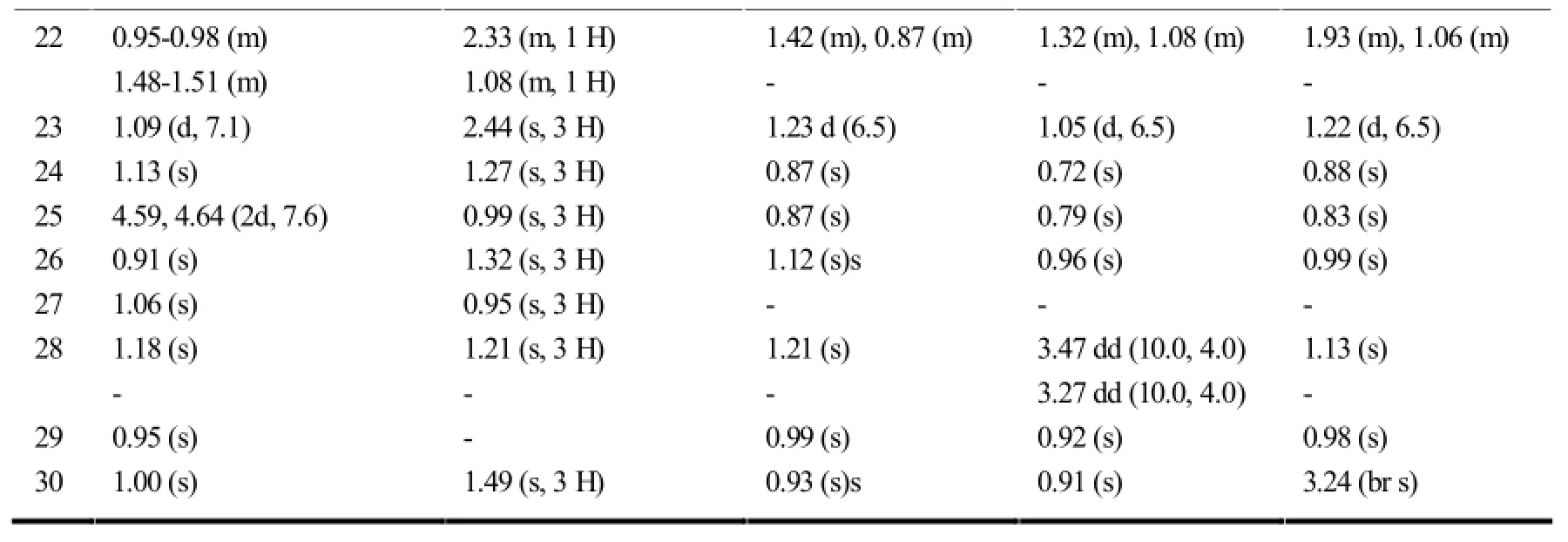
续表9
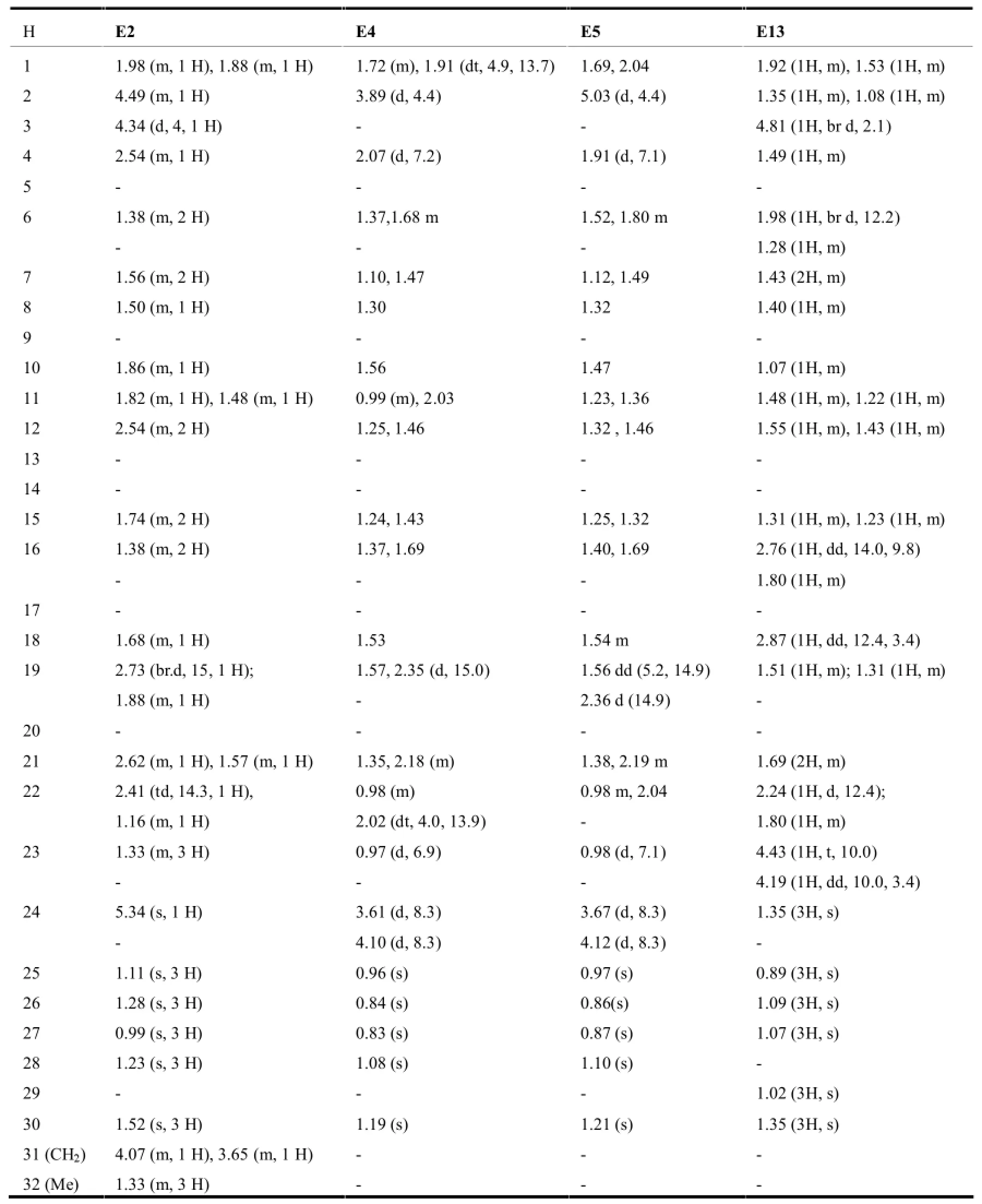
表10 环氧木栓烷型三萜的1H-NMR数据(mult,J in Hz)
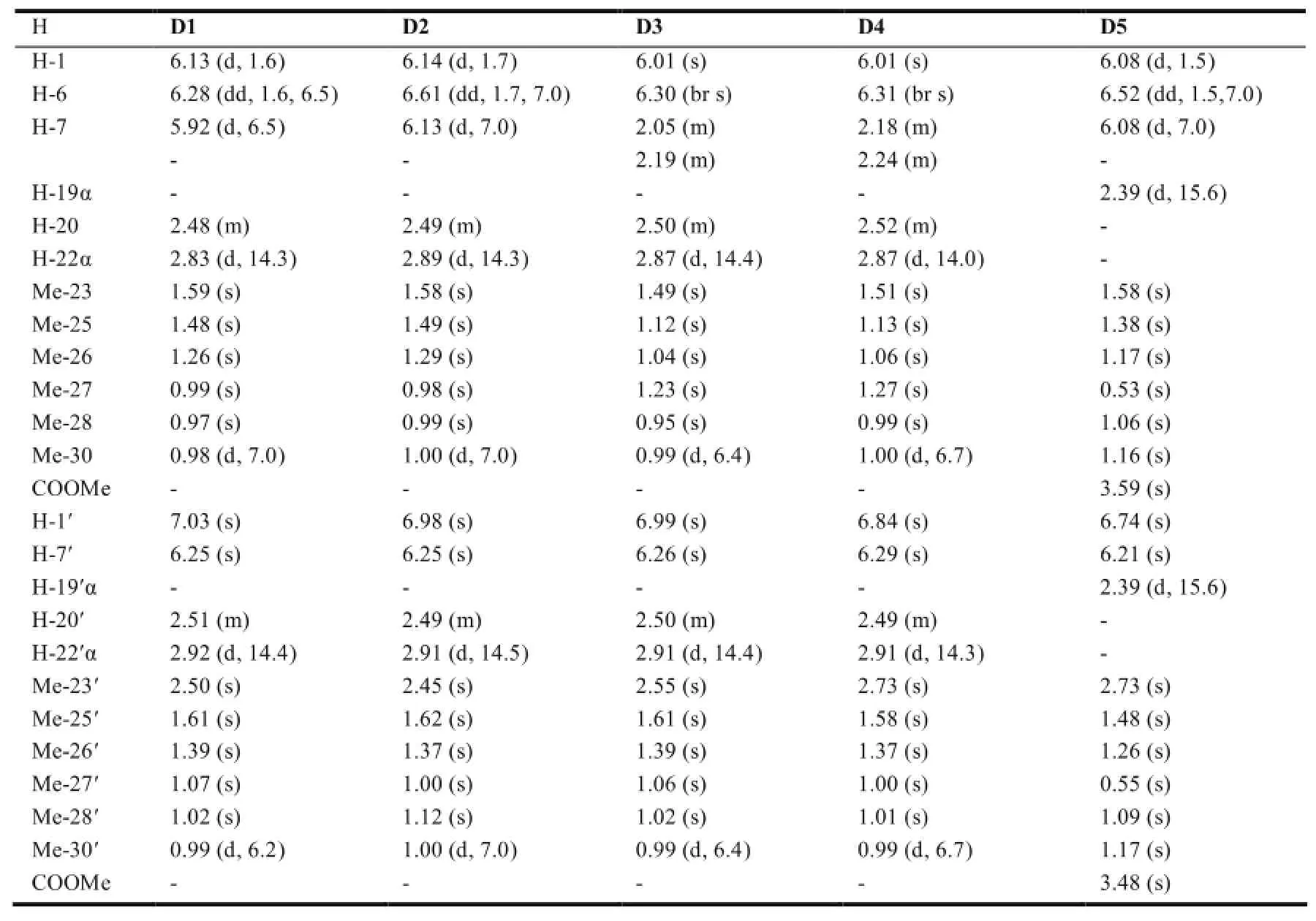
表11 二聚体类木栓烷型三萜的1H-NMR数据(mult,J in Hz)
3 结论
木栓烷型三萜是五环三萜中具有潜在药用价值的一类,其中一些化合物表现出了良好的生物活性,例如雷公藤红素(celastrol,N36)、卫矛酮(tingenone, N37)、扁塑藤素(pristimerin,N44)、violaic A(S10)、violaic B(S11)、violalide(S12)等,尤其是最近发现的结构独特且生物活性好的醌甲基化物降碳类、开环类、环氧类木栓烷型三萜,引起了许多相关研究者的极大关注。本文主要总结了近几十年来天然来源的木栓烷型三萜的结构特征,对这些化合物的NMR数据特征进行了分类归纳,以期对木栓烷型三萜化合物的结构解析研究提供一定的参考。
[1]Itokawa H,Takeya K,Hitotsuyanagi Y,et al.Anti Tumor Compounds Isolated from Higher Plants[J].Studies in Natural Products Chemistry,2000,24:269-350.
[2]Rios JL,Recio MC,Manez S,et al.Natural Triterpoids as Anti-inflammatory Agents[J].Studies in Natural Products Chemistry,2000,22:93-143.
[3]Chen K,Shi Q,Kashiwada Y,et al.Anti-AIDS Agents,6. Salaspermic Acid,an Anti-HIV Principle from Tripterygium wilfordii,and the Structure-Activity Correlation with Its Related Compound[J].Journal of Natural Products,1992,55(3):340-346.%
[4]Patra A,Chaudhuri SK.Studies on triterpenoids:Conversion of friedelanones into some secofriedelanes[J].Indian Journal of Chemistry,1989,28B:376-380.
[5]Nozaki H,Suzuki H,Lee KH,et al.Structure and StereochemistryofMaytenfolicAcidandMaytanfoliol,TwoNewAntileukemic Triterpenes from Maytenus dioversifolia:X-Ray Crystal Structures[J].Journal of the Chemical Society,Chemical Communications,1982,18:1048-1051.
[6]CarvalhoPRF,SilvaDHS,BolzaniVS,etal.Antioxidant Quinonemethide triterpenes from Salacia campestris[J].Chemistry&Biodiversity,2005,2:367-372.
[7]Gunatilaka AAL,Nanyakkara NPD,Sultanbawa MUS.Studies on terpenoids and Steroids.Part 1.Structure of six Novel 27-Hydroxy and 6β-Hydroxy Di-and Tri-oxygenated D:A-friedo-Oleanane Triterpenes fromKokoona zeylanica[J].Journal of the Chemical Society,Perkin Transactions 1,1983:2459-2469.
[8]Gunatilaka AAL,Nanyakkara NPD.Studies on terpenoids and steroids-2,Structure of two new tri-and tetra-oxygenated D:A-friedo-oleanane triterpenes fromKokoona zeylanica[J].Tetrahedron,1984,40(4):805-809.
[9]Klass J,Tinto WF.Friedelane Triterpenoids fromPeritassa compta:Complete1H and13CAssignments by 2D NMR Spectroscopy [J].Journal of Natural Products,1992,55(11):1626-1630.
[10]Betancor C,Freire R,Gonzalez AG,et al.Three Triterpenes and other terpenoids from Catha cassinoides[J].Phytochemistry,1980,19:1989-1993.
[11]Anjaneyulu ASR,Rao MN.Elaeodendrol and Elaedendradiol, New Nortriterpenes from Elaeeodendron glaucum[J].Phytochemistry,1980,19:1163-1169.
[12]Waeretunga C,Kumer V,Sultanbawa MS.Two new angular methyldioxygenatedD:A-friedo-oleananes[J].Tetrahe-dron Letters,1982,23(19):2031-2032.
[13]Weeratunga G,Kumar V,Sultanbawa MS.D:A-Friedelanes fromElaeodendron glaucum[J].Australian Journal of Chemistry, 1983,36(5):1067-1072.
[14]Weeratunga G,Kumar V,Sultanbawa MUS,et al.28,29-dihydroxyfriedelane-3-one,afriedelanewithtwooxygenated methyl groups,from Elaeodendron balae(Celastraceae)[J]. Journal of the Chemical Society,Perkin Transactions 1,1982, 2457-2459.
[15]Martinez MV,Corona MM,Velez CS,et al.Terpenoids from Mortonia diffusa[J].Journal of Natural Products,1988,51(4): 793-796.
[16]Nozaki H,Suzuki H,Lee KH,et al.Structure and stereochemistryofmaytenfolicacidandmaytenfoliol,twonewantileukemic triterpenes from Maytenus diversifolia:X-ray crystal structures[J].Journal of the Chemical Society,Chemical Communications,1982,18:1048-1051.
[17]Nozaki H,Suzuki H,Hirayama T,et al.Antitumour triterpenes of Maytenus diversifolia[J].Phytochemistry,1986,25(2):479-485.
[18]Nozaki H,Matsuura Y,Hirono S,et al.Maytensifolin-C,a friedelane alcohol from Maytenus diversifolia[J].Phytochemistry,1991,30(11):3819-3821.
[19]Queiroga CL,Silva GF,Dias PC,et al.Evaluation of the antiulcerogenic activity of friedelan-3β-ol and friedelin isolated from Maytenus ilicifolia(Celastraceae)[J].Journal of Ethnopharmacology,2000,72:465-468.
[20]Salazar GCM,Silva GDF,Duarte LP,et al.Two epimeric friedelane triterpenes isolated from Maytenus truncata Reiss:1H and13C chemical shift assignments[J].Magnetic Resonance in Chemistry,2000,38:977-980.
[21]Oliveira MLG,Duarte LP,Silva GDF,et al.3-oxo-12α-hydroxyfriedelane from Maytenus gonoclada:structure elucidation by1H and13C chemical shift assignments and 2D NMR spectroscopy[J].Magnetic Resonance in Chemistry,2007,45:895-898.
[22]Andrade de SF,Comunello E,Noldin VF,et al.Antiulcerogenic activity of fractions and 3,15-dioxo-21α-hydroxyfriedelane isolated from Maytenus robusta(Celastraceae)[J].Archives of Pharmacal Research,2008,31(1):41-46.
[23]Chavez H,Braun AE,Ravelo AG,et al.Friedelane triterpenoids from Maytenus macrocarpa[J].Journal of Natural Products,1998,61:82-85.
[24]ItokawaH,ShirotaO,IkutaH,etal.Triterpenesfrom Maytenus ilicifolia[J].Phytochemistry,1991,30(11): 3713-3716.
[25]Rodriguez FM,Perestelo NR,Jimenez IA,et al.Friedelanes from Crossopetalum lobatum.A New Example of a Triterpene Anhydride[J].Helvetica Chimica Acta,2009,92:188-194.
[26]Sousa de JR,Silva GDF,Pedersoli JL,et al.Friedelane and oleanane triterpenoids from bark wood of Austroplenckia populnea[J].Phytochemistry,1990,29(10):3259-3261.
[27]Filho SAV,Duarte LP,Santos MH,et al.Complete assignment of the1H and13C NMR pectral of a new polyester sesquiterpene from Austroplenckia populnea[J].Magnetic Resonance in Chemistry,2000,38:1023-1026.
[28]Silva GDF,Duarte LP,Filho SAV,et al.Epikatonic acid from Austro-plenckia populnea:structure elucidation by 2D NMR spectroscopy and X-ray crystallography[J].Magnetic Resonance in Chemistry,2002,40:366-370.
[29]Sun CR,Hu HJ,Xu RS,et al.A New Friedelane Type Triterpene from Euonymus hederaceus[J].Molecules,2009,14: 2650-2655.
[30]Prakash O,Roy R,Garg HS,et al.13C NMR studies of the friedelane series of triterpenoids and the conformation of the D and E ring in friedelan-7-one[J].Magnetic Resonance in Chemistry,1987,25:39-41.
[31]Sukumar E,Rao RB,Kundu AB.A friedelane triol from the roots of Pristimera grahamii[J].Phytochemistry,1990,29(9): 3044-3046.
[32]Wu XY,Qin GW,Fan AJ,et al.1-Hydroxy-2,5,8-trimethyl-9-fluorenone from Tripterygium wilfordii[J].Phytochemistry, 1994,36(2):477-479.
[33]MorotaT,YangCX,SasakiH,etal.Triterpenesfrom Tripterygium wilfordii[J].Phytochemistry,1995,39(5):1153-1157.
[34]Yang JH,Luo SD,Wang YS,et al.Triterpenes from Tripterygium wilfordii Hook[J].Journal of Asian Natural Products Research,2006,8(5):425-429.
[35]Zhang WJ,Pan DJ,Zhang LX,et al.Studies on triterpenoids of Tripterygium wilfordii Hook-f.[J].Acta Pharmaceutica Sinica,1986,21(8):592-598.
[36]Liu X,Wu D.Constituents of friedelane triterpenes fromCelastrus monospermus Roxb.[J].Chinese traditional and herbal drugs, 1993,24:395-397.
[37]Zhang K,Liu JL,Wang YH,et al.Constituents of triterpenes from Celastrus monospermus Roxb.[J].Acta Scientiarum Naturalium Sunyatseni,1998,37:85-88.
[38]Chen MX,Wang DY,Guo J.3-Oxo-11β-hydroxyfriedelane from the roots of Celastrus monospermus[J].Journal of Chemical Researches,2010,2:114-117.
[39]Somwong P,Suttisri R,Buakeaw A.A new 1,3-diketofriedelane triterpene from Salacia verrucosa[J].Fitoterapia,2011, 82:1047-1051.
[40]Silva FC,Rodrigues VG,Duarte LP,et al.A new friedelane triterpenoid from the branches of Maytenus gonoclada(Celastraceae)[J].Journal of Chemical Research,2011,10:555-557.
[41]Kaweetripob W,Mahidol C,Prawat H,et al.Lupane,friedelane,oleanane,andursanetriterpenesfromthestemof Siphonodon celastrineus Griff[J].Phytochemistry,2013,96: 404-417.
[42]Ardiles AE,González-Rodríguez A,Núñez MJ,et al.Studies of naturally occurring friedelane triterpenoids as insulin sensitizers in the treatment type 2 diabetes mellitus[J].Phytochemistry,2012,84:116-124.
[43]Chen MX,Wang DY,Guo J.3-oxo-11β-hydroxyfriedelane from the roots ofCelastrus monospermus[J].Journal of Chemical Research,2010,34(2):114-117.
[44]ChenMX.StudiesonFriedelaneTriterpenesofCelastrus monospermus Roxb.[D].Guangdong Pharmaceutical Uni-versity,2010.
[45]Sousa de GF,Soares DCF,Mussel W da N,et al.Pentacyclic Triterpenes from Branches of Maytenus robusta and in vitro Cytotoxic Property Against 4T1 Cancer Cells[J].Journal of the Brazilian Chemical Society,2014,25(8):1338-1345.
[46]RogersD,Phillips FL,Joshi BS,et al.Revised structures of the triterpenes Q,T,and U from Salacia prinoides DC;X-ray crystal structure of triterpene T[J].Journal of the Chemical Society,Chemical Communications,1980,22:1048-1049.
[47]Kumar V,Wazeer MIM,Wijeratne DBT.21α,26-Dihydroxy-D: A-friedo-oleanan-3-one from Salacia reticulata Var.Diandra (Celastraceae)[J].Phytochemistry,1985,24(9):2067-2069.
[48]Kumar V,Wijeratne DBT,Abeygunawardena C.21α,30-Dihydroxy-D:A-friedooleanan-3-one from Salacia reticulata Var.β-Diandra stem bark[J].Phytochemistry,1990,29(1):333-335.
[49]Gunatilaka AAL,Dhanabalasingham B,Karunaraine V.Studies on terpenoids and steriods.Part 27.Structure of a D:A-friedooleanane triterpenoid fromSalacia reticulata and revision of the structures of kokoonol and kokzeylanol series of triterpenoids[J].Tetrahedron,1993,49(45):10397-10404.
[50]Morikawa T,Kishi A,Pongpiriyadacha Y,et al.Structures ofnewfriedelane-typetriterpenesandeudesmanes-type sesquiterpene and aldose reductase inhibitors from Salacia chinensis[J].Journal of Natural Products,2003,66:1191-1196.
[51]Kishi A,Morikawa T,Matsuda H,et al.Structures of new friedelane-and nor-friedelane-type triterpenes and polyacylated eudesmane-type sequiterpene from Salacia chinesis Linn.(S. prinoides DC.,Hippocrateaceae)and radical scavenging activities of principal constituents[J].Chemical&Pharmaceutical Bulletin,2003,51(9):1051-1055.
[52]Agius BR,Vogler B,Stokes SL,et al.Inhibition of Cruzain by triterpenoids isolated from a Salacia species from Monteverde, Costa Rica[J].Natural Product Communications,2007,2(11): 1083-1084.
[53]Duarte LP,Miranda de RRS,Rodrigues SBV,et al.Stereochemistry of 16α-hydroxyfriedelin and 3-oxo-16-methylfriedel-16-ene established by 2D NMR spectroscopy[J].Molecules, 2009,14:598-607.
[54]Huang J,Guo ZH,Cheng P,et al.Three new triterpenoids from Salacia hainanensis Chun et How showed effective antia-glucosidase activity[J].Phytochemistry Letters,2012,5:432-437.
[55]Yu MH,Shi ZF,Yu BW,et al.Triterpenoids and α-glucosidase inhibitory constituents from Salacia hainanensis[J].Fitoterapia,2014,98:143-148.
[56]Anjaneyulu V,Ravi K.Terpenoids from Euphorbia antiquorum [J].Phytochemistry,1989,28(6):1695-1697.
[57]Wandji J,Wansi JD,Fuendjiep V,et al.Sequiterpene lactone and friedelane derivative from Drypetes molunduana[J].Phytochemistry,2000,54:811-815.
[58]Wandji J,Tillequin F,Mulholland DA,et al.Phenolic constituents from Drypetes armoracia[J].Phytochemistry,2003, 63:453-456.
[59]Chiozem DD,Dufat HTV,Wansi JD,et al.New Friedelane Triterpenoids with Antimicrobial Activity from the Stems of Drypetes paxii[J].Chemical&Pharmaceutical Bulletin,2009, 57(10):1119-1122.
[60]Fannang SV,Kuete V,Djama CM,et al.A new friedelane triterpenoid and saponin with moderate antimicrobial activity from the stems of Drypetes laciniata[J].Chinese Chemical Letters,2011,22:171-174.
[61]Wittayalai S,Mahidol C,Prachyawarakorn V,et al.Terpenoids from the roots of Drypetes hoaensis and their cytotoxic activities[J].Phytochemistry,2014,99:121-126.
[62]Li Y,Zuo WJ,Mei WL,et al.Three new terpenoids from Trigonostemon xyphophylloides(Croiz.)L.K.Dai and T.L.Wu [J].Phytochemistry Letters,2013,6:472-475.
[63]Awanchiri SS,Trinh-Van-Dufat H,Shirri JC,et al.Triterpenoids with antimicrobial activity from Drypetes inaequalis[J]. Phytochemistry,2009,70:419-423.
[64]Chen WH,Han CR,Hui Y,et al.Terpenoids from the Stems of Drypetes congestiflora[J].Helvetica Chimica Acta,2015, 98:724-730.
[65]Vincent C,Ange B,Serge R,et al.Composition and chemical variability of the triterpene fraction of dichloromethane extracts of cork(Quercus suber L.)[J].Industrial Crops and Products, 2002,15:15-22.
[66]Olmedo DA,perez JLL,Olmo del E,et al.A New Cytotoxic Friedelane Acid-Pluricostatic Acid-and Other Compounds from the Leaves of Marila pluricostata[J].Molecules,2008, 13:2915-2924.
[67]Lannang AM,Noudou BS,Sewald N.Ovalifolone A and B: New friedelane derivatives fromGarcinia ovalifolia[J].Phytochemistry Letters,2013,6:157-161.
[68]Giner RM,Gray AI,Gibbons S,et al.Friedelane Triterpenes from the Stem bark of Caloncoba glauca[J].Phytochemistry, 1993,33(1):237-239.
[69]Tane P,Tsopmo A,Ngnokam D,et al.New Friedelane triterpenes from Leppidobotrys staudtii[J].Tetrahedron,1996,52 (47):14989-14994.
[70]Laure F,Herbette G,Faure R,et al.Structures of new secofriedelane and friedelane acids from Calophyllum inphyllum of French Polynesia[J].Magnetic Resonance in Chemistry,2005, 43:65-68.
[71]Li LY,Huang XS,Sattler I,et al.Structure elucidation of a new friedelane triterpene from the mangrove plant Hibiscus tiliaceus[J].Magnetic Resonance in Chemistry,2006,44: 624-628.
[72]MensahIA,KumiSA,WaibelR,etal.AnovelD:A-friedooleanane triterpenoid and other constituents of the stem bark of Dichapetalum barteri Engl.[J].Arkivoc,2007,ix:71-79.
[73]Chen HY,Lin CW,Chen GY,et al.3β-hydroxyfriedelan-17β-carboxy-lic acid[J].Acta Crystallographica Section E: Structure Reports Online,2008,E64:o890.
[74]Setzer WN,Setzer MC,Peppers RL,et al.Triterpenoids Constituents in the Bark of Balanops australiana[J].Australian Journal of Chemistry,2000,53(9):809-812.
[75]Merfort I,Buddrus J,Nawwar MAM,et al.A triterpene from the bark of Tamarix aphylla[J].Phytochemistry,1992,31(11):4031-4032.
[76]Chang CW,Wu TS,Hsieh YS,et al.Terpenoids of Syzygium formosanum[J].Journal of Natural Products,1999,62:327-328.
[77]Ankli A,Heilmann J,Heinrich M,et al.Cytotoxic cardenolides and anti-bacterial terpenoids from Crossopetalum gaumeri[J]. Phytochemistry,2000,54:531-537.
[78]Oliveira DM de,Silva GD de F,Duarte LP,et al.Chemical constituentsisolatedfromrootsofMaytenusacanthophylla Reissek(Celastraceae)[J].Biochemical Systematics and Ecology,2006,34(8):661-665.
[79]Nakano K,Oose Y,Masuda Y,et al.A diterpenoid and triterpenes from tissue cultures of Tripterygium wilfordii[J].Phytochemistry,1997,45(2):293-296.
[80]Nakano K,Oose Y,Takaishi Y,et al.A novel epoxy-triterpene and nortriterpene from callus cultures ofTripterygium wilfordii[J].Phytochemistry,1997,46(7):1179-1182.
[81]Takaishi Y,Miyagi K,Kawazoe K et al.Terpenoids from Tripterygium wilfordii Var.regelii[J].Phytochemistry,1997,45 (5):975-978.
[82]Duan H,Takaishi Y,Momota H,et al.Immunosuppressive terpenoids from extract of Tripterygium wilfordii[J].Tetrahedron, 2001,57:293-296.
[83]YangGZ,LiCY,LiYC.Studyofanewtriterpenoid fromTripterygiumwilfordii[J].ChineseJournalofOrganic Chemistry,2006,26(11):1529-1532.
[84]Yang GZ,Li YC.Antitumor Triterpenoids fromTripterygium wilfordii Hook f.[J].Chemistry and Industry of Forest Products,2006,26(4):19-22.
[85]Yoshihisa T,Noriko W,Hideo T,et al.Triterpenoid inhibitors from Tripterygium wilfordii Var.regelii[J].Phytochemistry, 1997,45(5):969-975.
[86]LiKH,DuanHQ,KazuyoshiK,etal.Terpenoidsfrom Tripterygium wilfordii[J].Phytochemistry,1997,45(4):791-796.
[87]Duan H,Kawazoe K,Bando M,et al.Di-and Tri-terpenoids from Tripterygium hypoglaucum[J].Phytochemistry,1997,46 (3):535-543.
[88]MorotaT,YangCX,QinWZ,etal.D:A-friedo-24-noroleanane triterpenoids fromTripterygium wilfordii[J]. Phytochemistry,1995,39(5):1159-1163.
[89]Wu J, Zhou Y,Wang LY,et al.Terpenoids from root bark of Celastrus orbiculatus[J].Phytochemistry,2012,75:159-168.
[90]Ying YM,Li CY,Chen Y,et al.Lupane-and Friedelane-Type Triterpenoids from Celastrus stylosus[J].Chemistry& Biodiversity,2015,12:1222-1228.
[91]Chang FR,Hayashi KI,Chen IH,et al.Antitumor Agents. 228.Five New Agarofurans,Reissantins A-E,and Cytotoxic Principles from Reissantia buchananii[J].Journal of Natural Products,2003,66:1416-1420.
[92]CarvalhoPRF,SilvaDHS,BolzaniVS,etal.Antioxidant quinonemethild Triterpenes from Salacia campestris[J].Chemistry&Biodiversity,2005,2:367-372.
[93]He YF,Sun YW,Chen DL,et al.hainanenone A:a new friedelanetriterpenoidfromtheleavesandstemsof Drypetes hainanensis[J].Chemistry of Natural Compounds, 2015,51(2):273-275.
[94]Chen DL,Cheng X,Sun YW,et al.A New Friedelane Triterpenoid Possessing Cytotoxicity from the Leaves and Stems of Drypetes hainanensis[J].Chemistry of Natural Compounds, 2014,50(1):93-96.
[95]Mpetga JDS,He HP,Hao XJ,et al.Further cycloartane and friedelane triterpenoids from the leaves of Caloncoba glauca [J].Phytochemistry Letters,2014,7:52-56.
[96]Giner RM,Gray AI,Lavaud C,et al.30-Norfriedelane Triterpenes from the Stem bark of Caloncoba glauca[J].Phytochemistry,1992,31(1):223-225.
[97]Camacho MDR,Phillipson JD,Croft SL,et al.Assessment of the antipro-Tozoal activity of Galphimia glauca and the isolation of new nor-secofriedelanes and norfiredelanes[J].Journal of Natural Products,2002,65:1457-1461.
[98]Núñez MJ,Ardiles AE,Martínez ML,et al.Unusual D:B-friedobaccharane and oxygenated friedelane-type triterpenoids fromSalvadoreanCelastraceaespecies[J].Phytochemistry Letters,2012,5(2):244-248.
[99]Yang GZ,Yin XQ,Li YC.Chemical constituents of Tripterygium wilfordii[J].Helvetica Chimica Acta,2000,83(12): 3344-3350.
[100]Dai JJ,Tao HM,Min QX,et al.Anti-hepatitis B virus activities of friedelolactones from Viola diffusa Ging[J].Phytomedicine,2015,22:724-729.
[101]SutthivaiyakitS,NakornNN,KrausW,etal.Anovel 29-nor-3,4-seco-friedelane triterpene and a new guaiane sesquiterpene from the roots of Phyllanthus oxyphyllus[J]. Tetrahedron,2003,59:9991-9995.
[102]Setzer WN,Shen X,Bates RB,et al.A phytochemical investigation of Alchornea latifolia[J].Fitoterapia,2000,71:195-198.
[103]Duan H,Takaishi Y,Momota H,et al.Triterpenoids from Tripterygium wilfordii[J].Phytochemistry,2000,53(7):805-810.
[104]Anu SJ,Rao JM.New norfriedelene-1,3-dione from the root bark ofSalacia oblonga[J].Indian Journal of Chemistry SectionB-OrganicChemistryincludingMedicinalChemistry, 2003,42(5):1180-1182.
[105]Pradhan BP,Hassan A,Shoolery JN.Three new friedelane lactones from the bark ofGynocardia odorata(Flacourtiaceae) [J].Tetrahedron Letters,1984,25(8):865-868.
[106]Li YZ,Li ZL,Yin SL,et al.Triterpenoids from Calophyllum inophyllumandtheirgrowthinhibitoryeffectsonhuman leukemia HL-60 cells[J].Fitoterapia,2010,81:586-589.
[107]Shirota O,Morita H,Takeya K,et al.Five New Triterpene Dimers from Maytenus chuchuhuasca[J].Journal of Natural Products,1997,60:1100-1104.
[108]Itokawa H,Shirota O,Morita H,et al.New triterpene dimers from Maytenus ilicifolia[J].Tetrahedron Letters,1990,31: 6881-6882.
(本文编辑 苏维)
NMR Spectral Characteristics of Natural Friedelanes:A Review
LIU Xiangqian1,LI Xiaojun1,2,KIM Youn-chul2,YOOK Chang-soo3
(1.School of Pharmacy,Hunan University of Chinese Medicine,Changsha,Hunan 410208,China;
2.School of Pharmacy,Wonkwang University,Iksan 570-749,Korea;
3.School of Pharmacy,KyungHee University,Seoul 130-701,Korea)
The friedelane-type triterpenoids from natural products were studied in this paper including their chemical structures and spectral characteristics of13C-NMR,1H-NMR,so as to provide reference for reducing the blindness and repeatability of structure identification,and contribute to reducing some difficulties in the structure identification of friedelanetype triterpenoids,and provide theoretical basis for further research and analysis of friedelane-type triterpenoids.
friedelane-type triterpenoids;NMR spectral characteristics;13C-NMR;1H-NMR
R284.1
A
2016-04-12
湖南省中医药科研计划项目(2013136);湖南中医药大学药物分析学“十二五”校级重点学科建设项目;湖南省中药学重点学科建设项目。
刘向前,男,博士,教授,研究方向:天然产物活性成分研究,生药活性成分与质量评价研究,中药化学与分析;E-mail: lxq0001cn@163.com。
