Plasticity in Metamorphic Traits of Rice Field Frog (Rana limnocharis) Tadpoles: The Interactive Effects of Rearing Temperature and Food Level
2017-01-20TongleiYUGuifangYANGMichaelBUSAMandYaohuiDENG
Tonglei YU, Guifang YANG, Michael BUSAMand Yaohui DENG
1Department of Biology, College of Life Science, Xinyang Normal University, Xinyang 464000, Henan, China
2College of Agriculture and Natural Resources, University of Maryland, College Park, MD 20742, USA
Plasticity in Metamorphic Traits of Rice Field Frog (Rana limnocharis) Tadpoles: The Interactive Effects of Rearing Temperature and Food Level
Tonglei YU1*, Guifang YANG1, Michael BUSAM2and Yaohui DENG1
1Department of Biology, College of Life Science, Xinyang Normal University, Xinyang 464000, Henan, China
2College of Agriculture and Natural Resources, University of Maryland, College Park, MD 20742, USA
In organisms with complex life cycles, such as amphibians, morphological variation is strongly infuenced by environmental factors (e.g. temperature) and maternal effects (e.g. diet). Although temperature and food level exert a strong infuence on larval growth, little is known about the interacting effects of these factors on age and size at metamorphosis. In this study, plasticity in growth rates, survival, larval period, and size at metamorphosis were examined in Rice feld Frog (Rana limnocharis) under different combinations of rearing temperature and food level. Rearing temperature did not affect age at metamorphosis, but a signifcant interaction between temperature and food level revealed that of tadpoles feeding at a high food level, those reared at 32°C had a shorter length of larval period than those reared at 29°C or 26°C. Similarly, our results also showed high food level produced a larger growth rate and mass at metamorphosis at 32°C, but not at 29 and 26°C. Therefore, our results revealed that the effects of food level on larval growth and metamorphosis were highly dependent on developmental temperature.
metamorphosis, Rana limnocharis, larval period, phenotypic plasticity, rearing temperature, food level
1. Introduction
In animals with complex life cycles, such as amphibians, metamorphic size and timing of metamorphosis are important fitness components (Arnold and Wassersug, 1978; Wilbur, 1980). Larval amphibians are particularly likely to encounter variation in temperature and resource availability due to variation in aquatic breeding habitats (Morey and Reznick, 2004; Skelly, 2004). In addition to energy uptake, temperature can be considered the most important proximal cause of variation in size and age at metamorphosis (Newman, 1998; reviewed by Álvarez and Nicieza, 2002; Castano et al., 2010). Generally, larval anurans living in cold temperatures have prolonged developmental periods, but they are also larger as metamorphs than conspecifics living at warmer temperatures. Thus, some previous studies have found that age and size at metamorphosis may be larger in anurans living in cold temperatures (von Bertalanffy, 1960; Atkinson, 1996; Beck and Congdon, 2000; Merila et al., 2000; Álvarez and Nicieza, 2002; Laugen et al., 2003; Palo et al., 2003; Liess et al., 2013). However, in low temperatures, an increase in the time required to process food can set a limit for intake rates, which would reduce the gain associated with the higher energy value of the diet (reviewed by Álvarez and Nicieza, 2002).
Other factors affect metamorphic timing and growth rate, such as food supply, water level, and the type and density of competitors and predators (Rose, 2005). High food availability accelerates growth and developmental rates, thus allowing tadpoles either to maximize size at metamorphosis or minimize the length of the larval phase (Pandian and Marian, 1985; Arendt and Hoang, 2005; Peacor and Pfster, 2006). Contrarily, low resourceavailability due to low food level, high larval density, or both, would also constrain metamorphosis, such that if conditions were poor throughout the larval period, then tadpoles growing at a slow rate might take a long time to reach the minimum size for metamorphosis (viewed by Wilbur and Collins, 1973). However, water temperature has contradictory effects on growth rate and body size such as higher temperatures result in faster growth to a smaller size at metamorphosis. Few studies have examined how temperature and food level can interact to affect amphibian tadpoles (viewed by Castano et al., 2010).
In this study, we examined the effects of food level and temperature on size and age at metamorphosis of the Rice field Frog (Rana limnocharis). From mid-May to early September, Rice feld frogs breed in small bodies of water, from temporary or semi-permanent ponds to very short-lived rain pools (Fei and Ye, 2001). Consequently, larval viability relies on rapid development; eggs hatch after 3–4 days at 26–30°C (mean temperature = 28°C), which is the temperature range most often observed in the breeding ponds (Wu and Sun, 1981). We tested experimentally how sensitivity to temperature and its interaction with food level has infuenced variation both in the length of larval period and in the body size of R. limnocharis at metamorphosis.
2. Materials and Methods
2.1 Study species and rearing conditionWe collected 100 eggs from each of 10 egg masses in one population in Shihe County (32°08' N, 114°01' E, altitude 84 m), Henan, the central plains of China, in early May 2011. Each egg mass was kept in one 100-l plastic container with an automatic aerator, and all hatched on the same day. Tadpoles were at the same developmental stage (Gosner stage 26, absorption of external gills and fully formed spiracle; Gosner, 1960) at the start of the experiment. Room (air) temperature was kept at 26°C. In the experiments where temperature was manipulated, aquarium heaters were used to raise the temperature to 29°C and 32°C. Three temperatures (26°C, 29°C, and 32°C) were chosen because they fall within the range this species experiences in the feld or lab (Wu and Sun, 1981; Shi et al., 2012). Tadpoles were exposed to a 12L:12D photoperiod throughout the study period and the water in the containers was changed weekly.
2.2 Experiment designA 2×3 factorial design was used to examine the effects of food level and temperature on larval growth rates and post-metamorphic performance (Table 1). To evaluate the effects of food level, half of the tadpoles in each temperature treatment were placed on a low food regimen (LF, 6% of per tadpole mass per day) and half were placed on a high food regimen (HF, 12% per tadpole mass per day). Larvae were fed with commercial fish food (Bieyanghong, Biological Co. Ltd., Hangzhou, China, medium protein content, MPC; 30% protein, 10% lipids, 18% algae, 4% fber, 10% ash). A total of 420 tadpoles were randomly allocated into each of six experimental treatments (n = 70). For each temperature, 140 individual vessels (70 for each diet treatment), each of which is 0.3L (diameter = 77 mm), were randomly placed into two rectangular tanks (110 cm × 90 cm × 60 cm; L × W × H) flled with fresh water to a depth of 8 cm.

Table 1 A 2×3 factorial design was used in this study. HT: high temperature, MT: medium temperature, LT: low temperature, HF: high food level, LF: low food level
2.3 Data analysisAfter the first metamorph (defined as the emergence of the frst forelimb, stage 42; Gosner, 1960) was discovered, all tadpoles in the six plastic containers were checked daily. All metamorphs found were collected and kept in plastic vials with sand and 1 mm of water until tail re-sorption was completed. Several variables were measured: (1) age at metamorphosis (number of days from the beginning of the experiment until first metamorphosis); (2) mass at metamorphosis (Gosner stage 46; tail re-sorption was completed), weighed with an electric balance to the nearest 0.001 g; (3) growth rate (measured as the mass at complete metamorphosis divided by the age at metamorphosis); and (4) survival rate (the proportion of tadpoles surviving until metamorphosis).
Length of larval period, mass at metamorphosis, survival rate, and growth rate were analyzed with a generalized linear model (GLM) with type III mean squares using temperature and food level treatment as fxed factors. If the overall GLM results were signifcant, the data were analyzed with ANOVAs by using post hoc multiple comparisons (Fisher’s LSD) or a Chi-square test to evaluate differences between food levels or between temperatures (SPSS 13.0, SPSS Inc., 2004, Chicago, IL,USA). All P-values given are two-tailed, with values presented as means ± standard error.
3. Results
The effects of rearing temperature and food level on length of larval period were not signifcant (temperature, F2,293= 2.13, P = 0.12; food level, F1,155= 2.60, P = 0.11, Table 2, Figure 1), but revealed a signifcant temperature × food level interaction (F2,293= 10.95, P = 0.024). At 32°C, HF tadpoles had shorter larval period lengths than those reared at the 29°C and 26°C temperatures (Tukey’s Post hoc tests all P < 0.035), but no difference was observed in larval period length between 29°C and 26°C (P = 0.83). However, LF tadpoles had similar larval period lengths among the three experimental temperatures (Post Hoc all P > 0.086).
因为后验是以离散的质点近似的,在权重集中在一小部分质点时经过几次迭代更新后,该方程组就会遭遇抽样简并。权重方差的减少可确定简并度,可用近似(Arulampalamet al,2002):
Mass at metamorphosis was significantly affected by temperature (F2,293= 4.05, P = 0.018) and food level (F1,293= 10.21, P = 0.002). There was a signifcant interaction between food level and temperature (F2,293= 3.42, P = 0.034). Post hoc tests revealed significant differences between HF tadpoles reared at 32°C and 29°C (P = 0.004), and between 32°C and 26°C (P < 0.001, Figure 1). The effect of food level differed according to temperature. HF tadpoles were signifcantly larger than LF tadpoles at 32°C, but not at 29°C (P = 0.31) and 26°C (P = 0.38).
Although effects of temperature were not significant (F2,293= 0.64, P = 0.53) on growth rate, food level (F1,293= 4.41, P = 0.037) and temperature × food level interaction (F2,293= 5.82, P = 0.003) did affect growth rate. HF tadpoles had a greater growth rate than LF tadpoles at 32°C (P < 0.001), but not at 29°C (P = 0.34) or 26°C (P = 0.20). HF tadpoles reared at 32°C had a significantly or marginally signifcantly larger growth rate than those raised at 29°C and 26°C (all P < 0.059, Table 1), while there was no difference at 29°C or 26°C (all P > 0.06). Similarly, LF tadpoles reared at 29°C had a greater growth rate compared with those at 32°C and 26°C (P = 0.049), but not between 32°C and 26°C (P = 0.86).
Temperature and food level had a signifcant effect on survivorship to metamorphosis (all P < 0.001, Table 2, Figure 1), but the interaction between rearing temperature and food level was not signifcant (F2,414= 1.60, P = 0.20). Tadpoles at 26°C suffered from significantly reduced survival compared to those at 32°C and 29°C (P < 0.002), but not between 29°C and 32°C. HF larvae showed higher survival compared to LF larvae at 26°C (Chi-square test: χ2= 5.31, df = 2, P = 0.021), but not to larvae at 29°C (P = 0.18) or 32°C (P = 0.44).
4. Discussion
In this study, rearing temperature did not affect age at metamorphosis, but a significant interaction between rearing temperature and food level revealed HF tadpolesat 32°C had shorter larval period lengths than those reared at 29°C and 26°C. High food availability would likely cause faster growth, leading to both shorter larval periods and an increase in metamorphic size (Pandian and Marian, 1985), but our results revealed that the effects of food level on larval period were closely dependent on developmental temperature.

Table 2 The generalized linear model for the effects of temperature and food level on metamorphic traits in a Rana limnocharis population. T: temperature; F: food level.
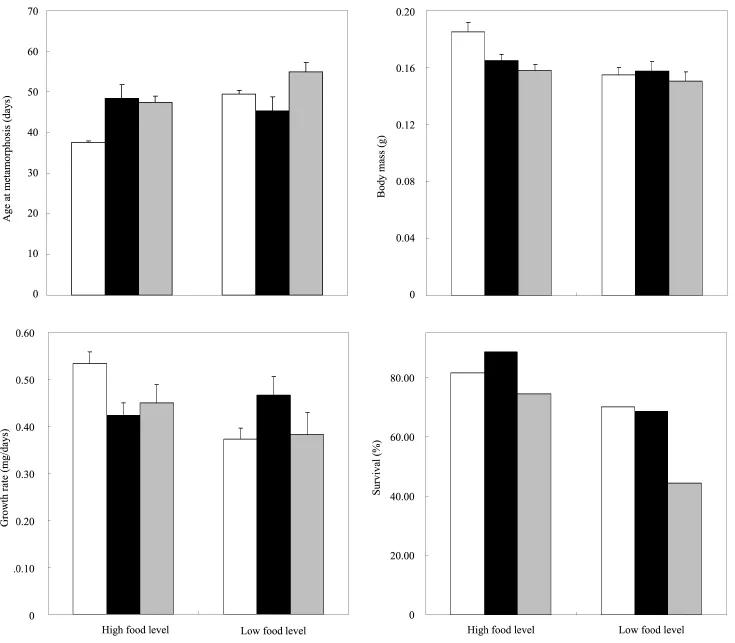
Figure 1 Infuences of temperature and food level on age, mass, growth rate, and survival at metamorphosis of Rana limnocharis (open columns, 32°C; black columns, 29°C; Gray columns, 26°C).
Our results also showed food level influenced the growth rate of Rice field frog tadpoles. An interesting finding was the significant interactions between temperature and food level, suggesting that HF tadpoles reared at 32°C had a significantly larger growth rate and mass at metamorphosis than those raised at 29°C and 26°C. This is similar to the pattern found in some populations of anurans (e.g. Laugen et al., 2005; Buchholz and Hayes, 2000; Castano et al., 2010). Therefore, an important fnding of the previous study was that the larvae with higher growth rates tended to have larger body sizes at any point in time including metamorphic climax, and tended reach metamorphosis early (Berven, 1982; viewed by Woodward et al., 1988; Riha and Berven, 1991; Loman, 2002). In addition to growth rates, metabolism may be an underlying mechanism for tadpoles at high food level to have a larger mass at high temperatures. High food availability will permit the elevated metabolic rate associated with higher temperatures (e.g. Arendt and Hoang, 2005; Lindgren and Laurila, 2009), whereas growth rate and metabolism may be more constrained atcool temperatures and low food levels.
Temperature and food level also influenced survivorship, with more tadpoles surviving at warmer temperatures and high food level. However, there was not a significant interaction between temperature and food level. Our results are in accordance with Sanuy et al. (2008) and Orizaola and Laurila (2009), but contrary to Castano et al. (2010), who found that survivorship was higher at cooler temperatures in wood frog tadpoles (Rana sylvatica). The differences may relate to adaptations for specific temperatures. Rice field frogs begin to breed in early summer and fnish in early autumn. Moreover, they prefer to lay eggs in small pools, which may reach higher temperatures during the summer; thus Rice field frog tadpoles may be better adapted to warmer temperatures. Taken together, if the growth condition is good, global climate change or local manipulations of the environment may promote growth and development of Rice feld frog tadpoles in natural ponds.
AcknowledgementsWe are very grateful to J. DU, Y. L. HE and T. ZHAO for assistance with feldwork. Work was approved by the Wildlife Protection Law of China. The study was funded by Program for Innovative Research Team (in Science and Technology) in universities of Henan Province (Grant No. 17IRTSTHN019) and Henan Scientific and Technological Project (Grant No.162102310124).
Álvarez D., Nicieza A. G. 2002. Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct Ecol, 16: 640–648
Arendt J., Hoang L. 2005. Effect of food level and rearing temperature on burst speed and muscle composition of Western Spadefoot Toad (Spea hammondii) Funct Ecol, 19: 982–987
Arnold S. J., Wassersug R. J. 1978. Differential predation on metamorphic anurans by garter snakes (Thamnophis): social behavior as a possible defense. Ecology, 59: 1014–1022
Atkinson D. 1996. Ectotherm life-history responses to developmental temperature. Animals and Temperature. Phenotypic and Evolutionary Adaptation (eds I. A. Johnston and A. F. Benett), pp. 183–204. Cambridge University Press, Cambridge
Beck C. W., Congdon J. D. 2000. Effects of age and size at metamorphosis on performance and metabolic rates of Southern Toad, Buffo terrestris, metamorphs. Funct Ecol, 14: 32–38
Berven K. A., Gill D. E. 1983. Interpreting geographic variation in life history traits. Am Zool, 23: 85–97
Buchholz D. R., Hayes T. B. 2000. Larval period comparison for the Spadefoot Toads Scaphiopus couchii and Spea multiplicata (Pelobatidae: Anura). Herpetologica, 56: 455–468
Castano B., Miely S., Smith G. R., Rettig J. E. 2010. Interactive effects of food availability and temperature on wood frog (Rana sylvatica) tadpoles. Herpetol J, 20: 209–211
Fei L., Ye C. Y. 2001. The colour handbook of amphibians of Sichuan. China Forestry Publishing House, Beijing
Gosner K. L. 1960. A simplifed table for staging anuran embryos and larvae with notes on identifcation. Herpetologica, 16: 183–190
Laugen A. T., Laurila A., Rasanen K., Merila J. (2003). Latitudinal countergradient variation in the common frog (Rana temporaria) development rates-evidence for local adaptation. J Evol Boil, 16: 996–1005
Liess A., Rowe O., Guo J., Thomsson G., Lind M. I. 2013. Hot tadpoles from cold environments need more nutrients-life history and stoichiometry reflects latitudinal adaptation. J Anim Ecol, 82: 1316–1325
Lindgren B., Laurila A. 2009. Physiological variation along a geographical gradient: is growth rate correlated with routine metabolic rate in Rana temporaria tadpoles? Biol J the Linn Soc, 98: 217–224
Loman J. 2002. Temperature, genetic and hydroperiod effects on metamorphosis of brown frogs Rana arvalis and R. temporaria in the feld. J Zool, 258: 115–129
Merila J., Laurila A., Laugen A. T., Rasanen K., Pahkala M. 2000. Plasticity in age and size at metamorphosis in Rana temporara comparison of high and low latitude populations. Ecography, 23: 457–465
Morey S. R., Reznick D. N. 2004. The relationship between habitat permanence and larval development in California spadefoot toads: field and laboratory comparison of developmental plasticity. Oikos, 104: 172–190
Nathan J. M., James V. G. 1972. The role of protozoa in the nutrition of tadpoles. Copeia, 1972: 669–679
Newman R. A. 1998. Ecological constraints on amphibian metamorphosis: interactions of temperature and larval density with responses to changing food level. Oecologia, 115: 9–16
Orizaola G., Laurila A. 2009. Microgeographic variation in temperature-induced plasticity in an isolated amphibian metapopulation. Evol Ecol, 23: 979–991
Palo J. U., O'Hara R. B., Laugen A. T., Laurila A., Primmers C. R., Merila J. 2003. Latitudinal divergence of common frog (Rana temporaria) life history traits by natural selection: evidence from a comparison of molecular and quantitative genetic data. Mol Ecol, 12: 1963–1978
Pandian T. J., Marian M. P. 1985. Predicting anuran metamorphosis and energetics. Physiol Zool, 58: 538–552
Peacor S. D., Pfster C. A. 2006. Experimental and model analyses of the effects of competition on individual size variation in wood frog (Rana sylvatica) tadpoles. J Anim Ecol, 75: 990–999
Riha V. F., Berven K. A. 1991. An analysis of latitudinal variation in the larval development of the wood frog (Rana syIvatica). Copeia, 1991: 209–221
Rose C. S. 2005. Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends Ecol Evol, 20: 129–135
Sanuy D., Oromí N., Galofré A. 2008. Effects of temperature on embryonic and larval development and growth in the natterjack toad (Bufo calamita) in a semi-arid zone. Anim Biodiv Conserv, 31: 41–46
Shi L. Q., Zhao L. H., Ma X. H., Ma X. M. 2012. Selected body temperature and thermal tolerance of tadpoles of two frog species (Fejervarya limnocharis and Microhyla ornata) acclimated under different thermal conditions. Acta Ecologica Sinica, 32: 465–471
Skelly D. K. 2004. Microgeographic countergradient variation in the wood frog, Rana sylvatica. Evolution, 58: 160–165
Steinwascher K., Travis J. 1983. Influence of food quality and quantity on early growth of two anurans. Copeia, 1983: 238–242
von Bertalanffy L. 1960. Principles and theory of growth. Pages 137–259 in W. W. Nowinski, ed. Fundamental aspects of normal and malignant growth. Elsevier, New York
Wilbur H. M. 1980. Complex life cycles. Ann Rev Ecol Syst, 11: 67–93
Wilbur H. M., Collins J. P. 1973. Ecological aspects of amphibian metamorphosis. Science, 182: 1305–1314
Woodward B. D., Travis J., Mitchell S. 1988. The effects of the mating system on progeny performance in Hyla crucifer (Anura, Hylidae). Evolution, 42: 784–794
Wu Y. L., Sun Y. H. 1981. A preliminary observation on the early embryonic development of Rana limnocharis. Chin J Zool, 3: 28–30
*Corresponding author: Dr. Tonglei YU, from Xinyang Normal University, Henan, China, with his research focusing on wildlife ecology and conservation.
E-mail: yutonglei_00000@163.com
Received: 29 September 2015 Accepted: 20 September 2016
猜你喜欢
杂志排行
Asian Herpetological Research的其它文章
- A New Species of Euphlyctis (Amphibia, Anura, Dicroglossidae) from the West Coastal Plains of India
- Osteology of Quasipaa robertingeri (Anura: Dicroglossidae)
- Genotoxicity of Three Avermectins on Polypedates megacephalus Tadpoles Using the Comet Assay
- Pathological Changes in Andrias davidianus Infected with Chinese Giant Salamander Ranavirus
- Effects of Feeding Time on the Growth Performance and Variation of RNA/DNA Ratio of the Chinese Soft-shelled Turtle, Pelodiscus sinensis
- Effects of Dietary Vitamins A, B2, and B6Supplementation on Growth and Feed Utilization of Juvenile Chinese Soft-shelled Turtle Pelodiscus sinensis according to an Orthogonal Array Experiment
