Pathological Changes in Andrias davidianus Infected with Chinese Giant Salamander Ranavirus
2017-01-20YiGENGMatthewJamesGRAYKaiyuWANGDefangCHENPingOUYANGXiaoliHUANGChangliangHEZhijunZHONGandZexiaoYANG
Yi GENG, Matthew James GRAY, Kaiyu WANG, Defang CHEN, Ping OUYANG, Xiaoli HUANG, Changliang HE, Zhijun ZHONGand Zexiao YANG
1College of Veterinary Medicine, Sichuan Agricultural University, Ya’an 625014, Sichuan, China
2Center for Wildlife Health, University of Tennessee, Knoxville TN 379, USA
3Department of Aquaculture, Sichuan Agricultural University, Ya’an 625014, Sichuan, China
Pathological Changes in Andrias davidianus Infected with Chinese Giant Salamander Ranavirus
Yi GENG1*, Matthew James GRAY2, Kaiyu WANG1, Defang CHEN3, Ping OUYANG1, Xiaoli HUANG3, Changliang HE1, Zhijun ZHONG1and Zexiao YANG1
1College of Veterinary Medicine, Sichuan Agricultural University, Ya’an 625014, Sichuan, China
2Center for Wildlife Health, University of Tennessee, Knoxville TN 379, USA
3Department of Aquaculture, Sichuan Agricultural University, Ya’an 625014, Sichuan, China
Chinese giant salamander ranavirus (CGSRV) is an emerging pathogen in captive populations of the Chinese giant salamander (Andrias davidianus). We processed 140 morbid Chinese giant salamanders from seven captive breeding populations over fve years, and describe the disease associated with CGSRV infection. The most common gross signs were signifcant swelling of the legs and coelomic cavity, erythema of the legs and ventrum in juveniles; cutaneous erosions and ulcerations in adults, particularly the limbs and the head; and marked petechial or ecchymotic hemorrhages of the internal organs, particularly the liver, spleen and kidney. Histological examination showed degeneration, necrosis, and infammation in many organs, particularly in the organs where hemorrhage was observed. There was evidence of eosinophilic inclusion bodies in degenerated and necrotic cells. We identifed virus particles and empty capsids without viral nucleoid in the inclusion bodies using electron microscopy. Virus particles were hexagonal or round shape, and appeared in paracrystalline arrays, aggregates, or singly. All enveloped viral particles were 140–160 nm. Polymerase chain reaction followed by sequencing verified that the virus particles were CGSRV. These results collectively support that CGSRV was the etiologic agent responsible for these mass die-offs of the Chinese giant salamander. The pathology described herein will be useful in diagnosing cases of ranaviral disease caused by CGSRV, and provide evidence that this pathogen is a signifcant threat to the Chinese giant salamander.
pathology, CGSRV, Andrias davidianus, ranavirus, China
1. Introduction
The Chinese giant salamander (CGS, Andrias davidianus) is one of only three extant species of cryptobranchid salamanders (the others being the Japanese giant salamander Andrias japonicus and the hellbender Cryptobranchus alleganiensis), which belongs to the order Caudata and family Cryptobranchidae. The CGS is listed in Category II of the National Protected Aquatic Wildlife (China), in CITES Appendix I, and as critically endangered by the IUCN (Cunningham et al., 2015; Wang et al., 2004). In 1988 it was designated a State protected species in China. Historical information indicates that the species was once widely distributed in the middle and lower tributaries of the Yangtze, Yellow and Pearl Rivers across 17 provinces (Fortuny et al., 2015; Wang et al., 2004; Wells, 2007). The wild CGSs have experienced a severe range-wide decline since the 1950s, being threatened primarily by overexploitation for food,, habitat loss and fragmentation, competition with introduced fsh and frogs, pesticides and other pollutants, and emerging pathogens (Cunningham et al., 2015; Wang et al., 2004). In the past 30 years, artificial breeding and farming has been used to protect the CGS in China. Approximately 2 million CGSs are bred in China each year; however, the emergence of CGSRV has thwarted production, negatively impacted conservation efforts, and had dire economic impacts (Chen et al., 2013; Dong et al., 2011; Geng et al., 2011; Jiang et al., 2011; Meng et al., 2014).
Ranaviruses (RVs) are important pathogens of ectothermic vertebrates, and have been associated with massive die-offs in wild and farmed populations of fsh, reptiles and amphibians across the globe (Duffus et al., 2015; Whittington et al., 2010; Williams et al., 2005). RVs are considered emerging pathogens, and have been listed as notifiable disease by the World Organization for Animal Health (Schloegel et al., 2010). Although the pathology of ranaviral disease has been described in several host species (Miller et al., 2015), there is limited histopathological information in CGSs. The present report describes the clinical and the pathological changes observed in CGSs infected with CGSRV from several outbreaks.
2. Materials and Methods
From May 2010 through April 2015, we collected approximately 220 live moribund CGSs from seven CGS farms in Shanxi, Gansu and Sichuan Provinces, China. Average body mass of juveniles was 83.4 g (SD = 31.7), and average mass of adults was 2385.6 g (SD = 781.3). Individuals that were collected generally had a swollen head and legs, cutaneous ulcers, limb necrosis, and ecchymosis on the body. Of the individuals collected, we randomly selected 140 (n = 20 from each of seven farms) for detailed pathological investigation.
From all individuals, we collected tissue samples from the liver, lung, spleen, kidney, heart, brain, skin and muscles and promptly fxed in 10% neutral-buffered formalin. The samples were processed routinely in an automatic tissue processor, then embedded in paraffin wax, sectioned at 4–5 μm and stained with haematoxylin and eosin (H&E). Additionally, separate tissues samples of kidney, liver and spleen were homogenized and frozen at –80°C for polymerase chain reaction (PCR) testing and viral isolation. Extracted gDNA from tissues was used as a template and amplifed by PCR using published primers (forward primer: 5′-GACTTGG CCACTTATGAC-3′; reverse primer: 5′-GTCTCTGGAGAAGAAGAA-3′) that targeted a 500-bp, highly conserved region of the major capsid protein (MCP) gene of CGSRV (Zhou et al., 2012). The PCR products were resolved by gel electrophoresis for determination of the presence or absence of CGSRV. For virus isolation, fltered liver, spleen and kidney tissue homogenates were inoculated on epithelioma papulosum cyprini (EPC) cells. The cells were incubated at 25°C and observed daily for cytopathic effects (CPE). After the appearance of CPE, the supernatant was stored at −80°C and used as a virus stock for virus passage. To verify that the isolated virus was CGSRV, DNA was extracted from cultured EPC cells exhibiting CPE, and PCR was performed as described above.
Tissue samples and EPC cells infected with CGSRV were used for electron microscopy. Briefly, the samples were cut into 1-mm3pieces, and fixed in 2% glutaraldehyde in phosphate buffer (pH 7.3, 0.1 M) at 4°C. After post-fixation with 1% osmium tetroxide, the pieces were dehydrated through a series of graded alcohols, embedded in epoxy resin, sectioned at 50 nm, stained with urany lacetate and lead citrate, and observed under a transmission electron microscope (TEM; JEM-1200EX, JEOL, Tokyo, Japan). The entire experimental procedure was approved by the Committee of the Ethics on Animal Care and Experiments at Sichuan Agricultural University and was carried out in accordance with the approved guidelines.
3. Results and Discussion
Liver,kidney and spleen tissues were PCR positive for CGSRV at all seven farms. Further, CPE on EPC cells was evident after 3–4 days at 25°C. To verify that the isolated virus was CGSRV, a 500-bp DNA fragment of the MCP was amplifed and sequenced. A GenBank BLAST search showed 100% sequence identity with CGSRV (Zhou et al., 2012).
Clear and consistent gross signs were observed in the majority of CGSs infected with CGSRV (Figure 1). Early clinical signs included the dark and speckled skin, anorexia, slow moving, bloody stools, and vomiting (occasionally bloody). Morbid juveniles often displayed erratic swimming, lethargy, and the lack of equilibrium. Gross lesions in juveniles included erythema on the head, ventral surface, legs (Figure 1A), swelling of the legs and marked abdominal distension (Figure 1B). In adults, erythema was often present on the legs and ventrum (Figure 1C), there were small, pale raised foci in the skin that accompanied swelling of the legs, head and body (Figure 1D), and cutaneous erosions and ulcers (Figure 1E). In some cases, necrosis and rotting occurred in the limbs (Figure 1F), and 10.0% of these cases show hyperemic and edematous orbits. Duration from the presence of initial gross signs to death, was 15–40 days across the farms. The mortality rate was 70%–90% in affected juveniles and 30%–45% in adults.
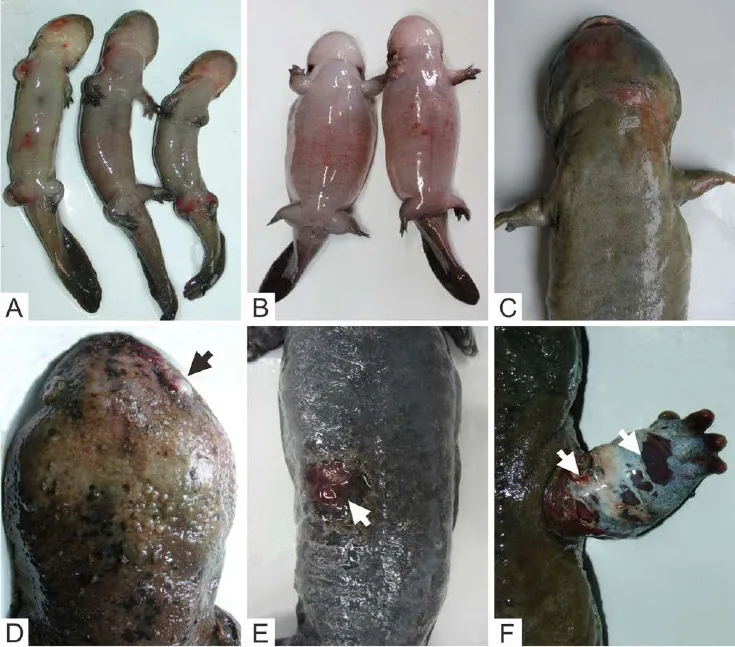
Figure 1 Gross lesions in infected Chinese giant salamanders (Andrias davidianus). A: Erythema on the ventral surface and legs in larvae; B: Abdominal distension in larvae; C: Erythema on the ventrum in adult; D: Swelling in the head, hemorrhage and edema in the orbit (arrow) of an adult; E: Cutaneous ulcer (arrow) in an adult; F: Ecchymoses, swelling and necrosis (arrows) in a forelimb of an adult.
At necropsy, the major visceral organs had various gross lesions. The most common intracoelomic lesions included petechial or ecchymotic hemorrhages of the internal organs, especially the liver (Figure 2A), kidney (Figure 2B), lung and spleen (Figure 2C). We also observed pale swollen livers, and swollen kidneys and spleens. In some cases, hemorrhage or ulcers appeared in oral mucosa (Figure 2D). In general, the gastrointestinal tracts were empty and the gall bladder was enlarged (Figure 2E), both of which were consistent with inappetence. Hemorrhage, erosion, and ulceration were observed in the gastric mucosa (Figure 2F). The intestine was thin-walled, mildly distended with clear or bloody fuid. The coelomic cavity often contained clear or bloody fuid.
Histological examination revealed that the pathologic lesions of CGSRV infection in CGSs are characterized by systemic hemorrhage, cellular degeneration and necrosis, likely resulting in multiple organ failure or dysfunction, particularly the kidney, liver, spleen and gastrointestinal tract. The histological lesions were similar to the described histologic changes of RVs infected salamanders in North America (Bollinger et al., 1999; Douglas et al., 2003). Vacuolar degeneration of the renal tubular epithelial cells, focal peracute tubular necrosis, and intracytoplasmic inclusions in the renal tubular epithelium (Figure 3A); acute glomerulonephritis and necrosis were present in the kidney (Figure 3B). Additionally, homogeneous red-stained protein formed protein casts or cell casts in the renal tubular. Hepatocellular swelling and vacuolar degeneration were common in the liver, and consisted of areas infiltrated with lymphocytes andmacrophages (Figure 3C). Hepatocytes also frequently contained viral inclusions, which were associated with either single degenerate cells or focal necrosis (Figure 3C). Diffuse acute necrosis of the spleen with lymphocytolysis, lymphoid depletion, deposition of fbrin, and obliteration of the normal splenic architecture were observed (Figure 3D). The lesions in the spleen were similar to fish infected with RVs (Gibson-Kueh et al., 2003). Whether amphibians and fsh infected with RVs have similar immunological responses needs further investigation.
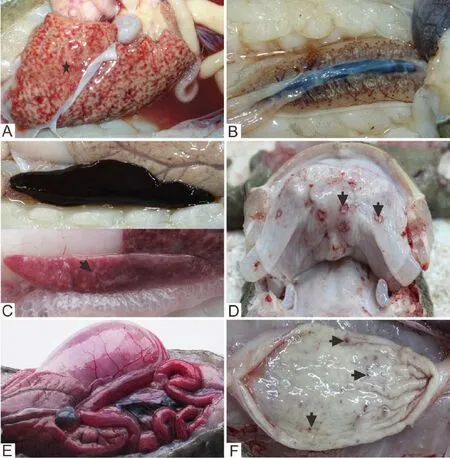
Figure 2 Gross lesions in infected Chinese giant salamanders (Andrias davidianus). A: The liver was pale and swollen with multifocal hemorrhages (star), and bloody fuid (arrow) in abdominal cavity; B: The kidney was pale and swollen with petechial hemorrhages; C: The spleen was swollen with congestion and focal necrosis (arrow) ; D: The oral mucosa showing hemorrhage, erosion and ulceration (arrows) ; E: The gastrointestinal tract was congested and empty, and the gall bladder was enlarged; F: The gastric mucosa showing hemorrhage, erosion and ulceration (arrows).
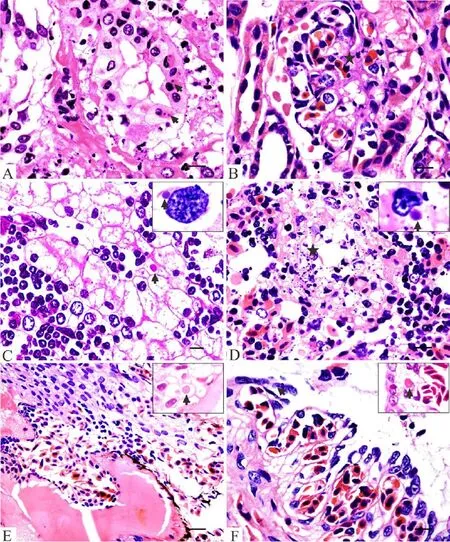
Figure 3 Histological lesions in infected Chinese giant salamanders (Andrias davidianus). A: Degeneration, necrosis and intracytoplasmic inclusions (arrows) in renal tubular epithelia (bar = 50 μm); B: Acute glomerulonephritis and necrosis (arrow) in the kidney (bar = 50 μm); C: Vacuolar degeneration, eosinophilic or basophilic inclusion bodies (arrows) in hepatocytes, and focal necrosis with lymphocytes and macrophages infltration in liver (bar = 30 μm); D: A focal necrosis area (star) of the spleen, with lymphocytolysis, lymphoid depletion and deposition of fbrin (bar = 50 μm); E: Intracytoplasmic inclusions (arrows) in epidermal cells of the skin; necrosis with infammatory cells infltration in muscles (bar = 25 μm); F: Congestion, necrosis and intracytoplasmic inclusion (arrow) in pulmonary alveoli epithelia (bar = 50 μm). (hematoxylin and eosin staining).
Skin lesions included intracellular and intercellular edema of the stratum spinosum, which frequently was associated with areas of degeneration and necrosis. Cytoplasmic inclusions were noted in the epithelium (Figure 3E). These lesions in the skin were thehistological counterpart of the pale foci seen in necropsy. In some cases, these lesions became ulcerations with necrosis of underlying muscles and inflammatory cell infiltration (Figure 3E). The histological lesions of skin were similar those described in other salamander species (Bollinger et al., 1999; Douglas et al., 2003) and lizards (Stöhr et al., 2013) infected with RVs. In the gastrointestinal tract, degeneration, necrosis, and sloughing of the epithelia, and congestion and edema of the submucosa and lamina propria were observed. Ulcers were frequently present and associated with hemorrhage in the lumen. Viral inclusions were occasionally seen in epithelial cells of the gastrointestinal tract. These lesions may have been responsible for the vomiting and bloody diarrhea that was observed clinically in some samples. The heart presented parenchymatous myocarditis, including the obvious fracture and necrosis of muscle fibers with lymphocytes and macrophages infiltration. Hemorrhagic necrosis was occasionally present in the lung. Viral inclusions were present and degeneration, necrosis, and sloughing of the pulmonary alveoli epithelia occurred (Figure 3F). Pathologic lesions in the brain were consistent with meningoencephalitis. Neuronal shrinkage, edema, lymphocytes, and mononuclear infiltration were found in the brain. Pathological changes associated with the brain may be responsible for the erratic swimming that was observed clinically, considering meningoencephalitis can result in neurological signs.
Tissue samples from the liver, spleen and kidney of the diseased CGSs, and the EPC cells infected with the virus were observed by electron microscopy. In these samples, virus-like particles were hexagonal or round, and present as paracrystalline arrays, aggregates, or free virions (Figure 4A). Virus was observed in the cytoplasm at different stages of assembly, ranging from complete icosahedral particles containing an electrondense core to incomplete particles containing empty to partially full cores. Virus particles and empty capsids without a viral nucleoid were found in inclusion bodies (Figure 4B). Enveloped particles were 140–160 nm in diameter. Virions were also observed budding from the infected cells (Figure 4B), where they acquired a hostderived membrane that encapsulated the virus. And some hexagonal viral particles were also observed inside the cell nucleus of infected cells. In addition, apoptotic bodies were observed in lymphocytes and EPC cells (Figure 4C), which has been reported for cells infected with red sea bream iridovirus (RSIV: Imajoh et al., 2004).
Collectively, our results indicate that CGSs collected during this pathological investigation were infected with CGSRV, and this pathogen was associated with gross signs and histological lesions. The pathological changes observed were similar to what has been described for several Ranavirus species among various amphibian, reptilian and fsh hosts (Miller et al., 2015). Therefore, we conclude that CGSRV was responsible for the mass dieoffs observed in the seven CGS farms in this study. It is possible that secondary invaders could have contributed to some of the cases of mortality. We occasionally detected conditional pathogenic bacteria (Aeromonas hydrophila, Aeromonas veronii, Citrobacter freundii, and Acinetobacter spp.,) in some of diseased CGSs;however, prevalence of these bacteria was inconsistent among samples. The occurrence and effect of bacteria or fungi (e.g., Batrachochytrium dendrobatidis) co-infecting CGSs with primary infections of CGSRV needs further investigation.
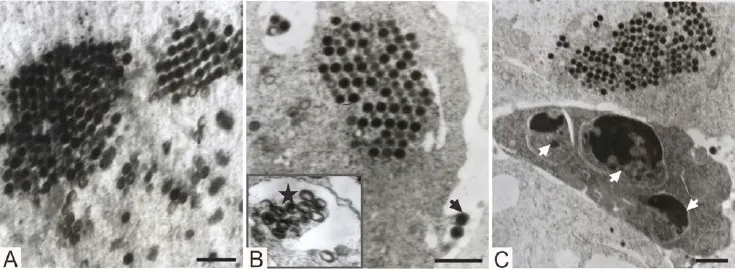
Figure 4 Electron microscopy observations. A: Viral particles appeared in paracrystalline arrays, or as free virions or aggregates in the renal tubular epithelia. (bar =800 nm); B: Viral particles and nucleocapsid aggregated in pseudocrystalline arrays in the hepatocyte; a virion is budding through the cell membrane to acquire an envelope (arrow); virus particles and empty capsids appeared in the inclusion body (star) (bar =1000 nm) ; C: Apoptotic bodies (arrows) appeared in infected EPC cells (bar =800 nm).
Our research and others (Cunningham et al., 2015; Dong et al., 2011; Geng et al., 2011; Jiang et al., 2011; Zhou et al., 2013) indicates that RVs are a threat to CGS conservation and farming. Future investigations need to document the source of CGSRV, and test different disease intervention strategies that attempt to interrupt the host-pathogen cycle. For example, decontaminating source water, reducing densities, and isolating infected individuals might help reduce CGS losses in farms. Additionally, the possible spillover effects to wild populations of CGSs need to be investigated, because most farms do not decontaminate wastewater (Cunningham et al., 2015). The effects of CGSRV on fsh, reptile and other amphibian species are unknown; hence, the impacts of this emerging pathogen might be more widespread than a single species.
AcknowledgementsThis work was supported by the Sichuan Technology Support Planning (No. 2014 NZ0027) and Sichuan Academic Leader Training Fund (No. 2015 RST0016).
Bollinger T. K., Mao J., Schoch D., Brigham R. M., Chinchar, V. G. 1999. Pathology, isolation, and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. J Wildlife Dis, 35: 413–429
Chen Z., Gui J., Gao X., Pei C., Hong Y., Zhang Q. 2013. Genome architecture changes and major gene variations of Andrias davidianus ranavirus (ADRV). Vet Res, 44: 101–124
Cunningham A. A., Turvey S. T., Zhou F., Meredith H. M., Guan W., Liu X. L., Sun C. M., WANG Z. Q., Wu M. Y. 2015. Development of the Chinese giant salamander Andrias davidianus farming industry in Shanxi Province, China: conservation threats and opportunities. Oryx, 49: 1–9
Dong W., Zhang X., Yang C., An J., Qin J., Song F., Zeng W. 2011. Iridovirus Infection in Chinese Giant Salamanders. Emerg Infect Dis, 17: 2388–2389
Douglas E. D., Carol U. M., Jun W., Mao J. H., Steven T. C., Chinchar V. G. 2003. Diagnostic and molecularevaluation of three iridovirus-associated salamander mortality events. J Wildlife Dis, 39: 556–566
Duffus A. L., Waltzek T. B., Stöhr A. C., Allender M. C., Gotesman M., Whittington R. J., Hick P., Hines M. K., Marschang R. E. 2015. Distribution and Host Range of Ranaviruses. In: Gray MJ, Chinchar VG (eds) Ranaviruses: lethal pathogens of ectothermic vertebrates. Springer, New York, pp: 9–58
Fortuny J., Marcé-Nogué J., Heiss E., Sanchez M., Sanchez M., Gil L., Galobart A. 2015. 3D bite modeling and feeding mechanics of the largest living amphibian, the Chinese giant salamander Andrias davidianus (Amphibia: Urodela). PloS ONE, 10: e0121885
Geng Y., Wang K., Zhou Z., Li C., Wang J., He M., Yin Z., Lai W. 2011. First report of a ranavirus associated with morbidity and mortality in farmed Chinese giant salamanders (Andrias davidianus). J Comp Path, 145: 95–102
Gibson-Kueh S., Netto P., Ngoh-Lim G., Chang S., Ho L., Qin Q., Chua F., Ng M., Ferguson H. 2003. The pathology of systemic iridoviral disease in fsh. J Comp Path, 129: 111–119
Imajoh M., Sugiura H., Oshima S. I. 2004. Morphological changes contribute to apoptotic cell death and are affected by caspase-3 and caspase-6 inhibitors during red sea bream iridovirus permissive replication. Virology, 322: 220–230
Jiang Y. L., Zhang M., Jing H. L., Gao L. Y. 2011. Isolation and characterization of an iridovirus from sick giant salamander (Andrias davidianus). Chin J Virol, 27: 274–282
Meng Y., Ma J., Jiang N., Zeng L.B., Xiao H. B. 2014. Pathological and microbiological fndings from mortality of the Chinese giant salamander (Andrias davidianus). Arch Virol, 159: 1403–1412
Miller D. L., Pessier A. P., Hick P., Whittington R. J. 2015. Comparative pathology of ranaviruses and diagnostic techniques. In: Gray MJ, Chinchar VG (eds) Ranaviruses: lethal pathogens of ectothermic vertebrates. Springer, New York, pp: 171–208
Schloegel L. M., Daszak P., Cunningham A. A., Speare R., Hill B. 2010. Two amphibian diseases, chytridiomycosis and ranaviral disease, are now globally notifiable to the World Organization for Animal Health (OIE): An assessment. Dis Aquat Organ, 92: 101–108
Stöhr A. C., Blahak S., Heckers K. O., Wiechert J., Behncke H., Mathes K., Günther P., Zwart P., Ball I., Rüschoff B. 2013. Ranavirus infections associated with skin lesions in lizards. Vet Res, 44: 1–10
Wang X. M., Zhang K. J., Wang Z. H., Ding Y. Z., Wu W., Huang S. 2004. The decline of the Chinese giant salamander Andrias davidianus and implications for its conservation. Oryx, 38: 197–202
Wells K. D. 2007. The ecology and Behaviour of Amphibians. The University of Chicago Press, pp:1148
Whittington R., Becker J., Dennis M. 2010. Iridovirus infections in finfish–critical review with emphasis on ranaviruses. J Fish Dis, 33: 95–122
Williams T., Barbosa-Solomieu V., Chinchar V. G. 2005. A decade of advances in iridovirus research. Adv Virus Res, 65: 173–248
Zhou Z., Geng Y., Liu X., Ren S., Zhou Y., Wang K., Huang X., Chen D., Peng X., Lai W. 2013. Characterization of a ranavirus isolated from the Chinese giant salamander (Andrias davidianus, Blanchard, 1871) in China. Aquaculture, 384: 66–73
Zhou Z., Geng Y., Ren S., Wang K., Huang X., Chen D., Liu X., Lai W. 2012. Ranavirus (family Iridoviridae) detection by polymerase chain reaction (PCR) in Chinese giant salamander (Andrias davidianus, Blanchard, 1871), China. Afr J Biotechnol, 11, 15130–15134
*Corresponding author: Prof. Yi GENG, from Sichuan Agricultural University, with his research focusing on aquatic animal diseases.
E-mail: gengyisicau@126.com
Received: 22 April 2016 Accepted: 6 September 2016
杂志排行
Asian Herpetological Research的其它文章
- A New Species of Euphlyctis (Amphibia, Anura, Dicroglossidae) from the West Coastal Plains of India
- Osteology of Quasipaa robertingeri (Anura: Dicroglossidae)
- Genotoxicity of Three Avermectins on Polypedates megacephalus Tadpoles Using the Comet Assay
- Plasticity in Metamorphic Traits of Rice Field Frog (Rana limnocharis) Tadpoles: The Interactive Effects of Rearing Temperature and Food Level
- Effects of Feeding Time on the Growth Performance and Variation of RNA/DNA Ratio of the Chinese Soft-shelled Turtle, Pelodiscus sinensis
- Effects of Dietary Vitamins A, B2, and B6Supplementation on Growth and Feed Utilization of Juvenile Chinese Soft-shelled Turtle Pelodiscus sinensis according to an Orthogonal Array Experiment
