A New Species of Euphlyctis (Amphibia, Anura, Dicroglossidae) from the West Coastal Plains of India
2017-01-20HebbarPRITIChandrakanthRukkappaNAIKKadabaShamannaSESHADRIRamitSINGALMadhavaKulkarniVIDISHAGudasalmaniRAVIKANTHandKotambyluVasudevaGURURAJA
Hebbar PRITI, Chandrakanth Rukkappa NAIK, Kadaba Shamanna SESHADRI, Ramit SINGAL, Madhava Kulkarni VIDISHA, Gudasalmani RAVIKANTHand Kotambylu Vasudeva GURURAJA,7,*
1Ashoka Trust for Research in Ecology and the Environment (ATREE), Royal Enclave, Sriramapura, Jakkur (P.O), Bangalore 560054, India
2Manipal University, Manipal 576104, India
3Wildlife Range, Kumbaravada, Nujji Section, Dandeli Anshi Tiger Reserve, Joida Taluk, 581187, India
4Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Block S3, Singapore
5Independent Researcher, B-14, Law Apartments, Karkardooma, Delhi 110092, India
6Gubbi Labs LLP, Science and Media Center, WS-5, I Floor, Entrepreneurship Center, Indian Institute of Science Campus, Bengaluru 560012, India
7Srishti Institute of Art, Design and Technology, N4, Yelahanka New Town, Bengaluru 560064, India
A New Species of Euphlyctis (Amphibia, Anura, Dicroglossidae) from the West Coastal Plains of India
Hebbar PRITI1,2, Chandrakanth Rukkappa NAIK3, Kadaba Shamanna SESHADRI4, Ramit SINGAL5, Madhava Kulkarni VIDISHA6, Gudasalmani RAVIKANTH1and Kotambylu Vasudeva GURURAJA6,7,*
1Ashoka Trust for Research in Ecology and the Environment (ATREE), Royal Enclave, Sriramapura, Jakkur (P.O), Bangalore 560054, India
2Manipal University, Manipal 576104, India
3Wildlife Range, Kumbaravada, Nujji Section, Dandeli Anshi Tiger Reserve, Joida Taluk, 581187, India
4Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Block S3, Singapore
5Independent Researcher, B-14, Law Apartments, Karkardooma, Delhi 110092, India
6Gubbi Labs LLP, Science and Media Center, WS-5, I Floor, Entrepreneurship Center, Indian Institute of Science Campus, Bengaluru 560012, India
7Srishti Institute of Art, Design and Technology, N4, Yelahanka New Town, Bengaluru 560064, India
The genus Euphlyctis is widely distributed across Southwestern Arabian Peninsula into parts of Southeast Asia. Five of the seven known Euphlyctis species are found within the Indian subcontinent. Here, we describe a new species, Euphlyctis karaavali sp. nov. from South-west coast of India, which was discovered during surveys engaging citizens. This species was identified to be distinct based on molecular and morphological evidence. We provide a detailed description of this species along with its call description and compare it with closest congeners. Previous studies in the region had identifed this species as E. hexadactylus but suggested the possibility of it being cryptic. Genetically E. karaavali sp. nov. is distinct from E. hexadactylus with a genetic divergence of 9.2% (12S and 16S) and shows a high divergence with E. kalasgramensis and E. ehrenbergii (13.04% each). Our fndings are discussed in the context of cryptic species discovery, citizen engagement in scientifc progress and conservation measures while suggesting future directions.
Karaavali skittering frog, citizen science, molecular identifcation, agriculture, cryptic species
1. Introduction
The genus Euphlyctis Fitzinger, 1843 is widespread across Southwestern Arabian Peninsula to South and Southeast Asia. It comprises of seven extant species viz., E.aloysii Joshy, Alam, Kurabayashi, Sumida and Kuramoto, 2009 known from West coast of India; E. cyanophlyctis (Schneider, 1799) a widespread species known from South-eastern Iran, Southern Afghanistan, Pakistan, Nepal, Bhutan, India, Pakistan, Sri Lanka, Myanmar, Malaysia and Vietnam; E. ehrenbergii (Peters, 1863) from South-western Arabian Peninsula, Pakistan and Yemen; E. ghoshi (Chanda, 1991) known only from Manipur, India; E. hexadactylus (Lesson, 1834) from India, Bangladesh, Pakistan and Sri Lanka; E. kalasgramensis Howlader, Nair, Gopalan, and Merilä, 2015, from Bangladesh and adjoining parts of India and E. mudigere Joshy, Alam, Kurabayashi, Sumida and Kuramoto, 2009 from Southern India and Sri Lanka (Frost, 2016). Five of these seven species are known from India.
In the monsoon season (June-August) of the year 2015, we encountered a species of Euphlyctis while undertaking a frog survey in the coastal region in Karnataka state, India. The frogs did not match any of the described Euphlyctis species and from initial morphologicalcomparisons; it appeared to be a new species. The molecular analysis provided further evidence, and here, we describe the new species as Euphlyctis karaavali sp. nov., provide its call description, geographic range and suggest IUCN Red List status.
2. Materials and Methods
2.1 Abbreviations usedCRN: Chandrakanth R Naik; KSS: KS Seshadri; RS: Ramit Singal; IUCN: International Union for Conservation of Nature; BNHS: Bombay Natural History Society, Mumbai; RBRL–Rondano Biodiversity Research Laboratory, St. Aloysius College, Mangaluru.
2.2 Study AreaIndividuals were observed vocalizing from Sanikatta, a coastal village in Kumta Taluk, Uttara Kannada District, Karnataka state (74.33783 °E, 14.55119 °N, 2 m amsl, datum WGS84). Subsequently, several individuals were encountered in six different localities within Karnataka State (Table 1). All these localities along 250 km of the West coast in the state of Karnataka have laterite formations interspersed with human habitation, estuarine habitats, and agriculture fields or fallow waterlogged land (Figure 1).
2.3 Specimen collectionSix adult individuals (5 males and 1 female) were collected for this study. Individuals were gently picked up by hand and were placed in a container with water. A small amount of 20% Benzocain gel (used as topical aesthetic) was applied on the ventral surface of the individual using a cotton swab to euthanize them. For molecular analysis, a small portion of the thigh muscle tissue was excised with sterilized scissors soon after the individual stopped moving and tissue was preserved in molecular grade ethanol. The specimen was fxed in about 4% formalin solution, which was also injected into the specimen using a hypodermal syringe. Specimens were retained in the formalin solution for a 24h period before being transferred to 70% ethanol. Individuals were photographed before and after fixing. Three individuals were collected from Kodanga, Herga Village by KSS and RS and three individuals were collected in Sanikatta by KSS and CRN on 26thand 27thJune 2015 respectively from waterlogged shallow rice paddy fields. Specimens were deposited at the Bombay Natural History Society Museum, Mumbai, India under the accession numbers (BNHS 5985-5990). Tissue from only two specimens (BNHS 5986 and BNHS 5988) were used for molecular analysis, and the sequences have been submitted to GenBank under accession numbers KU870372-KU870375. Tissues were excised from two individuals of E. hexadactylus collected from its type locality Pondicherry (BNHS 5992-5993) and one from E. aloysii collected from Kodanga, Herga Village (BNHS 5995).
2.4 MorphologyWe used Mitutoyo® digital slide calliper for morphological measurements. Values were rounded to the nearest 0.1 mm. Measurement and terminology follow Priti et al. (2016). Abbreviations used are as follows: snout–vent length (SVL); head depth, height of the head measured at post-orbital region (HD); head width, at the angle of the jaws (HW); head length, from the rear of the mandible to the tip of the snout (HL); inter upper eyelid width, i.e. the shortest distance between the upper eyelids (IUE); maximum upper eyelid width (UEW); snout length, measured from the tip of the snout to the anterior orbital border of the eye (SL); eye length, i.e. the horizontal distance between the bony orbital borders of the eye (EL); internarial distance, i.e. least distance between the inner margins of nares (IN); nostril–snout distance, i.e. distance between middle of nostril and tip of snout (NS); eye to nostril distance, i.e. distance between anterior-most point of eye and middleof nostril (EN); tympanum–eye distance, i.e. anterior rim of tympanum to posterior of eye (TYE); distance from the rear of the mandible to the nostril (MN); distance from the rear of the mandible to the anterior orbital border of the eye (MFE); distance from the rear of the mandible to the posterior orbital border of the eye (MBE); distance between anterior corner of eyes, i.e. the shortest distance between the anterior orbital borders of the eyes (IFE); distance between posterior corner of eyes, i.e. the shortest distance between the posterior orbital borders of the eyes (IBE); largest tympanum diameter, horizontal (TYD H); largest tympanum diameter,vertical (TYD V); forelimb length, measured from the elbow to the base of the outer palmar tubercle (FLL); hand length, measured from the base of the outer palmar tubercle to the tip of the third finger (HAL); thigh length (TL); shank length (ShL); foot length, measured from the base of the inner metatarsal tubercle to the tip of the fourth toe (FOL); distance from the heel to the tip of the fourth toe (TFOL); lengths of fngers I, II, III and IV measured from base of proximal subarticular tubercle to fingertip (FL I, II, III and IV); tibia width, i.e. width of tibia at its widest region (TW); length of toes I, II, III, IV and V measured from base of proximal subarticular tubercle to tip of toe (ToL I, II, III, IV and V); length of inner metatarsal tubercle (IMT); distance from distal edge of metatarsal tubercle to maximum incurvature of web between fourth and ffth toe (MTFF); distance from distal edge of metatarsal tubercle to maximum incurvature of web between third and fourth toe (MTTF); distance from maximum incurvature of web between fourth and ffth toe to tip of fourth toe (FFTF); distance from maximum incurvature of web between third and fourth toe to tip of fourth toe (TFTF); width at groin (WG).
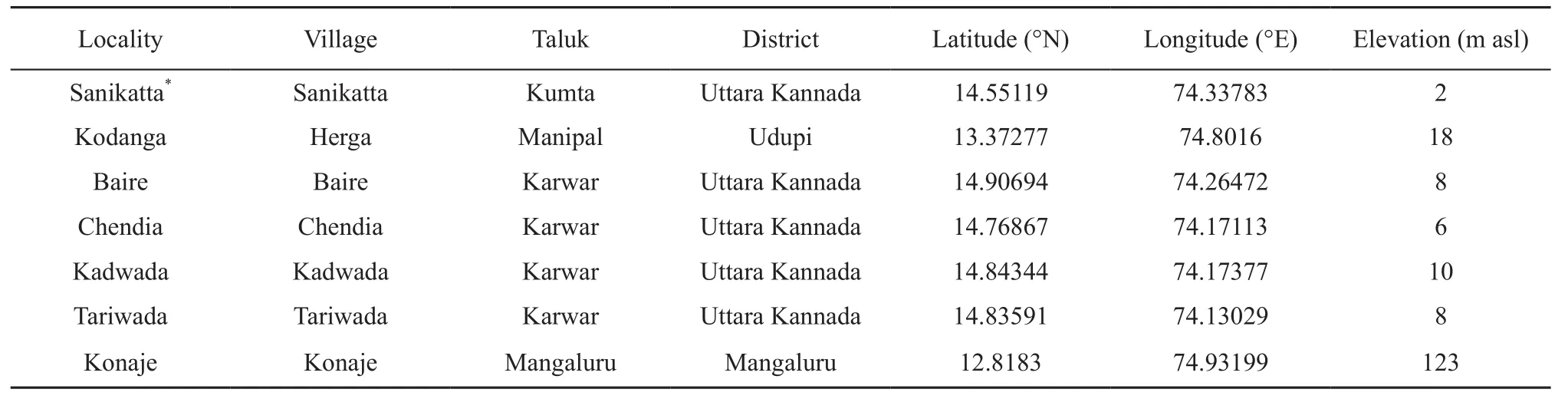
Table 1 Localities where E. karaavali sp. nov. was encountered. All localities were within Karnataka State. Asterisk indicates type locality.
2.5 Advertisement call recording and analysisCall recordings were made using Sennheiser K6®unidirectional microphone coupled with a Marantz PMD 660®solid state recorder. Calls with low signal to noise ratio were manually selected from different individual call records and were analysed using Audacity Ver.1.3 (Beta) and Raven Pro 1.5. Twenty-three calls from four individuals of Euphlyctis karaavalisp. nov.were selected for the analysis. Duration, inter-call interval duration, dominant frequency and number of pulses of each call was recorded. Call terminology was based on (Kok and Kalamandeen, 2008). Air temperature and relative humidity were recorded using TFA®digital Thermo-Hygrometer.
2.6 Molecular analysisDNA was extracted from thigh muscle tissue (n = 2) using the method described by Vences et al. (2012). PCR amplifcation and sequencing of 16S and 12S rRNA genes were carried out following Gururaja et al. (2014). The primers 12Sai, 12Sbi and 16 SA-L, 16SB-H (Simon et al., 1994; Palumbi et al., 2002) respectively were used for amplification. A total reaction volume of 20µl was used for amplifying DNA and contained 1.2µl of DNA, 0.2µl 1,000 Units/ml Taq polymerase, 2.5µl of reaction buffer, 2.5µl of 1mM dNTPs, 1.5µl of 5pmole/µl forward and reverse primers, and 10.6µl of autoclaved MilliQ water. The PCR products were sent for purification and sequencing to Chromous Biotech, Bangalore, India.
The 16S and 12S sequences were aligned separately using MAFFT algorithm (Katoh et al., 2002) along with available sequences of other Euphyctis species retrieved from GenBank (Table 2). Neighbour Joining trees were run for each dataset to check their topology. As there was no significant difference in their topology, the 16S and 12S sequences were combined and sequence divergence for the combined dataset was calculated in MEGA version 5.1 (Tamura et al., 2011) with complete deletion option where indels were not used for calibration. Sequences are deposited in GenBank (Accession numbers: KU870372–KU870375). Detail of sequences of six Euphlyctis species are given in Table 2 and combined sequences are given in Supplementary Table 1S. Sequences of E. ghoshi wasunavailable. The fnal dataset consisted of 895 base pairs in length.
Maximum likelihood (ML) algorithm and Bayesian inference methods were used for phylogenetic analysis. The ML analysis was executed in RaxML v1.3 (Silvestro and Michalak, 2012) with TIM2ef+Gmodel selected as the best-fit nucleotide substitution model in jModel test (Posada, 2008) for 1000 bootstrap replicates. The Bayesian analysis was performed in MrBayes 3.2.4 (Ronquist et al., 2012). The Markov chain Monte Carlo analysis of the dataset was run for 50 million generations and trees were sampled every 500 cycles. The convergence of the runs was analyzed by assessing the split frequency standard deviations (< 0.001) and potential scale reduction factor (PSRF ~1.0). The frst 10% of the sampled trees were discarded as burn-in and remaining samples were used to generate majority rule consensus tree. Uncorrected pair-wise sequence divergence between the species was calculated in MEGA 5.10.
2.7 ComparisonsComparisons were based on descriptions provided in publications (Boulenger, 1920; Roy and Elepfandt, 1993; Khan, 1997; Kurabayashi et al., 2005; Alam et al., 2008; Joshy et al., 2009; Howlader et al., 2015) and morphology of specimens deposited in BNHS (Voucher No. 5123-5126; 5127-5130) and RBRL (Catalogue No. 030606-01).
2.8 Maps and geographic range estimationMaps and geographic ranges were generated using QGIS® Pisa Ver. 2.10. Data was sourced from www.gadm.org for an administrative boundary and SRTM 90 m Database (http://srtm.csi.cgiar.org) for elevation. The area under minimum convex hull was computed on occurrence points of frogs to estimate the extent of occurrence.
3. Results
3.1 Molecular analysisEuphlyctis karaavali sp. nov. is distinct from all other known congeners as indicated by high genetic distance values (Table 3 and Figure 2). It differed between 9 – 13% from the six Euphlyctis species. The highest divergence was with E. kalasgramensis and E. ehrenbergii (13.04% each) and lowest with E. hexadactylus (9.21%, GenBank: AF215389). There was no divergence between E. karaavali and one of the E. hexadactylus sample from GenBank (AB167941)
3.2DiagnosisThis new species is assigned to the genus Euphlyctis as it is a large adult aquatic/semi-aquatic frog; snout pointed and elongated; obtuse canthus rostralis;eyes positioned more towards top of head; tympanum large; strong skin fold from eye to shoulder; paired lateral vocal sac; digits without discs; prominent webbing in feet; ffth toe is free up to the base; well-developed inner metatarsal tubercle; spatulate tongue, free behind and bifd.

Figure 2 Maximum Likelihood tree for seven Euphlyctis species and Hoplobatrachus tigerinus as an outgroup. Numbers above and below indicate Bayesian Posterior Probabilities and Maximum Likelihood Bootstrap values respectively. Asterisk (*) indicates values < 0.5 and < 50. For a, b and numbers given after species names, refer Table 2.
Euphlyctis karaavali sp. nov. can be distinguished from all other congeners by the following suite of morphological characters: (1) large adult size (SVL = male: 61.9 ± 7.1mm; female: 106.3 mm); (2) snout obtusely pointed in dorsal and ventral view, projected beyond mouth; (3) tongue spatulate, bifd without lingual papilla (4) large distinct tympanum with prominent supra-tympanic fold from back of eye to shoulder; (5) head wider than long; (6) skin glandular and spinular on dorsum; (7) nuptial pad present in male individuals; (8) Two dark blackish purple vocal sacs present; (9) complete webbing in feet; (10) Ventral surface with brown reticulation, denser at abdomen and hindlimbs, sparse on chest and throat.
3.3 Description of holotype (Adult Male BNHS 5989; All measurements in mm, Table 4, Figures 3 and 4):Euphlyctis karaavali sp. nov.
Suggested common name:KARAAVALI SKITTERING FROG
Holotype:BNHS 5989, an adult male collected from fallow paddy fields inundated with water in Sanikatta village, Kumta Taluk, Uttara Kannada District by CRN and KSS at 18:30 on 27thJune 2015.
Paratypes:Two males (BNHS 5988, BNHS 5990) collected in same locality and time as holotype by CRN and KSS. Two males (BNHS 5985, BNHS 5986) and one female (BNHS 5987) collected from Kodanga, Herga Village, Manipal Taluk, Udupi District, Karnataka on 26thJune 2015 at 20:15 by RS and KSS.


Figure 3 Holotype (BNHS 5989) of Euphlyctis karaavali sp. nov. A. dorsal view; B. ventral view; C. lateral profle of head; D. ventral view of forelimb; E. ventral view of foot and F. posterior view of thighs.
A large sized adult (SVL = 70.9), head arched, width larger than length (HW = 25.9 mm; HL = 24.5 mm). Snout obtusely pointed in both dorsal and ventral view, protrudes beyond mouth in ventral view, acute in lateral profle. Snout length 1.4 times the eye length (SL = 11.5 mm; EL = 8.5 mm). Canthus rostralis obtuse, loreal region concave. Interorbital space flat, less than upper eyelid width and internarial distance (IUE = 3.3; UEW = 5.6; IN = 3.7). Internarial distance between posterior margins of eyes 1.7 times that of anterior margins (IFE = 9.3; IBE = 16.1). Nostrils rounded, slightly protruding, with a small flap, closer to tip of snout than to eye (NS = 5.3; EN = 6.1). Symphysial knob prominent. Distinct tympanum, rounded (TYDH = 6.1, TYDV = 5.1) and is 1.4 times the eye length (TYDH = 6.1, EL = 8.5). Supratympanic fold distinct. Paired lateral vocal sac with a pair of openings at the base of lower jaw. Vomerine teeth present, oblique between choane. Tongue large bifid, spatulate, without lingual papilla. Eyes moderately large (EL = 8.5), protruding, pupil horizontal. Two vocal slits on lower mandible, near the base of jaws.
Forelimb length 1.3 times the hand length (FLL = 11.9; HAL = 15.7). Dermal fringes weak, on both sides of the fingers. Webbing between fingers rudimentary. Relative lengths of fingers II<I<IV<III (FL I = 5.9; FL II = 4.6; FL III = 6.9; FL IV = 5.3). Finger tips without any discs. Subarticular tubercles distinct (fnger: i = 1, ii = 1, iii = 2, iv =2) rounded and thenar tubercle indistinct, palmar tubercle round. Supernumerary tubercles absent. Nuptial pad present on frst fnger. Hindlimbs moderately long, heels do not overlap when folded at right angles to body. Shank 2.8 times longer than wide (ShL = 29.2; TW = 10.3), shorter than thigh length (TL = 29.7) and shorter than foot length (FOL = 31.6). Heel to tip of fourth toe (TFOL = 46.7) about 2.7 times longer than fourth toe length (ToL IV = 17.3). Relative toe lengths I<II<III<V<IV (ToL I = 5.6; ToL II = 8.4; ToL III = 12.4; ToL IV = 17.3; ToL V = 12.9). Toes without any discs. Webbing full, reaching the tip of all toes and sharply incised (MTTF = 23.5, MTFF = 23.8, TFTF = 9.6, FFTF
= 10.8). Inner metatarsal tubercle 1.3 times frst toe length (IMT = 4.3). Outer metatarsal tubercle absent, tarsal tubercle present (toe: i = 1, ii = 1, iii = 2, iv = 3, v = 2).
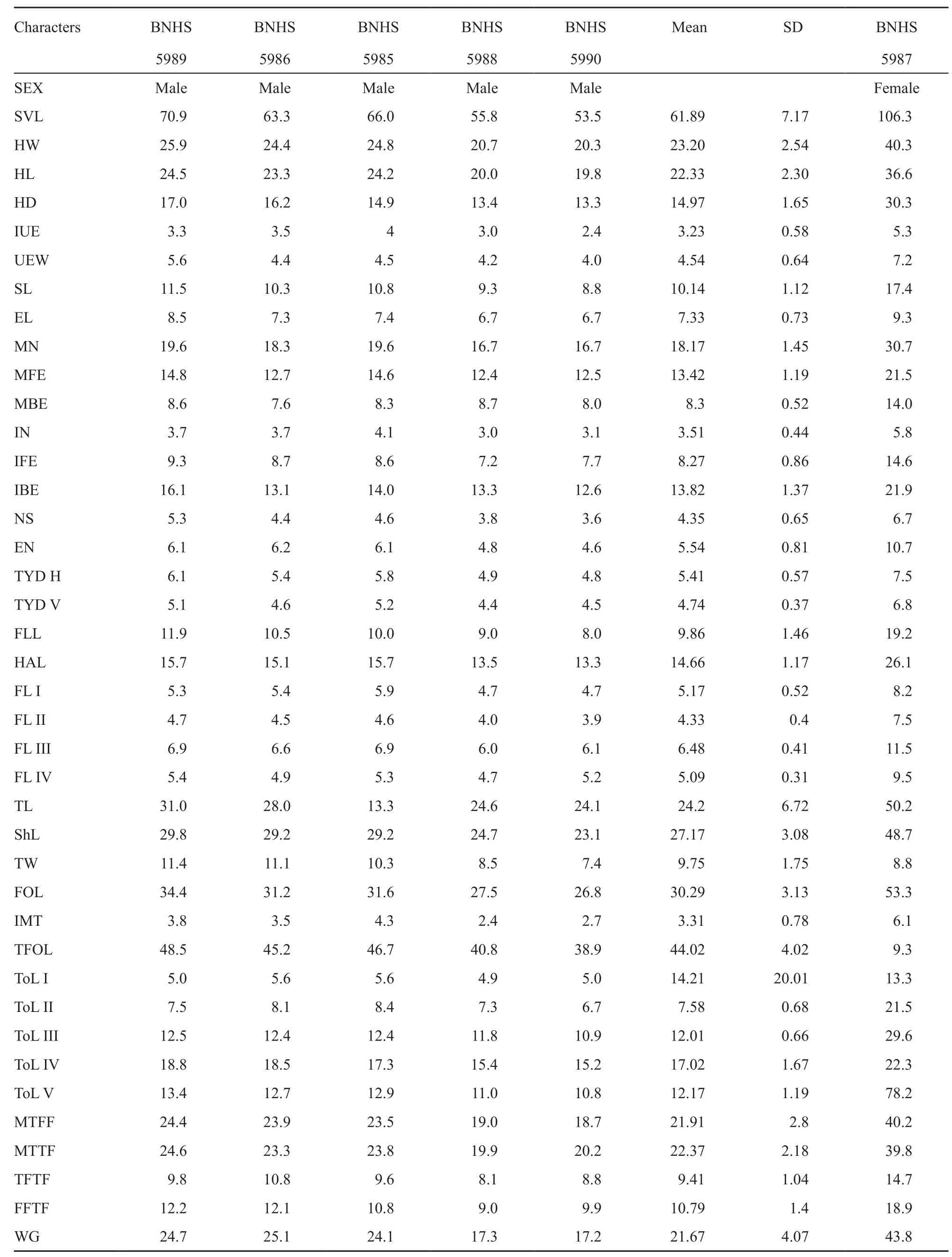
Table 4 Morphometric data of Euphlyctis karaavali sp. nov. (Holotype and Paratypes). Measurements in mm.
Skin:Dorsum shagreened with glandular tubercles and spinules. Dorsum of snout, inter orbital region, upper eyelid, sides of head, loreal region, forelimb, hindlimb with spinules. Spinules are absent on ventral surface, posterior part of hindlimb, forelimb and hand. Spinules are present on ventral surface of all toes and dorsal surface of fifth toe. Supratympanic fold distinct. Tympanum shagreened. Glandular tubercles behind eyes till groin. Glandular tubercles near groin and vent. Flanks shagreened. Skin on ventral surface shagreened. Glandular warts on throat, belly and ventral parts of thigh. Glandular warts forming two lines from base of forelimb to groin. Vocal sac shagreened. Glandular folds and macro glands are absent. Nuptial pad present. A distinct dermal fold along the frst and ffth toe.
Color in Preservative:Dorsum, forelimbs and hindlimbs dark brown. Tympanum light brown. Pupil whitish. The lower part of eyes, throat, ventral surface of forelimbs buff white. A whitish-yellow stripe on flank starting from behind tympanum to groin. A brown stripe from supratympanic fold to groin, tapering as groin. Vocal sac dark blackish purple. On dorsum, two light brown stripes from behind eyes to vent. Between light brown stripe and yellow stripe on the flank, a light brown broken stripe from mid fank to groin. Ventral surface of thigh, shank, tarsus, belly and throat with brown reticulation. Brown reticulation is sparse on throat and dense on thigh and belly. Ventral surface of hand and foot dark brown with few yellowish white mottling. Lips not barred. Groin yellowish white with brown reticulation. Webbing brown. A yellow stripe from vent along the posterior part of thigh terminating on the ventral surface at the intersection of the thigh and shank. Another yellow stripe below vent along the posterior part of thigh terminating midway on the ventral surface of thigh.
Color in Life:Overall, uniform green colored dorsum with dark yellow stripes. Snout, loreal region, upper eyelids, sides of head, forelimb, hindlimb and tympanum uniform green. Dark green between eyes and nostrils. Dark green patches on forelimb and hindlimb. Pupil black. Iris black interspersed with golden yellow vermiculations. Translucent nictitating membrane. A pale yellow band below eye. Ventral surface ivory in color with brown reticulation. Vocal sac dark blackish purple. Yellow coloration on groin, anterior part of thigh, flank and armpit. Throat buff white with sparse brown reticulation. A pale green stripe on fank starting from behind tympanum to groin. A dark green stripe from supratympanic fold to groin, tapering towards the groin. Two pale green stripes from behind eyes to vent on dorsum. Pale to dark green stripes on the fank, a light green broken stripe from mid-flank to groin. Ventral surface of thigh, shank, tarsus, belly and throat with brown reticulation. Brown reticulations are sparse on throat and dense on thigh and belly. Ventral surface of hand and foot dark brown with few yellowish-white mottling. Ventrally, fingers, toes and webbing brown. A yellow stripe from vent along the posterior part of thigh terminating at ventral surface of thigh and shank junction. Another yellow stripe below vent along the posterior part of thigh terminating midway on the ventral surface of thigh.
Variations:Variations in morphological measurements of this species from four males and a female are provided in Table 4. Females lack nuptial pad and dark blackish purple vocal sac. Males with nuptial pad. Some individuals have a pale green colored mid-dorsal line from tip of snout to the vent (Figure 4).
Etymology:The specific epithet ‘Karaavali’ is derived from the Kannada language, a name given to the coastal region. The species name Karaavali is a noun in apposition to the generic name.
Comparisons
Euphlyctis karaavali sp. nov. can be distinguished from all other known congeners in this genus using a suite of characters. It can be clearly distinguished from its closest congener, E. hexadactylus in having a smaller ratio of HW/SVL (E. karaavali sp. nov. vs. E. hexadactylus: 0.36 vs. 0.40); ratio of HD/SVL small vs. larger (0.24 vs. 0.3); smaller MN/SVL vs. larger (0.27 vs. 0.29); smaller FLL/ SVL vs. larger (0.16 vs. 0.19); smaller HAL/SVL vs. larger (0.22 vs. 2.88); and larger ToL I/SVL vs. smaller (0.7 vs. 0.1). Further, E. karaavali sp. nov. is distinct from E. hexadactylus in having dark blackish purple vocal sacs in life (grey in preservative) vs. translucent straw yellow in life (yellowish in preservative); ventral surface with brown reticulations vs. reticulations absent; prominent black lateral stripe from base of forelimb vs. absent. The large genetic distance supports these morphological differences as well.

Figure 4 Live individuals of Euphlyctis karaavali sp. nov. A. Paratype BNHS 5986; B Paratype BNHS 5985.
Amongst the other species in this genus, E. aloysii differs from E. karaavali sp. nov. in having a small adult male (up to 45.2 mm) vs. large adult male (70.9 mm); posterior surface of thigh with three, brown horizontal stripes interspersed with white vs. posterior surface of thigh brown, with ivory speckles; dorsum with elliptical markings vs. markings not elliptical. Euphlyctis cyanophlyctis differs from E. karaavali sp. nov. in having a small adult size (40 mm, adult male from Trivandrum, Boulenger, 1920) vs. relatively large size (SVL of male = 70.9 mm); snout not extending beyond lower jaw in dorsal view vs. snout extending well beyond lower jaw in dorsal view; first and second finger equally long vs. first finger longer than second; toe tips dilated vs. pointed. Euphlyctis mudigere differs from E. karaavali sp. nov. in having small sized adult male vs. (SVL = 31.1 mm) vs. large sized adult male (SVL = 70.9 mm); nostrils nearer to eye than to tip of snout (EN = 2.6 mm, NS = 3.0 mm) vs. nostrils closer to snout than to eye (EN = 6.1 mm, NS = 5.3 mm); ventral surface immaculate vs. ventral surface with brown reticulations. Euphlyctis ghoshi differs from E. karaavali sp. nov. in having a rounded snout vs. obtusely pointed; small but prominent subarticular tubercles vs. big and distinct subarticular tubercles; dorsum with irregular blotches vs. blotches absent. Euphlyctis kalasgramensis differs from E. karaavali sp. nov. small adult male (SVL = 37.9 mm) vs. larger adult male (SVL = 70.9 mm); skin on throat smooth vs. skin on throat granular; dorsal surface without any markings vs. dorsum with irregular mottling.
Euphlyctis ehrenbergii differs from E. karaavali sp. nov. in smaller body size (male, SVL = 60–66 mm; female, SVL = 75–92 mm) vs. larger body size (male, SVL = 53.5–70.9 mm; female, SVL = 106.3 mm); equal I and II Finger vs. I longer than II Finger; equal snout and eye length vs. snout length 1.4 times longer than eye length; traverse striations on abdomen vs. brown reticulation on ventral surface. Advertisement call characteristics – long duration (0.93 ± 0.21s) vs short duration (0.324 ± 0.02 s); more number of pulses (11 ± 2) vs less number of pulses (5–10) and dominant frequency in two frequency ranges (937 ± 74 and 2406 ± 39 Hz) vs. 2677.36 ± 18.93 Hz.
3.4 Advertisement call analysisThe advertisement calls of E. karaavali sp. nov. were recorded on 26thJune 2015 between 17:30–18:30 h; Air Temperature: 27.9°C; Relative Humidity: 95% at Manipal 13.3593°N, 74.7979°E, 50 m asl. The advertisement call spectrogram of
E. karaavali sp. nov. is given in Figure 5. Calls of E. karaavali sp. nov. had 5-10 pulses in each call (Mean ± SE, 6.4 ± 0.35, n = 23). Average dominant frequency was 2677.36 ± 18.93 Hz (range: 2530–2804 Hz) and call duration was 0.324 ± 0.02 s (range: 0.2–0.5 s). We observed 2ndharmonics in E. karaavali sp. nov. at 5542.87 ± 24.86 Hz (range: 5388–5742 Hz). A sample video of E. karaavali sp. nov. is given as Supplementary video clip 1.
3.5 Natural HistoryEuphlyctis karaavali sp. nov. is known from coastal plains along Karnataka state in India. It is a common frog species in the region and can found
calling from rainwater inundated fallow agriculture felds,
small manmade tanks, pools and puddles around human habitations. Advertisement calls resemble calls of whitethroated Kingfisher bird (Halcyon smyrnensis). Other anuran species like Hoplobatrachus tigerinus, Fejervarya sp., Duttaphrynus melanostictus, Microhyla ornata, M. laterite, Euphlyctis cyanophlyctis and E. aloysii co-occur with E. karaavali in sampled localities.
3.6 Geographic range and IUCN statusEuphlyctis karaavali sp. nov. was found in coastal plains along Karnataka state in India (Figure 1). The geographic extent of occurrence as determined from minimum convex hull was 2574.27 km2. We estimated about 2-4 individuals/100m2in Sanikatta, Baire and Herga during rainy season. However, there is limited information on population size, fluctuation and trends, number of individuals and generation length. As per IUCN Red List criteria based on extent of occurrence, a few known localities, less number of individuals and severely
fragmented habitats, this species qualifes to be listed asEndangered (EN) under B1ab(i)(iii)(iv).

Figure 5 Advertisement call spectrogram of E. karaavali sp. nov. A. amplitude B. spectrogram C. amplitude of single call and D. spectrogram of single call.
4. Discussion
We encountered a species of Euphlyctis in Karnataka state in Southwestern part of India which topically resembled E. hexadactylus. Previous studies in this region have suggested the presence of a E. hexadactylus subpopulation (Alam et al., 2008; Joshy et al., 2009) or considered it to be cryptic (Howlader et al., 2015) but do not attempt to investigate further. We report the discovery of a new cryptic lineage which we described as E. karaavali. on the basis of molecular analysis of two genes and morphological comparisons. Our finding supports earlier claims of a yet to be described Euphlyctis in South-western India. With the description of E. karaavali, there are now eight species of Euphlyctis and we suspect much more to be revealed by systematic surveys using an integrative taxonomic approach.
The molecular analysis in our study depicts a high genetic divergence among E. hexadactylus sequences available in GenBank. However, gene sequence of E. karaavali matched with a sequence published by Kurabayashi et al. (2005) (AB167941, Catalogue Number: 030606–01 deposited at RBRL). Kurabayashi et al. (2005) assigned the sequence AB167941 to E. hexadactylus hpEB haplotype based on the 16S genetic divergence of 8.75% with a Sri Lankan E. hexadactylus (AF215389) provided in Vences et al. (2000). Subsequently, Joshy et al. (2009) used the same specimen mentioned in Kurabayashi et al. (2005) (Catalogue No. 030606-01, collected from Adyar, Mangaluru) as E. hexadactylus without providing the basis for considering it as E. hexadactylus. We suggestthe name of AB167941 sequence to be E. karaavali on the basis of the phylogenetic tree presented in this study and morphological comparisons with a specimen at RBRL (Catalogue No. 030606-01). The identity of E. hexadactylus is not certain owing to the original type series of E. hexadactylus being lost. Seshadri et al., (Unpublished) addresses this problem wherein a collection of Euphlyctis individuals from the type locality“Pondicherry” (now called Puducherry) that matched the original morphological description of E. hexadactylus given in Lesson (1834) and Boulenger (1920) are assigned as type series with detailed morphological measurements. Seshadri et al. (unpublished) used morphological differences in size (SVL) and size ratios to other parts of the body, vocal sac and body coloration, and genetic differences to assign it to E. hexadactylus. The comparisons provided in this paper illustrate E. hexadactylus to be distinct from E. karaavali. There is one more haplotype under the name E. hexadactylus (Meenakshi et al., 2009) that shows high genetic divergence from E. hexadactylus collected from type locality (6%) suggesting that there are more cryptic species that is needed to be described under Euphlyctis genus.
Another highlight of our study is the discovery of a new species in largely human-dominated landscapes and agricultural areas. A vast landscape of India is humandominated and amphibian diversity is known to be high outside of forests with a protected status within the Western Ghats (Das et al., 2006). Efforts to reconcile this high diversity of life in human-dominated landscapes have been discussed (See: Rosenzweig, 2003) and require the participation of several stakeholders. The areas in which we found the new species are largely unprotected and disregarded habitats. Several developmental activities like expressways are poised to destroy the habitat in which the frogs were found. Efforts for a systematic survey with a focus on mitigating damages from a further increase in anthropogenic activities are imminent. Engaging citizens in such initiatives will prove valuable.
AcknowledgementsThanks to SUDHIRA H. S., Director, Gubbi Labs LLP, for providing office space. We thank Raghuvansh SAXENA, Country Director, Earthwatch Institute India for supporting the travel. Rahul KHOT, curator BNHS assisted in deposition of vouchers. KSS was supported by The Mohamed bin Zayed Species Conservation Fund and the Chicago Board of Trade (CBOT) Endangered Species Fund.
Alam M. S., Igawa T., Khan M. M., Islam M. M., Kuramoto M., Matsui M., Kurabayashi A., Sumida M. 2008. Genetic divergence and evolutionary relationships in six species of genera Hoplobatrachus and Euphlyctis (Amphibia: Anura) from Bangladesh and other Asian countries revealed by mitochondrial gene sequences. Mol Phylogenet Evol, 48(2): 515–527
Boulenger G. A. 1920. A monograph of the South Asian, Papuan, Melanesian and Australian frogs of the genus Rana. India: Zoological Survey of India
Das A., Krishnaswamy J., Bawa K. S., Kiran M. C., Srinivas V., Kumar N. S., Karanth K. U. 2006. Prioritisation of conservation areas in the Western Ghats, India. Biol Conserv, 133(1): 16–31
Frost D. R. 2016. Amphibian Species of the World: an Online Reference. Version 6.0. American Museum of Natural History, New York, USA. Available: http://researchamnhorg/herpetology/ amphibia/indexhtml
Gururaja K. V., Dinesh K. P., Priti H., Ravikanth G. 2014. Mudpacking frog: a novel breeding behaviour and parental care in a stream dwelling new species of Nyctibatrachus (Amphibia, Anura, Nyctibatrachidae). Zootaxa, 3796: 33–61
Hartop E. A., Brown B. V., Disney R. H. 2015. Opportunity in our Ignorance: Urban Biodiversity Study Reveals 30 New Species and One New Nearctic Record for Megaselia (Diptera: Phoridae) in Los Angeles (California, USA). Zootaxa, 3941: 451–484
该基础的防水板与独立基础一样,通过素混凝土垫层与地基相连,相互影响,采用“抗”和“消”相结合的结构抗浮设计,该类防水板下设聚苯板软垫层一般能承担30%左右的上部荷载,通过调整软垫层,达到竖向向下合力作用下防水板的变形与独立基础沉降变形相对沉降差为零或最小,从而使防水板不承担或者承担最少量的地基反力,最终达到平衡受力状态。独立基础加防水板下设聚苯板软垫层的基础形式是独立基础和防水板整体浇注在一起,只要二者存在变形差异,就必须将相互影响,产生协调变形,存在内力重分布达到平衡受力状态。当独立基础沉降时,聚苯板软垫层收到压缩,防水板只受软垫层的弹力,因此不会开裂。
Howlader M. S., Nair A., Gopalan S. V., Merila J. 2015. A new species of Euphlyctis (Anura: Dicroglossidae) from Barisal, Bangladesh. PLoS ONE, 10(2): e0116666
Joshy S. H., Alam M. S., Kurabayashi A., Sumida M., Kuramoto M. 2009. Two new species of the genus Euphlyctis (Anura: Ranidae) from Southwestern India as revealed by molecular and morphological comparisons. Alytes, 26(1-4): 97–116
Katoh K., Misawa K., Kuma K. I., Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic acids res, 30(14): 3059–3066
Khan M. S. 1997. A new subspecies of common skittering frogEuphlyctis cyanophlyctis (Schneider, 1799) from Balochistan, Pakistan. Pakistan J Zool, 29: 107–112
Kok P. J., Kalamandeen M. 2008. Introduction to the taxonomy of the amphibians of Kaieteur National Park, Guyana. Belgian Development Cooperation
Kosuch J., Vences M., Dubois A., Ohler A., Bohme W. 2001. Out of Asia: Mitochondrial DNA evidence for an oriental origin of tiger frogs, genus Hoplobatrachus. Mol Phylogenet Evol, 21: 398–407
Kurabayashi A., Kuramoto M., Joshy H., Sumida M. 2005. Molecular Phylogeny of the Ranid Frogs from Southwest India based on the Mitochondrial Ribosomal RNA Gene Sequences. Zool Sci, 22: 525–534
Meenakshi K., Sujith VG., Sanil G. 2009. DNA barcoding of some amphibians of Western Ghats. 3rdInternational Barcode of life Conference (In Press)
Palumbi S., Martin A., Romano S., McMillan W., Stice L., Grabowski G. 2002. The simple fool’s guide to PCR. Honolulu USA
Posada D. 2008. jModelTest: phylogenetic model averaging. Mol Biol Evol, 25: 1253–1256
Priti H., Roshmi R. S., Ramya B., Sudhira H. S., Ravikanth G., Aravind N. A., Gururaja K. V. 2016. Integrative Taxonomic Approach for Describing a New Cryptic Species of Bush Frog (Raorchestes: Anura: Rhacophoridae) from the Western Ghats, India. PLoS ONE, 11(3): e0149382
Ronquist F., Teslenko M., van der Mark P., Ayres D. L., Darling A., Höhna S., Larget B., Liu L., Suchard M. A., Huelsenbeck J. P. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol, 61(3): 539–542
Rosenzweig M. L. 2003. Reconciliation ecology and the future of species diversity. Oryx, 37(02): 194–205
Roy D., Elepfandt A. 1993. Bioacoustic analysis of frogs from northeast India. J Biosci, 18(3): 381–393
Seshadri K.S., Ramit S., Priti H., Ravikanth G., Vidisha M.K., Saurabh S., Pratik M., Gururaja K.V. 2016. Microhyla laterite sp. nov., A New Species of Microhyla Tschudi, 1838 (Amphibia: Anura: Microhylidae) from a Laterite Rock Formation in South West India. PLoS ONE, 11(3): e0149727. doi:10.1371/journal. pone.0149727
Silvestro D., Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol, 12(4): 335–337
Simon C., Friati F., Beckenbach A., Crespi B., Liu H., Flook P. 1994. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am ,87(6): 651–701
Tamura K., Petersonm D., Peterson N., Stecher G., Nei M., Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol, 28: 2731–2739
Vences M. 2000. Phylogenetic studies of ranoid frogs (Amphibia: Anura), with a discussion of the origin and evolution of the vertebrate clades of Madagascar. PhD Thesis submitted to Universitaet Bonn
Vences M., Nagy Z., T, Sonet G., Verheyen E. 2012. DNA barcoding Amphibians and reptiles. Kress, WJ & Erickson, DL (eds) DNA Barcodes: Methods and Protocols, Methods in Molecular Biology Springer Science+Business Media, LLC 2012: 79–108
(This article is a submission to the WCH8 conference)
*Corresponding authors: Kotambylu Vasudeva GURURAJA, from Gubbi Labs, Indian Institute of Science Campus, Bengaluru, India, with his research focusing on anuran ecology, behaviour and conservation. E-mail: gururaja@gubbilabs.in
Received: 6 March 2016 Accepted: 9 September 2016
猜你喜欢
杂志排行
Asian Herpetological Research的其它文章
- Isolation and Characterization of 15 Microsatellite DNA Loci for the Alpine Stream Frog Scutiger boulengeri (Anura: Megophryidae)
- A Field Observation and Signifcant Range Extension of Manouria impressa in Myanmar
- Major Factors Affecting the Distribution of Anuran Communities in the Urban, Suburban and Rural Areas of Shanghai, China
- Effects of Dietary Vitamins A, B2, and B6Supplementation on Growth and Feed Utilization of Juvenile Chinese Soft-shelled Turtle Pelodiscus sinensis according to an Orthogonal Array Experiment
- Effects of Feeding Time on the Growth Performance and Variation of RNA/DNA Ratio of the Chinese Soft-shelled Turtle, Pelodiscus sinensis
- Plasticity in Metamorphic Traits of Rice Field Frog (Rana limnocharis) Tadpoles: The Interactive Effects of Rearing Temperature and Food Level
