Genotoxicity of Three Avermectins on Polypedates megacephalus Tadpoles Using the Comet Assay
2017-01-20BaorongGENGLinglingZHANGYunJIANGXiupingHUANGandJinmeiDAI
Baorong GENG, Lingling ZHANG, Yun JIANG, Xiuping HUANG and Jinmei DAI
College of Life Sciences, Fujian Normal University, Fuzhou 350117, Fujian, China
Genotoxicity of Three Avermectins on Polypedates megacephalus Tadpoles Using the Comet Assay
Baorong GENG*, Lingling ZHANG, Yun JIANG, Xiuping HUANG and Jinmei DAI
College of Life Sciences, Fujian Normal University, Fuzhou 350117, Fujian, China
Avermectins are a new class of macrocyclic lactones derived from mycelia of the soil actinomycete, and are used as effective agricultural pesticides and antiparasitic agents. However, run-off from crops treated with avermectins may contaminate various bodies of water, and accumulated to certain concentrations to impact the development of aquatic animals. Here, we tested the genotoxicity of three avermectins (abamectin, ABM; ivermectin, IVM; and emamectin benzoate, EMB) on Polypedates megacephalus tadpoles by the alkaline single-cell gel electrophoresis assay. Tadpoles were treated for 48 h in the laboratory with different concentrations of these three agents, 0.006, 0.012, 0.018, 0.024, 0.030 mg/L for ABM, 0.003, 0.006, 0.009, 0.012, 0.015 mg/L for IVM and 0.04, 0.06, 0.08, 0.10, 0.12 mg/L for EMB, and then measured their DNA damage by the Comet assay tail factor %. The concentrations of resulted in highly signifcant increases in DNA damage of the tadpoles were found above the concentration threshold of 0.012 mg/ L ABM, 0.003 mg/L IVM and 0.06 mg/L EMB and linear correlations between the intensity of DNA damage and the concentrations of these three avermectins. Our results showed clearly that avermectins caused dose dependent DNA damage on amphibian tadpoles, and there might be a control on the misuse of avermectins.
Polypedates megacephalus, tadpole, avermectins, abamectin, ivermectin, emamectin benzoate, DNA damage, comet assay
1. Introduction
Amphibian decline in almost all over the world (Stuart et al., 2004; Xie et al., 2007), with diverse speculations regarding the causes (Ankley et al., 1998; Davidson, 2004; Wang and Jia, 2009). Chemical contamination in aquatic environment as a consequence of pesticide application continues to be postulated as a contributing factor for the decline (Berrill et al., 1997; Mann and Bidwell, 2001). Indeed, amphibians may be at greater risk of the toxic effects of pollutants than other aquatic vertebrates due to their special physiological and life history characteristics. Amphibian skins are highly absorptive, contaminants have the potential to easily permeate the epidermis (Tyler, 1994), and some amphibians often prefer to breed in shallow, lentic, or ephemeral water bodies, where contaminants may accumulate without dilution (Duellman and Trueb, 1994). Avermectins and their derivatives are very effective agricultural pesticides and antiparasitic agents, and nowadays are used widely in veterinary, and agricultural fields. About 2500 tons of avermectins is produced annually in China, with production expected to increase in the future (Sun and Meng, 2009). Avermectins are a new class of macrocyclic lactones derived from mycelia of the soil actinomycete, Streptomyces avermitilis, with four closely related major components, A1a, A2a, B1a and B2a, and four minor components, A1b, A2b, B1b and B2b, which are lower homologs of the corresponding major components (Danishefsky et al., 1989). These compounds were reported to be possessing insecticidal, acaricidal and nematicidal properties and the mechanism of toxicity is fundamentally different from those associated with current natural and synthetic pesticides (Putter et al., 1981). Among these components, the B1 fractions (ABM, abamectin, a blend of B1a and B1b avermectins) display the most effective antiparasitic activities (Egerton et al., 1979) and was selected fordevelopment to control phytophagous mites and insect pests on a variety of agricultural and horticultural crops worldwide (Reddy, 2013). Ivermectin (IVM, 22, 23-dihydroavermectins B1) is semisynthetic derivatives of avermectins B1 with the same effective antiparasitic activity and registered and widely used in veterinary medicine against scab mites (Currie and McCarthy, 2010). Emamectin benzoate (EMB), 4'-deoxy-4'-epi-methyl amino benzoate salt of avermectins B1, is structurally similar to natural fermentation products. It is a mixture of two avermectin homologues: a major constituent (≥90%) MAB1a and a minor constituent (≤ 10%) MAB1b. It has unprecedented potency against a broad spectrum of lepidopteron pests and is used for controlling lepidopteron pests in agricultural felds (Singh et al., 2013).
More and more frequent application and broad array of uses, the potential negatively impact of these three agents needs to be carefully considered. After the investigation of acute toxicities, the genotoxicity of these avermectins to Polypedates megacephalus tadpoles were evaluated in this study using the Alkaline Single-Cell Gel Electrophoresis Assay (SCGE) or Comet assay, an effective and sensitive assay for testing DNA damage caused by mutagens (Tice, 1995).
2. Materials and Methods
2.1 ChemicalsNormal-melting-point agarose (NMA), Low-melting-point agarose (LMA), Triton X-100, and Tris (Tris hydroxymethyl) aminonethane hydrochloride were obtained from BBI (Ontario, Canada). Dimethylsulfoxide (DMSO) and ethidium bromide (EtBr) were purchased from Amersco (UKAS), while methylmethane sulfonate (MMS) and Trypan-blue dye was obtained from Sigma (St. Louis, MO). Other general reagents and chemicals used for the comet assay were purchased from Sangon Biotech (Shanghai) Co., Ltd. ABM and IVM were provided by Chengdu Aikeda Chemical Product Co., Ltd. (Chengdu, China), and EMB was provided by Yinnong Biochemical Industry Co., Ltd. (Huizhou, China).
2.2 AnimalsPolypedates megacephalus (Anura: Rhacophoridae) is a medium-sized treefrog, widely distributed in southeastern China. It was chosen as the test animal for this study due to its presence in many disturbed agricultural areas, and its reproductive period is relatively long (Cai, 1979). The chance for the tadpoles to contact these agents is very high. The tadpoles were collected from farm felds in Geling Town, about 50 km southwest from Fuzhou, Fujian Province, China, and reared to Gosner-stage 37–38 tadpoles in the laboratory (Gosner, 1960).
2.3 TreatmentAll tadpoles were held in glass tanks in dechlorinated water and fed with eel fodder and yolk. After 5–7 days of acclimation, healthy tadpoles with the same stage were selected for the genotoxic tests.
The maximum test concentrations used in the assay were based on approximate 60% of the 48 h LC50concentrations for ABM (0.030 mg/L), IVM (0.015 mg/ L) and EMB (0.120 mg/L), and then a series of dilutions were made from these concentrations (Table 1). These 48 h LC50concentrations were derived from a report on acute toxicity in P. megacephalus tadpoles that will be published separately (in preparation). 60% of the 48 h LC50concentrations for these agents were chosen as the maximum test concentrations since more than the concentrations could induce a part of tadpole death and disturbed experimentation. A 48-h exposure to all concentrations resulted in 100% tadpole survival.
A total of 408 tadpoles were divided into three parts (i.e., replicated 3 times) with each part consisting of 136 individuals. There were 8 tadpoles per group including negative, positive controls and various treated groups were conducted in the dark in 2 L beakers containing 1500 mL of dechlorinated water, 1500 mL of 3.125 mg/L MMS, or 1500 mL of the various concentrations of these avermectins.
2.4 Alkaline Comet AssayThe procedure described by Ralph et al. (1996) and Geng et al. (2010) was employed, with some modifications. All animals were processed individually. The animals were truncated tails and placed immediately into 1 mL of cold phosphate buffered saline (PBS, calcium- and magnesium- free) for 5 min. Each roughened microscope slide was coated with 200 μL of 0.7 % NMA at 37°C, and then covered with a coverslip and transferred to a humidified box at 4°C for 25 min to allow the solidification of agarose. The erythrocytes (30 μL) were then mixed with 0.7 % LMA (100 μL) and this suspension was pipetted onto fully frosted slides and covered with coverslips. The slides were stored in the dark at 4°C for 30 min to allow complete polymerization of the agarose. The coverslips then were removed and the slides were immersed into freshly made lysing solution (pH = 10) and incubated at 4°C in the dark for 2 h. After lysis, the slides were drained and placed in an alkaline electrophoresis buffer for 30 min. For the electrophoresis, the power supply was set at 20 V and the current adjusted to 200 mA by slowly changing the buffer level in the tray. Slides were electrophoresed in the dark at 4°C for 30 min. After electrophoresis, the slides were placed in a stainingtray and covered with a pH 7.5 Tris-HCl neutralizing buffer in the dark for 15 min. This last step was repeated 3 times. The slides were drained, overlayered with 20 μg/ mL EtBr, covered with coverslips, and examined at 400× using a fluorescence microscope. All slides were coded and examined blindly. Routinely, 100–120 cells were examined per animal.
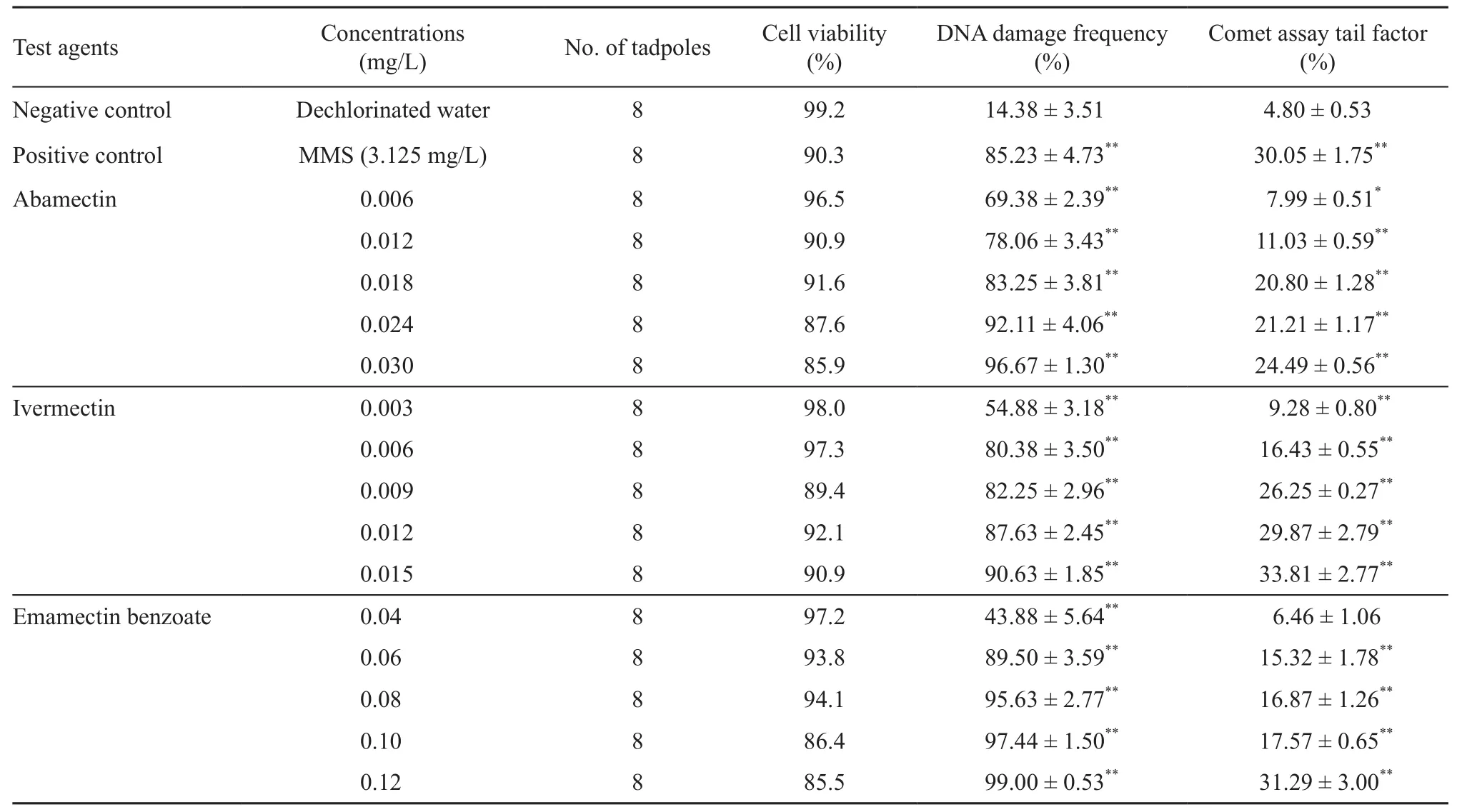
Table 1 Detection of DNA damage with DNA damage frequency and cell viability in erythrocytes of Polypedates megacephalus tadpoles (Gosner-stage 37-38) after a 48 h exposure to different concentrations of abamectin, ivermectin and emamectin-benzoate.
2.5 Statistical AnalysisThe standard of classify comets as 0–4 class (Collins et al., 1995) was used according to degree of DNA damage using software CASP in the study (Figure 1). Comet assay tail factor % was used as DNA damage degree according to Valic et al. (2004). Comet assay tail factor % = ∑i×Fi (The “i” was coeffcients of the various classify comets, with 2.5,12.5, 30, 67.5 and 97.5, respectively; and the “Fi” was the percent of various class damages). Prior to any statistical tests all variables were tested for normality using the Kolmogorov- Smirnov test and for homogeneity of variances using Bartlett’s test. The results of the different treatment groups relative to the negative control groups were compared using non-parametric comparisons (Kruskal-Wallis test). Alpha levels of 0.05 and 0.01 were used to determine significance in all statistical analysis. Linear regression analyses were carried out to establish correlations between dose and DNA damage (Comet assay tail factor %). All data processing was made using statistical software SPSS 19.0.

Figure 1 Classifcation of comets as 0–3 class in erythrocytes of Polypedates megacephalus tadpoles.
3. Results
No death and morbidity of the tadpoles were observed after the treatment. DNA damage degrees (Comet assay tail factor %) with DNA damage frequency and cell viability in each treatment group were summarized in table 1.
As shown in table 1, Polypedates megacephalus tadpoles exposed to the lower concentrations of EMB (0.040 mg/L) did not show a significant increase in the mean Comet assay tail factor % compared to those of the negative control (P > 0.05). However, the tadpoles exposed to the lower concentrations of ABM (0.006 mg/ L) showed a significant increase in DNA damage (P <0.05), and the tadpoles exposed to other concentrations of the three avermectins showed a highly significant increase in DNA damage (P < 0.01). Similarly, the tadpoles exposed to MMS (3.125 mg/L) showed a strong signifcant increase in DNA damage (P < 0.01).
The three avermectins increased the DNA damage observed in the tadpoles in a dose-responsive manner. There were strong linear correlations between the DNA damages and the concentrations of the three test substances (Figure 2). The cellular distributions of DNA damages in tadpoles are shown in Figure 3. Of the tadpoles treated with increasing concentrations of the three test substances, higher proportions of cells had greater amount of DNA damage than those of the negative control.

Figure 2 Linear correlations between the DNA damages (Comet assay tail factor %) of tadpoles and the concentrations of abamectin, ivermectin and emamectin benzoate.
4. Discussion
Cell viability was found to be more than 85 %, measure up the most current internationally accepted standards for conducting the comet assay (Tice et al., 2000), using the Trypan-blue dye exclusion technique. DNA damage frequency and DNA damage degree were evaluated in the testes of three avermectins-exposed Polypedates megacephalus tadpoles. The results indicate that the comet assay can detect DNA damage induced by exposing P. megacephalus tadpoles to avermectins.
Although numerous studies report on the toxicities of avermectins (Madsen et al., 1990; Herd, 1995; Davies et al., 1998; Katharios et al., 2002; Jensen et al., 2003; Jencic et al., 2006; Sanderson et al., 2007; Yu et al., 2007; Fanigliulo and Sacchetti, 2008; Jiang et al., 2008; Römbke et al., 2009; Egeler et al. 2010; Römbke et al., 2010; Tang et al., 2011; Prichard et al., 2012; Bansod et al., 2013), little information is available on their genotoxicities. The 96-h LC50values of ABM to Brachydanio rerio, Oncorhynchus mykiss and Pelophylax nigromaculatus were 55.1 μg/L (Tisler and Erzen, 2006), 3.2 μg/L (Jencic et al., 2006) and 43.2 μg/L (Wang and Zhao, 2013), respectively. The 96-h LC50values of IVM to Salmo gairdneri and Xenopus laevis larvae were 3.3 μg/L (Bloom and Matheson, 1993) and 5.5 μg/L (Martini et al, 2012), respectively. The 96 h-LC50values of EMB to Brachydanio rerio and Rana zhenhaiensis tadpoles were 0.113 mg/L (Wei et al., 2008) and 0.129 mg/L (Chen et al., 2011) , respectively. The adverse effectof ABM was found on male rat fertility (Elbetieha and Da’as, 2003) and it might have reproductive toxicity (Bing, et al., 2008). Wang and Zhao (2013) found that ABM can induce micronucleus and nuclear anomalies in erythocytes of Pelophylax nigomaculatus. Zhang et al. (2014) recently reported an in situ assay for quantifying genotoxicity of IVM to the tadpoles at Gosner stage 30-33 in laboratory conditions using alkaline SCGE, and EMB was also found to produce genotoxicity on Rana zhenhaiensis tadpoles (Fang et al., 2010).

Figure 3 Distribution of DNA damage (based on damage class of DNA patterns pooled across 8 tadpoles in each dose group) observed at the cellular level in Polypedates megacephalus tadpoles after exposure for a 48 h period to selected concentrations of abamectin, ivermectin and emamectin benzoate.
According to these results above and our fnding that avermectins can cause DNA damage in tadpoles at the concentrations below the recommended applied levels (Xu et al., 2010), we consider it possible that avermectins are carcinogenic, and confrm it has the negative impact on the development of tadpoles. Amphibian tadpoles were found to be susceptible to genetic damage caused by short-term exposure to low concentrations of chemicals(Ralph et al., 1996; Clements et al., 1997). Our study also shows amphibian tadpoles may be considered as a sensitive biomonitor for detecting the genotoxic potential of avermectins.
In conclusion, because of their genotoxic effects at relatively low concentrations, dose- dependent responses, frequent application and broad array of uses, avermectins likely pose a threat to organisms inhabiting in small water bodies.
AcknowledgementsWe thank Dr. Xiaohong HUANG for her helping to improve the English of this article. The research was granted by the Natural Science Foundation of Fujian, China (2015J01124).
Ankley G. T., Tietge J. E., Defoe D. L., Jensen K. M., Holcombe G. W., Durhan E. J., Diamond S. A. 1998. Effects of ultraviolet light and methoprene on survival and development of Rana pipiens. Environ Toxicol Chem, 17: 2530–2542
Bansod Y. V., Kharkar S. V., Raut A., Choudalwar P. 2013. Abamectin: an uncommon but potentially fatal cause of pesticide poisoning. Int J Res Med Sci, 1: 285–286
Berrill M., Bertram S., Pauli B. 1997. Effects of pesticides on amphibian embryos and tadpoles. In: Green D. M. (ed). Amphibians in decline: Canadian studies of a global problem. Society for the Study of Amphibians and Reptiles, St. Louis, MO. 233–245
Bing X., Ru S. G., Zhou W. L., Jia Y. G. 2008. Avermectin’s safety evaluation of environmental estrogenic activity and reproductive toxicity. J Wuhan Univ, 54: 745–750 (In Chinese)
Bloom R. A., Matheson J. C. 1993. Environmental assessment of avermectins by the US Food and Drug Administration. Vet Parasitol, 48: 281–294
Cai M. Z. 1979. Observations on reproductive habits of thirty-two anuran species of Fujian Province. J Fujian Nor Univ, (1): 71–79 (In Chinese)
Chen Z. X., Fang X. Q., Lin L., Geng B.R. 2011. Acute toxicity of emamectin benzoate on Rana zhenhaiensis tadpoles. J Ningde Teach Coll, 23: 21–23 (In Chinese)
Clements C., Ralph S., Petras M. 1997. Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline sing-cell gel DNA electrophoresis (Comet) assay. Environ Mol Mutagen, 29: 277−288
Collins A. R., Ma A. G., Duthie S. J. 1995. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutation Res, 336: 69–77
Currie B. J., McCarthy J. S. 2010. Permethrin and Ivermectin for Scabies. N Engl J Med, 362: 717–725
Danishefsky S. J, Armistead D. M., Wincott F. E., Selnick H. G., Hungate R. 1989. The total synthesis of avermectin-A1A. J Am Chem Soc, 111: 2967–2980
Davidson C. 2004. Declining downwind: Amphibian population declines in California and historical pesticide use. Ecol Appl, 14: 1892−1902
Davies I. M., Gillibrand P. A., McHenery J. G., Rae G. H. 1998. Environment risk of ivermectin to sediment dwelling organisms. Aquaculture, 163: 29–46
Duellman W. E., Trueb L. 1994. Biology of amphibians. Baltimore: The John Hopkings University Press
Egeler P., Gilberg D., Fink G., Duis K. 2010. Chronic toxicity of ivermectin to the benthic invertebrates Chironomus riparius and Lumbriculus variegates. J Soils Sedime, 10: 368–376
Egerton J. R., Ostlind D. A., Blair L. S., Eary C. H., Suhayda D., Cifelli S., Riek R. F., Campbell W. C. 1979. Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob Agents Chemother. 15: 372–378
Elbetieha A., Da’as S. I. 2003. Assessment of antifertility activities of abamectin pesticide in male rats. Ecotoxicol Environ Saf, 55: 307–313
Fang X. Q., Chen Z. X., Lin L., Geng B. R. 2010. Genotoxicity of emamectin benzoate on Rana zhenhaiensis tadpoles. J Ningde Teach Coll, 22: 373–376 (In Chinese)
Fanigliulo A., Sacchetti M. 2008. Emamectin benzoate: new insecticide against helicoverpa armigera. Commun Agric Appl Biol Sci, 73: 651–653
Geng B. R., Lin L., Zhang Q. J., Zhong B.J. 2010. Genotoxicity of the pesticide dichlorvos and herbicide butachlor on Rana zhenhaiensis tadpoles. Asian Herpetol Res, 1: 118–122
Gosner K. L. 1960. A simplifed table for staging anuran embryos and larvae with notes on identifcation. Herpetologica, 16: 183–190
Herd R. 1995. Endectocidal drugs: ecological risks and countermeasures. Int J Parasitol, 25: 875–885
Jencic V., Cerne M., Erzen N. K., Kobal S., Cerkvenik-Flajs V. 2006. Abamectin effects on rainbow trout (Oncorhynchus mykiss). Ecotoxicol, 15: 249–257
Jensen J., Krogh P. H., Sverdrup L. E. 2003. Effects of the antibacterial agents tiamulin, olanquindox and metronidazole and the anthelmintic ivermectin on the soil invertebrate species Folsomia fimetaria (Collembola) and Enchytraeus crypticus (Enchytraeidae). Chemosphere, 50:437–443
Jiang M., Peng Z. X., Wu H., Hu K., Huang X. X. 2008. Application of ivermectin in aquaculture and corresponding aqua-ecosystem risk. Fish Modern, 35: 47–50 (In Chinese)
Katharios P., Iliopoulou-Georgudaki J., Kapata-Zoumbos K., Spiropoulos S. 2002. Toxicity of intraperitoneally injected ivermectin in sea bream, Sparus aurata. Fish Physiol Biochem, 25: 99–108
Krieger R. I. 2001. Handbook of pesticide toxicology second edition volume 1. American: Academic Press
Madsen M., Overgaard-Nielsen B., Holter P., Pedersen O. C., Brøchner-Jespersen J., Vagn-Jensen K. M., Nansen P., Grønvold J. 1990. Treating cattle with ivermectin: effects on the fauna and decomposition of dung pats. J Appl Ecol, 27: 1–15
Mann R. M., Bidwell J. R. 2001. The acute toxicity of agricultural surfactants to the tadpoles of four Australian and two exotic frogs. Environ Poll, 1l4: 195–205
Martini F., Tarazona J. V., Pablos M. V. 2012. Are fish and standardized FETAX assays protective enough for amphibians? A case study on Xenopus laevis larvae assay with biologically active substances present in livestock wastes. Sci World J, 2012: 1–6
Prichard R., Ménez C., Lespine A. 2012. Moxidectin and the avermectins: Consanguinity but not identity. Int J Parasitol: Drugs and Drug Resistance, 2: 134–153
Putter J. G., Mac Connell F. A., Preiser F. A., Haidri A. A., Rishich S. S., Dybas R. A. 1981. Avermectins: novel class of insecticides, acaricides and nematicides from a soil microorganism. Experientia, 37: 963–964
Ralph S., Petras M., Pandrangi R., Vrzoc M. 1996. Alkaline single cell gel (comet) assay and genotoxicity monitoring using two species of tadpoles. Environ Mol Mut, 28: 112–120
Reddy P. P. 2013. Recent advances in crop protection. Springer: 13–24
Römbke J., Floate K. D., Jochmann R., Schäfer M. A., Puniamoorthy N., Knäbe S., Lehmhus J., Rosenkranz B., Scheffczyk A., Schmidt T., Sharples A., Blanckenhorn W. U. 2009. Lethal and sublethal toxic effects of a test chemical (ivermectin) on the yellow dung fly Scathophaga stercoraria based on a standardized international ring test. Environ Toxicol Chem, 28: 2117–2124
Römbke J., Krogh K. A., Moser T., Scheffczyk A., Liebig M. 2010. Effects of the veterinary pharmaceutical ivermectin on soil invertebrates in laboratory tests. Arch Environ Contam Toxicol, 58: 332–340
Sanderson H., Laird B., Pope L., Brain R., Wilson C., Johnson D., Bryning G., Peregrine A. S., Boxall A., Solomon K. 2007. Assessment of the environmental fate and effects of ivermectin in aquatic mesocosms. Aquat Toxicol, 85: 229–240
Singh G., Chahil G. S., Jyot G., Battu R. S., Singh B. 2013. Degradation dynamics of emamectin benzoate on cabbage under subtropical conditions of Punjab, India. Bull Environ Contam Toxicol, 91:129–133
Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S., Fischman D. L., Waller R. W. 2004. Status and trends of amphibian declines and extinctions worldwide. Science, 306: 1783–1786
Sun J., Meng S. Q. 2009. Status and development trend of avermectin in Chinese market. World Pestic, 31(Suppl. 2): 18–21
Tang W. W., Lu Y. X., Mu B., Yin X. F., Zhang L. L. 2011. Research progress in emamectin benzoate toxicology. Chin J Foren Med, 26: 210–212
Tice R.R. 1995. Applications of the single cell gel assay to environmental biomonitoring for genotoxic pollutants. In: Butterworth B. M., Corkum L. D., Guzma´n-Rinco´n J. ed. Biomonitoring and Bio-markers as Indicators of Environmental Change. New York: Plenum Press, 69–79
Tice R. R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J. C., Sasaki Y. F. 2000. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mut, 35: 206–221
Tisler T., Erzen N. K. 2006. Abamectin in the aquatic environment. Ecotoxicol, 15: 495–502
Tyler M. J. 1994. Australian frogs: A natural history. Chatswood: Reed Books
Valic E., Jahn O., Päpke O., Winker R., Wolf C., Rüdiger.W. H. 2004. Transient increase in micronucleus frequency and DNA effects in the comet assay in two patients after intoxication with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Int Arch Occup Environ Heal, 77: 301–306
Wang D. D., Zhao E. M. 2013. Study on toxicological effect of avermectins on Pelophylax nigromaculatus. Sichuan J Zoo, 32: 334–342 (In Chinese)
Wang M. Z., Jia X. Y. 2009. Low levels of lead exposure induce oxidative damage and DNA damage in the testes of the frog Rana nigromaculata. Ecotoxicol, 18: 94–99
Wei F. L., Zhu J. W., Li S. N., Zhu G. N. 2008. Acute toxicity of emamectin benzoate on environmental organism. Pestic Sci Admin, 29: 19–24 (In Chinese)
Xie F., Lau M. W. N., Stuart S. N., Chanson J. S., Cox N. A., Fischman D. L. 2007. Conservation needs of amphibians in China: A review. Sci Chin Series C: Life Sciences, 50(2): 265–276
Xu H. R., Yang R. B., Fu Q., Liao H. Y. 2010. Abamectin residue in water, soil and rice. Environ Sci Manage, 35: 35–37 (In Chinese)
Yu X. L., Cheng C. H., Zhang Q. 2007. The primary study about immune effects of 1.8 % AVM latex on mice. Acta Acad Med Zunyi, 30: 254–256 (In Chinese)
Zhang L. L., Li Q. Y., Geng B. R. 2014. Genotoxicity of ivermectin on Polypedates megacephalus tadpoles. J Fujian Nor Univ, 30: 106–110 (In Chinese)
*Corresponding author: Prof. Baorong GENG, from College of Life Sciences, Fujian Normal University, with his research mainly focusing on taxonomy, ecology and ecotoxicology of amphibians.
E-mail: brgeng@fjnu.edu.cn
Received: 10 September 2015 Accepted: 9 June 2016
杂志排行
Asian Herpetological Research的其它文章
- A New Species of Euphlyctis (Amphibia, Anura, Dicroglossidae) from the West Coastal Plains of India
- Osteology of Quasipaa robertingeri (Anura: Dicroglossidae)
- Pathological Changes in Andrias davidianus Infected with Chinese Giant Salamander Ranavirus
- Plasticity in Metamorphic Traits of Rice Field Frog (Rana limnocharis) Tadpoles: The Interactive Effects of Rearing Temperature and Food Level
- Effects of Feeding Time on the Growth Performance and Variation of RNA/DNA Ratio of the Chinese Soft-shelled Turtle, Pelodiscus sinensis
- Effects of Dietary Vitamins A, B2, and B6Supplementation on Growth and Feed Utilization of Juvenile Chinese Soft-shelled Turtle Pelodiscus sinensis according to an Orthogonal Array Experiment
