Brain targeted delivery of paclitaxel using endogenous ligand
2017-01-19SanjeevAcharyaPadmanahaReddy
Sanjeev R.Acharya*,Padmanaha R.V.Reddy
aDepartment of Pharmacognosy,Nirma University,Ahmedabad,Gujarat 382481,India
bDepartment of Pharmacy,Institute of Pharmacy,Nirma University,Ahmedabad,Gujarat 382481,India
Brain targeted delivery of paclitaxel using endogenous ligand
Sanjeev R.Acharyaa,*,Padmanabha R.V.Reddyb
aDepartment of Pharmacognosy,Nirma University,Ahmedabad,Gujarat 382481,India
bDepartment of Pharmacy,Institute of Pharmacy,Nirma University,Ahmedabad,Gujarat 382481,India
A R T I C L EI N F O
Article history:
Received 24 June 2015
Received in revised form 21
September 2015
Accepted 19 November 2015
Available online 17 December 2015
Glutathione
The objective of the present investigation was to formulate nanoparticles constructed using PLGA polymer for the effective targeted delivery to brain via nasal route.The PLGA nanoparticles were optimized using novel design of experiment technique by 23full factorial design.Drug:polymer ratio(X1),surfactant concentration(X2)and stirring speed(X3)were identifed as critical process parameters,and its impact on particle size(Y1)and%entrapment effciency(Y2)was studied.The optimized nanoparticle formulation was conjugated with glutathione as an endogenous ligand by using carbodiimide chemistry using(1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide)(EDAC)as linker molecule.From Ellman’s assay, it was found that a total of 691.27±151 units of glutathione were conjugated upon each PLGA nanoparticle.Thein vitrorelease studies as well asex vivostudies revealed biphasic pattern of drug release with initial burst release followed by slow exponential release of drug over a period of 24 h.Thein vivobiodistribution studies were conducted on rat following nasal administration of the nanoparticle formulation(conjugated and unconjugated) and were compared with plain paclitaxel suspension.The results clearly demonstrated that the brain targeting effciency was enhanced with the glutathione conjugated formulation (387.474%)as compared to the unconjugated nanoparticle formulation(224.327%).Further, thein vitro in vivocorrelation studies revealed good relationship(R2>0.95)as obtained from the levy plot.Glutathione proves to be an effcient vector for the successful transport of poor bioavailable drug to the brain.
©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Nanoparticulate delivery systems[1],such as those based on poly(lactic-co-glycolic acid)(PLGA)have been studied extensively for many years.For the past three decades,lot of researchers has explored PLGA to fabricate drug delivery systems for pharmaceutical and biomedical applications due to its biocompatible and biodegradable properties.PLGA, further,has the advantage of being well characterized andcommercially used for microparticulate and nanoparticulate drug delivery systems[2].PLGA polymer is one of the most common biodegradable polymers used for the controlled delivery of drugs due to its early use and approval as a compatible biomaterial in humans.Lewis(1990)reported that,by varying the molecular weight and lactide/glycolide ratio,the degradation time of the PLA and PLGA and the release kinetics of the active agent can be controlled[3].In aqueous media,degradation of PLGA is triggered by hydrolysis of its ester linkages. Presence of methyl side groups in PLA makes it more hydrophobic than PGA,and hence lactide rich PLGA copolymers are less hydrophilic,absorb less water and degrade more slowly and control the release of drug for prolonged duration[4,5].
The major advantages of nanoparticles is improved bioavailability by enhancing aqueous solubility,increasing resistance time in the body(increasing half-life for clearance/ increasing specifcity for its associated receptors and targeting drug to specifc location in the body.This is why nanoparticles are increasingly used in variety of applications that includes drug carrier systems and to pass organ barriers such as the blood–brain barrier,cell membrane,etc.The cellular uptake, biodistribution and circulating half-life are the key factors which are infuenced by particle size of nanoparticles.Therefore,particle size becomes a primary concern while formulating a nanoparticulate system[6].Moreover the particle size thus obtained should be uniform because more uniform the distribution of particles more consistent will be the biodistribution,cellular uptake and drug release[7].
Nasal route is one of the alternative approaches for delivery of drugs to brain,where the drug is absorbed into the systemic circulation through nasal mucosa.Anatomically,nasal mucosa is comprised of tight junctions,and molecules with smaller size(less than 10 nm)can be able to traverse the junctions to reach the systemic circulation.Normally,many hydrophobic drugs or formulations(200 nm or more)opt transcellular route for delivery across the nasal mucosa. Nanoparticles above 500 nm size fall prey to the mucociliary clearance or by triggering immune response.The lower the particle size of nanoparticles,the more will be the rate of permeation across the nasal mucosa via transcellular pathway[8,9].
Paclitaxel(PTX)is one of the most widely used chemotherapeutic agents against breast and lung cancer,however, the brain delivery of paclitaxel is restricted owing to several bodily defense mechanisms.However,P-glycoprotein,an ATP-binding cassette(ABC)transporter,acts as a barrier in cancer treatment by chemotherapy by multidrug resistance(MDR)at cellular/non-cellular level offered by the tumor cells[10].Therefore,the absorption of paclitaxel is minimized due to rapid effux of the drug out of the tumor cell because of the overexpression of the plasma membrane,P-glycoprotein(P-gp)[11,12].Hence,nanoparticles consisting of Poly(Lactide-co-Glycolide(PLGA)have proven to possess potential delivery of insoluble drugs like paclitaxel to target areas,as they can be endocytosed/phagocytosed by cell resulting in the internalization of the encapsulated drug in the cell.PLGA,due to its biodegradability and biocompatibility,has attracted considerable attention for developing polymeric nanoparticles.PLGA possesses many advantages,such as able to encapsulate hydrophilic and hydrophobic drugs,improves interaction with biological matters and imparting stealthness,enhancing stability of drug,etc.[13,14].
Glutathione(GSH)is a hydrophilic endogenous tripeptide molecule that performs antioxidant function against reactive species and toxic metabolites of the cell[15].Glutathione helps in transporting few endogenous substances across the blood–brain barrier(BBB)by interacting with the membrane proteins in the BBB[16].Normally,P-glycoprotein(P-gp),the gatekeeper of BBB,helps in transporting the glutathione coupled compounds across the BBB[17].This mechanism is exploited for the delivery of paclitaxel to the brain by coupling it with glutathione.
Paclitaxel,belonging to BCS class IV,exhibits limited absorption due to its poor solubility and permeability characteristics.Paclitaxel,due to its poor permeability to brain,is primarily indicated for breast cancer and small cell lung cancer. Hence,paclitaxel is a suitable candidate for formulating nanoparticles due to its safe and effcient targeting of drug to the brain.In view of pursuing the objective of fostering the development of size controlled nanoparticles with enhanced entrapment effciency,novel quality by design concept was utilized.Further,the optimized nanoparticles were conjugated with glutathione and assessed the functionalization of nanoparticles with respect to its ability to transport drug across BBB.
The objective of the present investigation emphasizes the preparation of nanoparticles by using novel design of experiment concepts and subsequently conjugating with glutathione on the surface of nanoparticles.Hence,an attempt is being made in the current research to enhance the brain delivery of paclitaxel by loading PLGA nanoparticles conjugated with glutathione,which was further assessed byin vivobiodistribution studies.
2.Materials and methods
2.1.Materials
PLGA copolymer RG 502-H(lactide:glycolide ratio of 50:50,molecular weight 7–17 kDa),Resomer RG 504-H(lactide:glycolide ratio of 50:50,molecular weight 38-54 kDa)was obtained as gift sample from Evonik Industries AG,Germany.Poloxamer 188 was supplied as a gratis sample from Sandoz Pvt.Ltd,Mumbai. DCM(purity NLT 99%by GC),Paclitaxel was obtained as gratis sample from MAC-CHEM products Pvt.Ltd,Mumbai.Glutathione was purchased from SD Fine Chemicals,Mumbai.Dialysis membrane–Himedia LA401-5MT,Acetone(purity NLT 99%by GC),methanol(HPLC grade)were procured from Merck and Co., Germany.Double distilled water used was fltered through 0.22 μm flter from Millipore(Mumbai,India).All other cited chemicals used were of analytical grade.
2.2.Animals
Adult maleWistar rats weighing 200–240 g acquired from dedicated animal house of Institute of Pharmacy,Nirma University after receiving approval from the Institutional Animal Ethics Committee(IAEC)dated 15/06/2013 for studying the biodistribution potential of the formulation(Protocol No.IP/pcog/phd/13-1/021).All the experiments were performed as per the guidelines laid down by CDSCEA,India.
2.3.Preparation of paclitaxel loaded PLGA nanoparticles
The nanoparticles were developed by employing nanoprecipitation technique[18]using PLGA poly(lactic-co-glycolic acid) copolymer.Briefy,an organic solution containing specifed amount of PLGA polymer and Paclitaxel was added to an aqueous solution containing a suitable surfactant in a drop wise manner under vigorous stirring followed by magnetic stirring at room temperature.Later,the dispersion was kept for magnetic stirring(200±50 RPM)for 6 h at room temperature to evaporate the organic solvent.The precipitated nanoparticles were separated by ultracentrifugation and dried using vacuum drying to obtain free fowing dried nanoparticles[19]and stored at 4°C for further studies.
In the present investigation,for establishment of design space,a 23full factorial design was employed to assess the infuence of three factors,i.e.,Drug:polymer ratio(X1),a concentration of Poloxamer 188(X2)and stirring speed(X3)on particle size(Y1)and percentage entrapment effciency(Y2)of nanoparticles.The response(s)obtained are measured for each experiment and analyzed by either linear or multiple regression models.However,the change in one independent variable and its infuence on the responses can be studied by employing response surface methodology(RSM).The RSM and contour plots can be obtained by employing the regression equation, and the impact of each variable on the response variables can be studied effectively.Regression analysis was performed to develop a polynomial model for the estimation of the average particle size and%entrapment effciency.
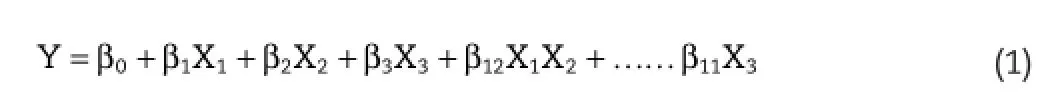
WhereYrepresents the response,while,X1,X2andX3denote the main effects of factors.Equation 1 above signifes simple regression equation for obtaining response surface and contour plots whereβ0is a constant andβ1,β2,β3are the coeffcients of the factors.TheP-values of the regression coeffcients as well as ANOVA were determined in order to evaluate the signifcance of the variables on the CQAs and signifcance of the model,respectively.
The design space may be obtained from overlay plots after plotting contour plots and response surface plots based on the desired range of values for the CQAs after which the formulation and process were optimized with respect to particle size and%entrapment effciency.Several trials were conducted to ascertain the correlation between the predicted and the practical values in order to validate the optimized process or formulation[20].
2.4.Conjugation of PLGA nanoparticles with glutathione
The paclitaxel loaded PLGA nanoparticles were conjugated with glutathione on their surface by utilizing the carbodiimide chemistry.This involves two steps,frst step comprises the activation of carboxyl groups on the surface of the nanoparticles by EDAC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide)followed by the nucleophilic addition of glutathione on to the activated functional groups of nanoparticles.The carboxyl group activation process involves incubation of prepared nanoparticles (100 mg)with 4 ml of EDAC solution(50 mg/ml)for 45 min at room temperature.Later,the free unreacted EDAC was separated by centrifugation for 15–20 min at 3500 rpm and resuspended the activated nanoparticles in 4 ml of buffer solution containing glutathione(50 mg/ml)under gentle stirring(200–250 rpm)for 6 h.The un-conjugated glutathione was then separated by centrifugation at 3500 rpm for 15–20 min and vacuum dried to obtain free fowing glutathione conjugated nanoparticles[21–23].The prepared formulation was transferred to clean vials and stored for further evaluation studies.
2.5.Entrapment effciency,particle size and size distribution analysis
Quantitative determination of Paclitaxel in nanoparticles was performed using HPLC(JASCO with PDA detector and manual injector with 20μl loop).Briefy,30 mg of nanoparticles formulation was taken in 10 ml of methanol,and the drug entrapped in the nanoparticles was extracted at room temperature by using sonication technique for 3 h.After 3 h,the undissolved matter was separated by fltration through 0.2 μ polytetrafuoroethylene membrane flters,and the amount of drug was analyzed by HPLC.Entrapment effciency(EE)of the nanoparticles was calculated from equation 2:
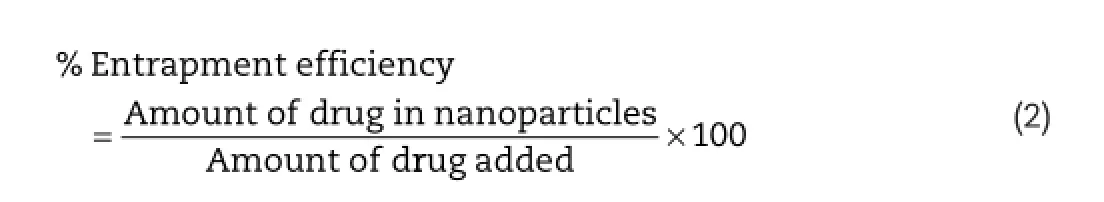
The average particle size and size distribution of Paclitaxel loaded PLGA nanoparticles was determined using particle size analyzer which works on the principle of dynamic light scattering technique(Nanophox,Sympatec GmbH,System-Partikel-Technik,Clausthal-Zellerfeld,Germany).The measurements recorded indicate average volume mean diameter 30 of samples conducted in triplicate.5g of the prototype nanoparticulate formulation was diluted in 100 ml of double distilled water and sonicated for about 3–5 min to declump the aggregates,and the resulted dispersion was analyzed for particle size at 250C. The width of the particle size distribution is articulated by means of span value(equation 3)which can be calculated by using the formula[24,25]:
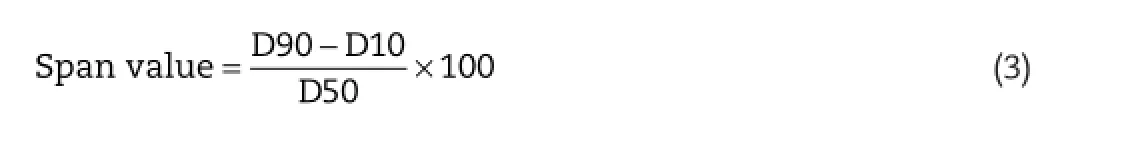
2.6.Zeta potential analysis
Zeta potential refers to the charge on the surface of the nanoparticles which in turn plays a pivotal role in the stability of colloidal dispersions.The zeta potential value designates the extent of repulsive forces between the particles in dispersion which aids in preventing the agglomeration of particles upon storage.Lower zeta potential value leads to focculated dispersion as the attractive forces dominate the repulsive forces and the particles unite to form aggregates.Zeta potential of the Paclitaxel loaded PLGA nanoparticles was analyzed usingZetasizer Nano(Malvern instruments,UK)by dispersing the sample in double distilled water followed by gentle sonication for disaggregation of particles.
2.7.Quantifcation of glutathione in conjugated PLGA nanoparticles by Ellman’s assay
Ellman’s reagent(5,5’-dithiobis-(2-nitrobenzoic acid)or DTNB) is used to quantitatively determine the number of glutathione units conjugated on the surface of each nanoparticle surface.Ellman’s reagent was added to 5 ml of phosphate buffer pH 7.2 containing 60 mg of conjugated and un-conjugated nanoparticles and 1 mM EDTA and kept for 15–20 min at room temperature.The supernatant was collected by centrifugation for 15–20 min at 3500 rpm,and the thiol content was estimated by HPLC after addition of 2 ml of sodium hydrogen phosphate(0.2 M)against unconjugated nanoparticles as blank. The number of glutathione units conjugated on the surface of the PLGA nanoparticles was calculated by using the formula (equation 4)[26,27]:

Where,n=thiol functionality of glutathione unit per nanoparticle;a=mole of thiol per gram of PLGA;d=density of nanoparticles;r=mean radius of nanoparticles and N=6.011×1023(Avogadro number).Density of PLGA nanoparticles was determined by mercury porosimetry analysis,and the size,shape and diameter of the nanoparticles was obtained from laser light scattering studies.
2.8.In vitro release study
Thein vitrorelease of Paclitaxel from optimized PLGA nanoparticles was performed using dialysis bag technique.Dialysis bag(Himedia,molecular weight cut-off 12000 Da,Mumbai, India)was activated by soaking in the release medium overnight.Phosphate buffer pH 6.0+0.5%SLS,pH 4.0+0.5%SLS and pH 7.4+0.5%SLS were used as diffusion media which mimics the nasal mucosal microenvironment pH,brain microenvironment pH as well as physiological pH,respectively.The brain targeted polymeric nanoparticles,administered via intranasal route,enter the systemic circulation from the nasal mucosa and later reach the brain.Hence,the release behavior of the nanoparticles is studied at the respective pH. Nanoparticles equivalent to 5 mg of Paclitaxel were placed in the dialysis bag and immersed in the release medium after sealing both the ends with clips and kept at 370C under continuous stirring at 75 rpm.2 ml aliquots were withdrawn from the medium at predetermined intervals of time,fltered with 0.45 μ polytetrafuoroethylene membrane flters and assayed for drug content by HPLC[28,29].Same amount of diffusion medium was replenished in order to maintain the sink condition to promote diffusion of drug into surrounding medium.
2.9.Ex vivo permeation study
The permeability potential of PLGA nanoparticles conjugated with glutathione was assessed byex vivostudies on sheep nasal mucosa which was procured from animal slaughter house.In order to correlate thein vitrorelease behavior of Paclitaxel from PLGA nanoparticles,ex vivostudies were performed using Franz diffusion cell.The receptor compartment was flled with phosphate buffer pH 6.0+0.5%SLS,and the nasal mucosa was mounted on the receptor compartment on which the optimized formulation was placed,and the study was performed at 37°C,50–100 RPM.Phosphate buffer pH 6.0 was selected for the study as it mimics the nasal environment pH.Periodically at predetermined intervals of time,2 ml of sample was withdrawn from the receptor compartment and subsequently replenished with fresh buffer solution to maintain sink condition.Further,the samples were diluted appropriately and analyzed by HPLC against blank nanoparticles.The amount of Paclitaxel released from the formulation at different time points was calculated[30,31].Further,the release model kinetics was determined by using DD solver add in program.
2.10.In vivo biodistribution study
The targeting effciency of glutathione conjugated PLGA nanoparticles following nasal administration was investigated in rats.A total of 18 healthy adult Wistar rats each weighing around 200–350 g was used for the study.The animal studies were conducted as per the protocol and guidelines approved and laid down by the Institutional Animal Ethics Committee(IAEC)and Committee for the Purpose of Control and Supervision of Experiments on Animals(CPSCEA)guidance.Animals were kept in large spacious cages to provide enough movement of animal and were suffciently fed with food and water and maintained on 12:12 h light/dark cycle.The rats were divided into three groups representing six animals in each group.First group was kept as control and received plain Paclitaxel solution.Group II received Paclitaxel loaded PLGA nanoparticles,whereas the Group III with glutathione conjugated Paclitaxel loaded PLGA nanoparticles.In the experimental context,the formulations were administered intranasally at a dose of 2 mg/kg body weight of Paclitaxel.During nasal administration,the animal was held in the back in slant posture, and the formulation was administered in each nostril swiftly using micropipette attached with LDPE tubing,having 0.1 mm internal diameter at the delivery site,thus allowing the animal for proper breathing[32].At specifed intervals,the biodistribution study was evaluated by checking the uptake of Paclitaxel by different organs like brain,liver,spleen,lungs and kidneys.Hence,the animal was sacrifced and the organs are separated and washed completely with phosphate buffer saline pH 7.4 in order to isolate all the adsorbed fuorescein and is stored at−20°C for further studies.The organs separated were then homogenized in phosphate buffer saline pH 7.4 for fuorescein extraction from the organs.Later,the suspension was centrifuged,and the supernatant obtained was quantifed by HPLC to analyze the total amount of fuorescein distributed in the organs[33,34].
The pharmacokinetics of the developed nanoparticle formulation was compared with plain paclitaxel suspension for assessment of its biodistribution potential.The ability of the formulation to cross the blood–brain barrier and reaching the brain is assessed by calculating the brain targeting effciency of the respective formulation.The brain targeting effciency(BTE)of paclitaxel loaded nanoparticulate formulations was assessed by using formula(equation 5)[35]:
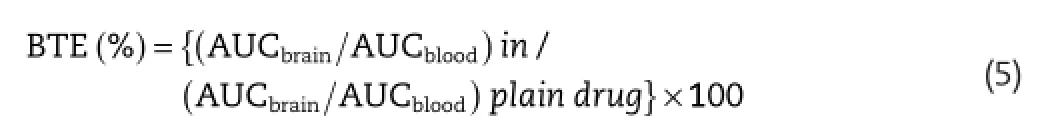
In vitro in vivocorrelation(IVIVC)is used to establish a relationship betweenin vitrodissolution andin vivodata.IVIVC, according to FDA,is a predictive model describing relation betweenin vitroproperty andin vivoresponse[36].IVIVC was obtained by plotting percentage of drug absorbedin vivoagainst percentage of drug dissolvedin vitro.This plot is called Levy plot,and the percentage of drug absorbed(%Fa)is obtained by Wagner-Nelson method by using equation 6[37]:
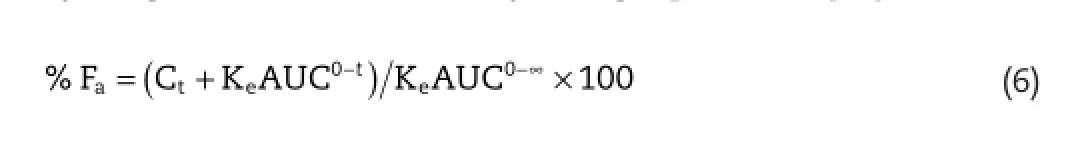
Where,Fa=percentage of drug absorbed,Ct=concentration of drug in plasma at time t,Ke=elimination rate constant, AUC0-tand AUC0-∞=area under curve from the drug plasma concentration–time curve from 0–48 h and 0-∞,respectively.
While performing IVIVC analysis,linear regression analysis was also implemented in order to relate thein vivodrug absorption to release of drugin vitro.The data are reported in the form of mean±standard deviation,and statistical analysis was conducted usingt-test.The data withP<0.05 was regarded as statistically signifcant.
2.11.Statistical analysis
All the experiments were performed in triplicate,and the results obtained are presented as mean±SD of the population.One way analysis of variance(ANOVA)and regression analysis was performed to evaluate the statistical signifcance of the differences by consideringP<0.05 for demonstrating statistically signifcant procedure.All the statistical evaluations were conducted using Minitab®16 statistical software(USA).
3.Results and discussion
3.1.Particle size,distribution and zeta potential
Particle size is an important factor in the biodistribution of the formulation among different organs in the body.The particle size of paclitaxel loaded PLGA nanoparticles was found to be in the range of 92.6 nm to 1080 nm,and several parameters were found to affect particle size.The screening studies of independent variables established the infuence of various factors on particle size and entrapment effciency of paclitaxel loaded PLGA nanoparticles.Acetone was selected as anti-solvent as it produced nanoparticle with average particle size of 159.7±4.6 nm,whereas ethanol and dichloromethane produced large nanoparticles with particle size of 210.7±4.8 nm and 1080±5.4 nm,respectively.This is due to the rapid diffusion of acetone in water than ethanol and dichloromethane. Similarly,poloxamer 188 gave nanoparticles with narrow size distribution as compared to SLS and tween 80.Hence,it was selected for the study,and the results suggest that increasing the surfactant concentration from 0.1%w/v 0.3%w/v,the f was found decreasing from 129.9±4.2 nm to 92.6±4.6 nm. Since the poloxamer molecules overlay on the surface of droplets and assist in preventing the coalescence of droplets by reducing the shear stress during homogenization process.As a result,the stabilizers which preferentially adsorb at the interface of droplets tend to decrease the particle size of nanoparticles by merely reducing the tension at interface.From the results,it was observed that on increasing the polymer concentration from 5 mg/ml to 20 mg/m,the particle size was found to increase from 104.2±5.7 nm to 151.2±5.1 nm.This effect can be attributed to the greater number of polymeric chains per unit volume of solvent which is consequently responsible for the formation of aggregates or larger particles due to increased polymer–polymer interaction and increased viscosity of organic phase.The amount of acetone as anti-solvent signifcantly impacted the particle size of nanoparticles as the turbidity increased on increasing the volume of anti-solvent (more than 20 ml)producing larger particles.Among the independent variables,stirring speed was revealed to have impact on the particle size of the nanoparticles.High rate of stirring led to enhanced mass transfer and rate of diffusion between the multiphase,which induced high homogenization supersaturation and rapid nucleation to produce smaller sized nanoparticles.Zeta potential,representing the surface charge, is indicative of stability of the formulation.Paclitaxel loaded PLGA nanoparticles exhibited zeta potential in the range of−38 to−42 mV,and this low value of surface charge implicit good stability by preventing coalescence of particles.
3.2.Entrapment effciency
The entrapment effciency of paclitaxel in the PLGA nanoparticles was found to be infuenced by several factors. Surfactant concentration was found to be crucial in affecting the entrapment effciency signifcantly.As the surfactant concentration was increased(from 0.1%w/v to 0.3%w/v),the entrapment effciency was found to decrease(from 44.1±6.3% to 37.6±5.5%),which can be attributed to the diffusion of paclitaxel from inner core to outer phase of nanoparticles.From the results,acetone was selected as solvent and poloxamer 188 was selected as stabilizer as they showed good entrapment effciency.The rate of addition of anti-solvent(water)was found insignifcant in altering the entrapment effciency of drug in polymer when compared to the composition of organic phase (P>0.05).Increasing the concentration of acetone has led to slight increase in entrapment effciency of paclitaxel from 39.2±5.6%to 42.7±6.4%.The concentration of PLGA was found to have proportionate direct relationship with entrapment effciency of drug.Due to the greater number of polymer chains, the entrapment effciency was found to improve.Also,at higher rates of stirring,the entrapment effciency increased due to enhanced the mass transfer and rate of diffusion between the multiphases.Drying process is found to be insignifcant in affecting the entrapment effciency of drug in NPs(P>0.05).
3.3.Optimization of nanoparticulate formulation using 23full factorial design
From the preliminary screening studies Drug:polymer ratio(X1), poloxamer concentration(X2)and stirring speed(X3)wereidentifed as critical process parameters that exhibited considerable effect on the CQAs.Table 1 shows the list of factors selected for the study along with their levels.In the current investigation,a design space was obtained for effective designing of formulation that eventually meets the target specifcation of the fnal product by utilizing the novel design of experiment(DOE)approach.The critical process parameters,thus,established were subsequently evaluated for simultaneous optimization using 23full factorial design as shown in Table 2.
From the results,the particle size varied from 92.6 nm to 1080 nm representing substantial dependence of quality attributes on the independent variables.From the polynomial equation for particle size,it was clear that the independent Drug:polymer ratio(X1),poloxamer concentration(X2)and stirring speed(X3)have signifcant impact(P<0.05)on particle size and entrapment effciency of nanoparticles.
From the polynomial equation,it was clear that drug: PLGA concentration,poloxamer concentration and stirring speed were inversely proportional to particle size of nanoparticles.Whereas,drug:PLGA concentration and stirring speed have positive impact on entrapment effciency. The relationship between the factors and their coeffcients were determined mathematically with their respectivePvalues by employing regression analysis,and the factors obtainingPvalues<0.05 were considered as signifcant.The results of ANOVA are tabulated in Table 3.From the results of ANOVA,it was evident that the independent factors possess signifcant impact on the particle size as thePvalues were well below 0.05.The polynomial equation(equation 7 and 8)is used to obtain useful information in evaluation of coeffcient,while the polynomial model for the estimation of particle size was as below:


Table 1–Factors and factor levels studied in 23full factorial design.
After the estimation of polynomial equations,the design space was established by setting the target value for particle size(<100 nm)and process yield(>40%),and for this design, response surface plots along with the contour plots were established as shown in Fig.1 which represents the polynomial equation 7 and equation 8,respectively.The contour plot displays a nonlinear relationship between the particle size and the stirring speed,which indicates strong interactions between these process parameters,especially at high values.Although all contour plots disclosed some degree of interaction, the effect was more predominant in the drug:polymer ratio and with stirring speed contour plot.The optimization was performed and the graphs were obtained by using Minitab®16 statistical software(Minitab Inc,USA).
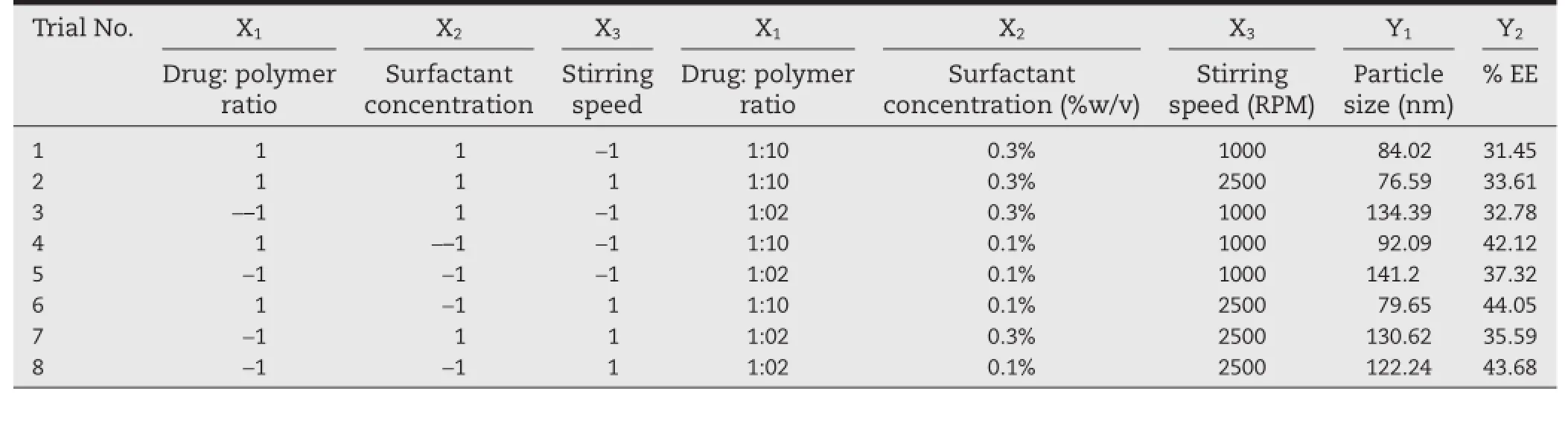
Table 2–Design layout of 23full factorial design for optimization of PLGA nanoparticles.
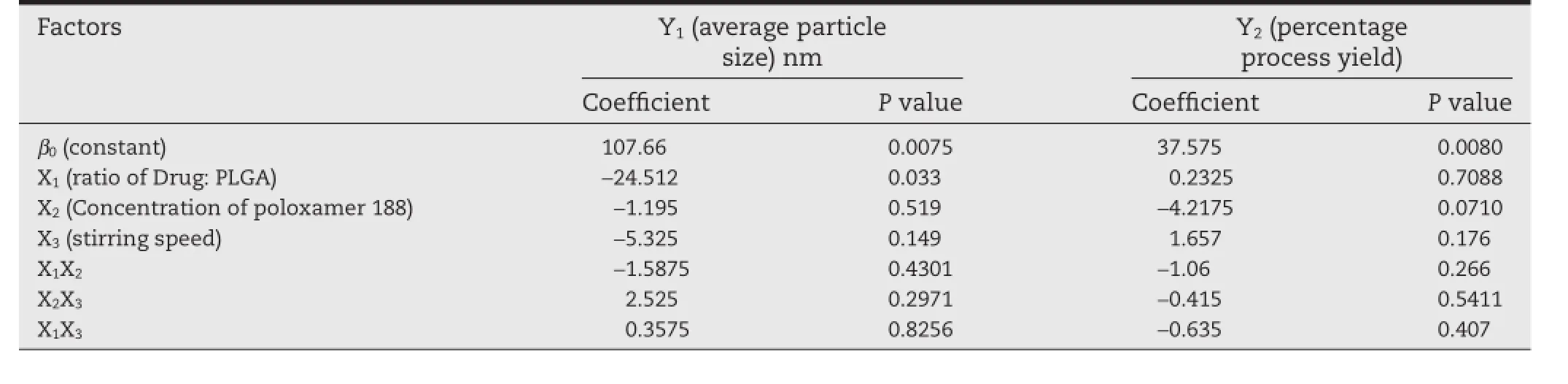
Table 3–Regression coeffcients and the respectivepvalues of the independent variables(n=3).
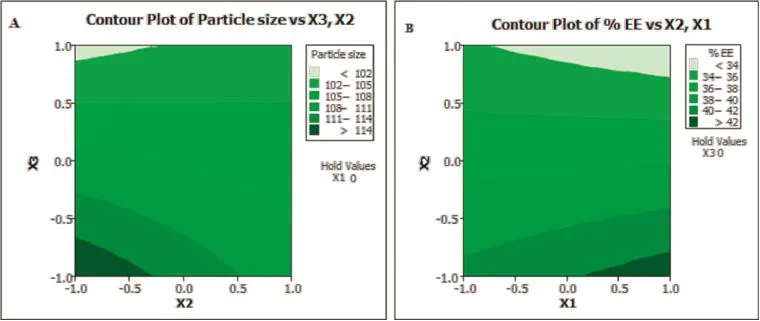
Fig.1–Two dimensional contour plots for(A)particle size of PLGA nanoparticles,and(B)%entrapment effciency of PLGA nanoparticles.

Fig.2–Overlay plot for simultaneous optimization of particle size and%entrapment effciency of PLGA nanoparticles.
The two dimensional plot obtained by contour plots were superimposed for simultaneous optimization of the independent variables.The desired values for the particle size(<100 nm) and process yield(>40%)were set to obtain the predicted values from the set coded values.From the predicted values obtained by overlay of contour plots of both the responses as depicted in Fig.2,the actual values were calculated and experimental trials were performed for ensuring the proper validation of the process.The observed and predicted values of optimized nanoparticles formulation were found to be similar from the checkpoint batches shown in Table 4,thus ensuring the reliability of the process in obtaining size controlled robust nanoparticles.
Checkpoint batches were prepared to check the predictive productivity of the regression equation,and a good correlation was observed between the observed and predicted values. The optimized formulation obtained exhibited encouraging results showing particle size of 79.65±3.2 nm as shown in Fig.3 and%entrapment effciency of 45.12%±2.4%(n=3),respectively.The scanning electron micrographs(SEM)of glutathione conjugated paclitaxel loaded PLGA nanoparticles are presented in Fig.3.The particles obtained were spherical in shape with slight aggregation.
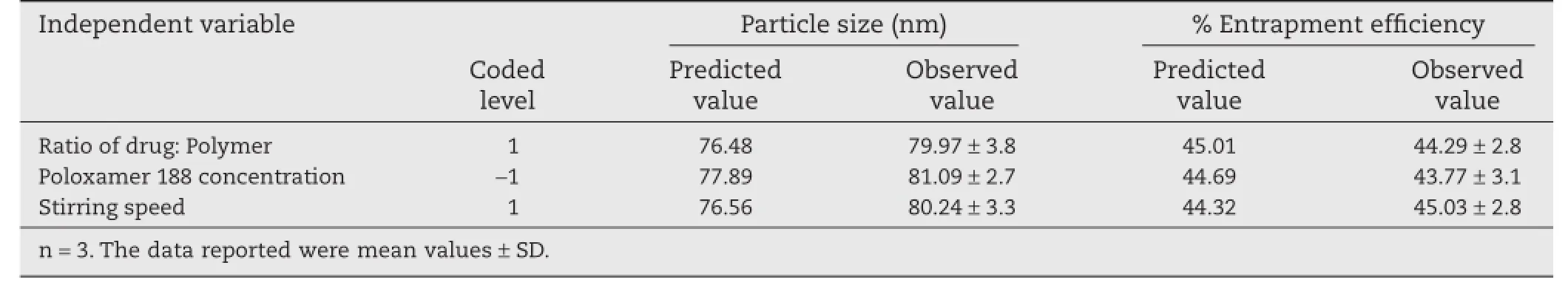
Table 4–Checkpoint batches for the validation of regression equation.
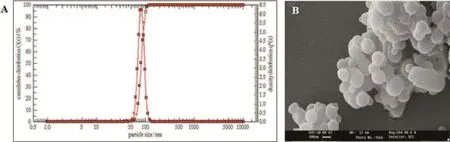
Fig.3–(A)Particle size distribution report and(B)scanning electron micrograph(100 K magnifcation)of optimized glutathione conjugated paclitaxel loaded PLGA nanoparticles formulation.
3.4.Conjugation of glutathione with PLGA nanoparticles
Glutathione,selected as model conjugating agent,was linked on the surface of the PLGA nanoparticles by using carbodiimide chemistry.The optimized formulation was subjected to conjugation reaction with different concentration of EDAC per 100 mg of nanoparticles.From the results,the concentration of 150 mg was selected as no signifcant difference was observed between the two concentrations(P=0.13).The optimized formulation exhibited 691.27±151 units of glutathione per nanoparticle as shown in Table 5.
3.5.In vitro release studies and release kinetics
The release of paclitaxel from the PLGA nanoparticles was determined by using diffusion bag method,and subsequently the release kinetics profle for the optimized nanoparticle formulation was obtained.Figure 4 clearly shows a biphasic release pattern of drug from the formulation with an initial burst release of drug within 2 h followed by sustained release of drug over a span of 24 h.The initial burst release of drug from nanoparticles was attributed to the drug molecules present on the outer surface of the nanoparticles which were dissolvedas soon as they came in contact with the diffusion medium. The latter sustained release of drug is due to the slow erosion of the polymer matrix thus releasing the drug in a controlled manner over 24 h.

Table 5–Infuence of EDAC concentration on conjugation of glutathione with PLGA nanoparticles.
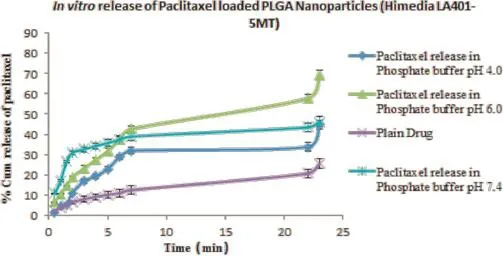
Fig.4–In vitrorelease of Paclitaxel from PLGA nanoparticles in phosphate buffer of different pH containing 0.5%SLS.The data reported were mean values±SD.
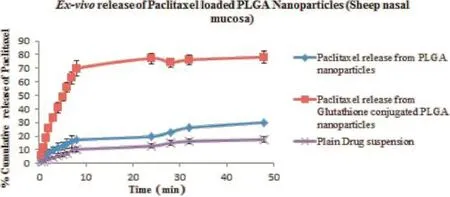
Fig.5–Ex vivorelease of Paclitaxel from glutathione conjugated and unconjugated PLGA nanoparticles in phosphate buffer of different pH containing 0.5%SLS.The data reported were mean values±SD.
3.6.Ex vivo release studies
Theex vivorelease of paclitaxel from glutathione conjugated and unconjugated nanoparticles was performed on excised nasal mucosa of sheep.The results ofex vivorelease studies, as depicted in Fig.5,revealed that the release pattern followed biphasic pattern as seen inin vitrorelease studies with initial burst release of drug followed by slow sustained release of drug over a period of 48 h.The results concluded that the glutathione conjugated nanoparticles exhibited better permeability releasing around 75%of drug across mucosal membrane as compared to unconjugated PLGA nanoparticles(around 25% drug).In vitrorelease studies due to the presence of barrier properties of nasal mucosal membrane.This may be attributed to the difference in the physicochemical properties of both conjugated and unconjugated nanoparticles.Moreover,glutathione plays an important role in opening of tight junctions of the mucosal membrane as well as in promoting the paracellular transport of molecules across mucosal membrane[38].Theex vivodrug release profle of the optimized paclitaxel loaded PLGA nanoparticles was ftted to various kinetic equations and the parameters like R2,AIC(Akaike information criterion),MSC (Model selection criteria)were calculated to determine the release kinetics for paclitaxel loaded PLGA nanoparticles.From Table 6,it is clear that the release kinetics of glutathione conjugated(R2=0.9736;AIC=92.14)and unconjugated PLGA nanoparticles(R2=0.9865;AIC=50.55)were found to be best ft for Weibull model among various kinetic models.
3.7.In vivo biodistribution studies
The results of percentagein vivobiodistribution studies are furnished in Fig.6.From the results,it can be concluded that most amount of paclitaxel in brain was achieved with glutathione conjugated nanoparticles(1.72±0.08 μg/g of brain)at the end of 8 h,when compared to unconjugated paclitaxel loaded PLGA nanoparticles(0.86±0.01 μg/g of brain)and plain paclitaxel suspension(0.22±0.04 μg/g of brain).From the results,it was clear that glutathione conjugation aided in enhancing the drug transport to brain across blood–brain barrier by achieving high concentrations of paclitaxel in brain as compared to plain drug suspension.Besides,the glutathione conjugated nanoparticles were taken up and was found to be accumulated in the reticulo endothelial cells comprising of spleen,kidney and lungs.
The brain targeting effciency of glutathione conjugated nanoparticles was found to be 1.72 times greater than the unconjugated PLGA nanoparticles and 7.8 times as that of plain paclitaxel suspension,thus demonstrating the potential behavior of glutathione in enhancing the drug delivery to brain by penetrating the blood–brain barrier.The pharmacokinetics,derived from the non-compartmental analysis,are shownin Table 7,and the results revealed that the AUC0-tof glutathione conjugated PLGA nanoparticles(19.42±0.22%)was considerably higher than the unconjugated nanoparticles (9.13±0.41%),thereby showing enhanced bioavailability of peptide conjugated formulation containing paclitaxel.The results corroborate the fndings that the glutathione conjugated PLGA nanoparticles can be effectively used as for brain delivery of drugs through nasal route.
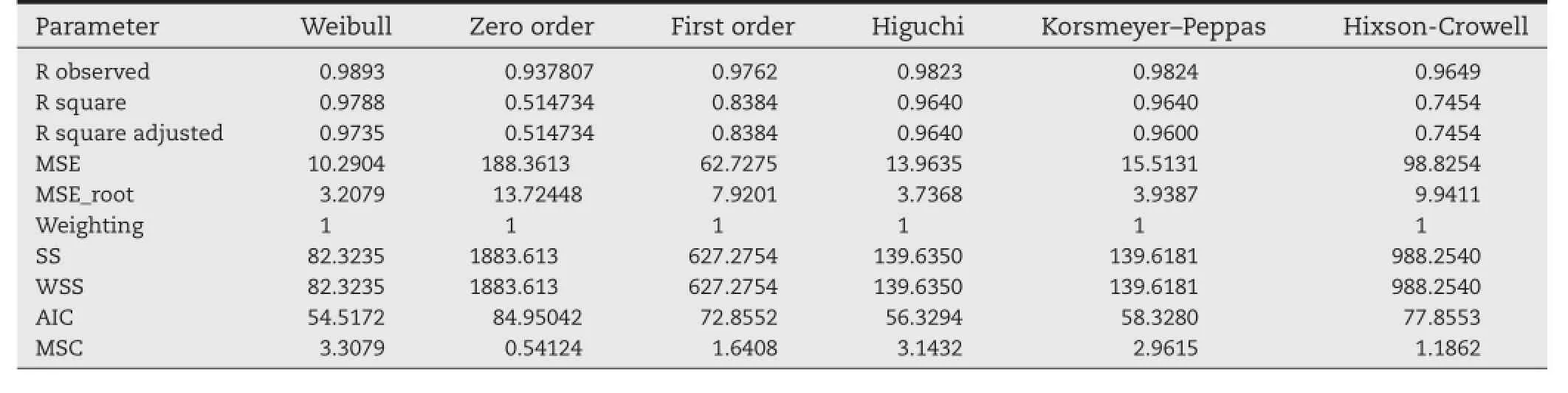
Table 6–Release kinetics of paclitaxel loaded PLGA nanoparticles.
From the results,it can be concluded that the prepared glutathione conjugated PLGA nanoparticles possessed signifcant drug delivery potential(P<0.05)to the brain as compared to the plain paclitaxel suspension.
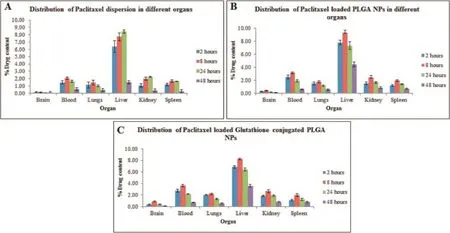
Fig.6–Bio distribution of(A)plain paclitaxel dispersion(B)paclitaxel loaded PLGA nanoparticles,and(C)glutathione conjugated paclitaxel loaded PLGA nanoparticles,in various organs at different time intervals.The data reported were mean values±SD.
3.8.In vitro-in vivo correlation(IVIVC)
IVIVC was performed to establish the relationship between thein vitrorelease and clinical data.Figure 7 shows levy plots obtained from bothin vitrorelease data andex vivorelease data plotted againstin vivoplasma drug concentration-time profle of glutathione conjugated paclitaxel loaded PLGA nanoparticles. Both the plots display good correlation between thein vitroandin vivodata(R2=0.9657)as well asex vivoandin vivodata (R2=0.9714).The data obtained from thein vitroas well asex vivostudies was convoluted to achieve the levy plot.Moreover,the percent of drug absorbed(in vivo)was calculated fromWagner-Nelson method,which was then plotted against thein vitrorelease andex vivorelease data(%drug dissolved)to obtain a plot,the linearity of which signifes the correlation between the two models.The prediction error was found to be less than 15%,which indicates good correlation between thein vivoandin vitromodels.Hence,it can be concluded from the data that thein vitroandex vivorelease data can be used as a predictive model forin vivobioavailability of the drug from PLGA nanoparticles.The levy plots were obtained from R project software for statistical computing(R-project.org).
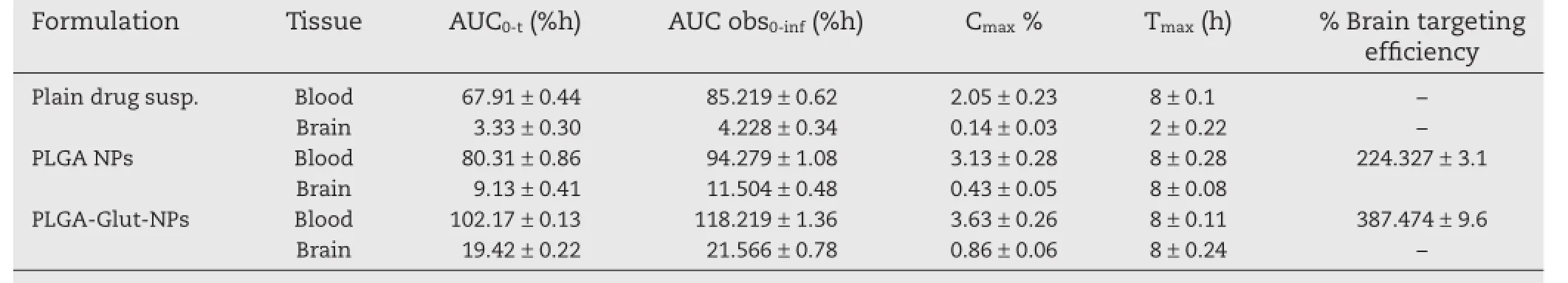
Table 7–Pharmacokinetic parameters in brain and blood and%brain targeting effciency of PLGA nanoparticles following intranasal administration of paclitaxel loaded nanoparticles.
The statistical analysis was performed by Tukey’s multiple comparison test for ensuring the signifcance of the model and was evaluated using ANOVA.It was found that the results of PLGA formulation were found signifcant(P=−0.4650)when compared to the plain drug suspension.Further,the results of ANOVA analysis on brain levels after each formulation dose suggest that the results for glutathione conjugated PLGA have come out signifcantly better(P=−0.245).
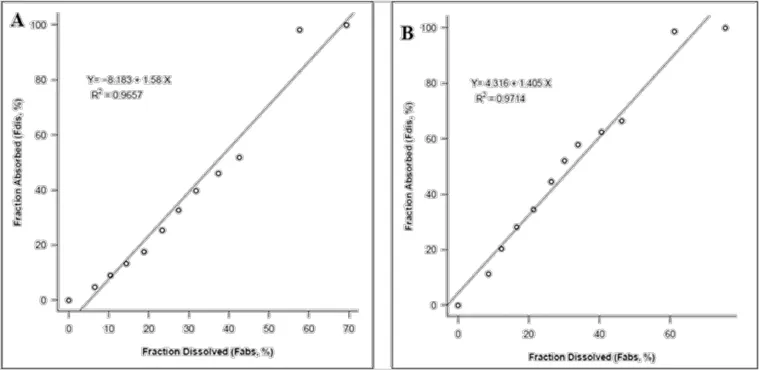
Fig.7–(A)Levy plot obtained from thein vitrodissolution as well as plasma drug concentration–time data of glutathione conjugated PLGA nanoparticles(B)Levy plot obtained from theex vivorelease data as well as plasma drug concentration–time data of glutathione conjugated PLGA nanoparticles.
4.Conclusion
In the present investigation,an attempt was made to obtain robust and rugged PLGA based nanoparticulate formulation loaded with paclitaxel by the aid of statistical DOE design.The preliminary experiments were conducted to identify the possible risk associated with the formulation of PLGA nanoparticles. Later,the impact of each factor on the critical quality attributes of the product was established by utilizing 23full factorial design,and design space was constructed using statistical tools. The results were further fortifed by statistical models,contour plots and response surface plot.Therefore,the objective of obtaining paclitaxel loaded PLGA nanoparticles with controlled size and improved EE can be successfully achieved through nanoprecipitation technique as well as by novel quality by design concept.EDAC was used for successful conjugation of glutathione with PLGA nanoparticles.Thein vivobiodistribution studies revealed excellent transport potential of glutathione in delivering the drug to the brain in the form of nanoparticles which was demonstrated to be 7.8 times as that of plain paclitaxel suspension.Hence,glutathione can be used as an effective vector in transport of drugs which have limited BBB permeation.
Acknowledgments
The author acknowledge to Institute of Pharmacy,Nirma University for providing the facility for carrying out this research work.
Declaration of interest
The authors report no declarations of interest.
R E F E R E N C E S
[1]Muthu MS.Nanoparticles based on PLGA and its copolymer:an overview.Asian J Pharm 2009;3(4):266–273.
[2]Allemann E,Leroux RG.Biodegradable nanoparticles of particles of poly(lactic acid)and poly(lactic-co-glycolic acid)for parenteral administration.In:Gregoridas G,editor. Pharmaceutical dosage form.New York,NY:Marcel Dekker; 1999.p.163–186.
[3]Lewis DH.Controlled release of bioactive agents from lactide/gylcolide polymers.In:Chasin M,Langer R,editors. Biodegradable polymers as drug delivery systems,vol.4.NY: Marcel Dekker;1990.p.1–41.
[4]Abhilash M.Potential applications of Nanoparticles.Int J Pharm Bio Sci 2010;1(1):1–12.
[5]Mirakabad FST,Koshki KN,Akbarzadeh A,et al.PLGA-Based nanoparticles as cancer drug delivery systems.Asian Pac J Cancer Prev 2014;15(2):517–535.
[6]Decuzzi P,Pasqualini R,Arap W,et al.Intravascular delivery of particulate systems:does geometry really matter?Pharm Res 2009;26:235–243.
[7]Gu F,Zhang L,Teply BA,et al.Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers.Proc Nat Acad Sci 2008;105:2586–2591.
[8]Illum L.Nanoparticulate systems for nasal delivery of drugs: a real improvement over simple systems?J Pharm Sci 2006;96(3):479–483.
[9]Mistry A,Stolnik S,Illum L.Nose-to-brain delivery: Investigation of the transport of nanoparticles with different surface characteristics and sizes in excised porcine olfactory epithelium.Mol Pharm 2015;12:2755–2766.
[10]Ping M,Mumper RJ.Paclitaxel nano-delivery systems:a comprehensive review.J Nanomed Nanotech 2013;4(2):1–16.
[11]Liang XJ,Chen C,Zhao Y,et al.Circumventing tumor resistance to chemotherapy by nanotechnology.Methods Mol Biol 2010;596:467–488.
[12]Lohcharoenkal W,Wang L,Chen Y,et al.Protein Nanoparticles as drug delivery carriers for cancer therapy. BioMed Res Int 2014;1–12.
[13]Danhiera F,Ansorenaa E,Silvaa JM,et al.PLGA-based nanoparticles:an overview of biomedical applications.J Contr Rel 2012;161(2):505–522.
[14]McCall RL,Sirianni RW.PLGA Nanoparticles formed by single-or double-emulsion with vitamin ETPGS.J Vis Exp 2013;82:1–8.
[15]Weperen WV,Gaillard P.Enhanced blood to brain drug delivery.Innov Pharm Tech 2015;55–57.
[16]Grover A,Hirani A,Sutariya V.Blood-brain barrier permeation of glutathione-coated nanoparticle.SOJ Pharm PharmSci 2014;1(1):1–4.
[17]Schulz JB,Lindenau L,Seyfried J,et al.Glutathione,oxidative stress and neurodegeneration.Eur J Biochem 2000;267:4904–4911.
[18]Fessi H,Puisieux F,Benita S,et al.Nanocapsule formation by interfacial polymer deposition following solvent displacement.Int J Pharm 1989;55(1):R1–R4.
[19]Govender T,Stolnik S,Illum L,et al.PLGA nanoparticles prepared by nanoprecipitation:drug loading and release studies of a water soluble drug.J Contr Rel 1999;57(2):171–185.
[20]Santos KC.Polymeric nanoparticles loaded with the 3,5,3′-triiodothyroacetic acid(Triac),a thyroid hormone:factorial design,characterization,and release kinetics.Nanotech Sci App 2012;5:37–48.
[21]Patel PJ,Acharya NS,Acharya SR.Development and characterization of glutathione-conjugated albumin nanoparticles for improved brain delivery of hydrophilic fuorescent marker.Drug Del 2013;20(3):1–13.
[22]Kafedjiiski K,Foger F,Schnurch AB,et al.Synthesis andin vitroevaluation of a novel chitosan–glutathione conjugate. Pharm Res 2005;22(9):1480–1488.
[23]Kocbek P,Obermajer N,Cegnar M,et al.Targeting cancer cells using PLGA nanoparticles surface modifed with monoclonal antibody.J Cont Rel 2007;120:18–26.
[24]Zhao D,Zhao X,Zu Y,et al.Preparation,characterization, and in vitro targeted delivery of folate-decorated paclitaxelloaded bovine serum albumin nanoparticles.Int J Nanomed 2010;5:669–677.
[25]Subedi RK,Kanga KW,Choi HK.Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin.Euro J Pharm Sci 2009;37:508–513.
[26]Nobs L,Buchegger F,Gurny R,et al.Surface modifcation of poly(lactic acid)nanoparticles by covalent attachment of thiol groups by means of three methods.Int J Pharm 2003;250:327–337.
[27]Nobs L,Buchegger F,Allémann E,et al.Poly(lactic acid) nanoparticles labeled with biologically active Neutravidin for active targeting.Eur J Pharm Biopharm 2004;58(3):483–490.
[28]Mona MA,Mottaleb A,Lamprecht A.Standardizedin vitrodrug release test for colloidal drug carriers using modifed USP dissolution apparatus I.Drug Dev Ind Pharm 2011;37(2):178–184.
[29]Lima SC,Resende M,Silvestre R,et al.Characterization and evaluation of BNIPDaoct-loaded PLGA nanoparticles for visceral leishmaniasis:in vitroandin vivostudies.Nanomed 2012;1–11.
[30]Sharma D,Maheshwari D,Philip G,et al.Formulation and optimization of polymeric nanoparticles for intranasal delivery of lorazepam using Box-Behnken design:in vitroandin vivoevaluation.Biomed Res Int 2014;1–14.
[31]Seju U,Kumar A,Sawant KK.Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery:in vitroandin vivostudies.Acta Biomat 2011;7(12):4169–4176.
[32]Abdelbary GA,Tadros MI.Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers:In vitrocharacterization,ex vivoestimation of nasal toxicity andin vivobiodistribution studies.Int J Pharm 2013;452(1):300–310.
[33]Patel S,Chavhan S,Soni H,et al.Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route.J Drug Targ 2011;19(6):468–474.
[34]Alam S,Khan ZI,Mustafa G,et al.Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting:a pharmacoscintigraphic study.Int J Nanomed 2012;7:5705–5718.
[35]Misra A,Vyas TK,Babbar AK,et al.Preliminary braintargeting studies on intranasal mucoadhesive microemulsions of sumatriptan.AAPS Pharm Sci Tech 2006;7(1):E1–E9.
[36]Sirisuth N,Eddington ND.In vitro in vivo correlation defnitions and regulatory guidance.Int J Gen Drugs 2012;1–11.
[37]Wu K.Pharmacokinetics,brain distribution,release and blood-brain barrier transport of shunaoxin pills.J Ethnopharmacol 2014;151:1133–1140.
[38]Clausen AE,Kast CE,Schnurch AB.The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm Res 2002;19(5):602–608.
*< class="emphasis_italic">Corresponding author.
.Department of Pharmacognosy,Institute of Pharmacy,Nirma University,Gandhinagar Highway,Ahmedabad,Gujarat 382481,India.Tel.:+91 2717 241916.
E-mail address:sanjeev.acharya@nirmauni.ac.in(S.R.Acharya).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.11.121
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Biodistribution
PLGA nanoparticles
In vitro in vivocorrelation(IVIVC)
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- PEGylation in anti-cancer therapy:An overview
- Near-infrared light-responsive inorganic nanomaterials for photothermal therapy
- Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole
- Povacoat affecting solid-state polymorphic changes of indomethacin after co-evaporation from different types of solvents via conventional and microwave drying techniques
- Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying:Physicochemical and in silico studies
- Preparation and evaluation of PEGylated phospholipid membrane coated layered double hydroxide nanoparticles
