Povacoat affecting solid-state polymorphic changes of indomethacin after co-evaporation from different types of solvents via conventional and microwave drying techniques
2017-01-19
Department of Biotechnology and Pharmaceutical Technology,Yuanpei University of Medical Technology,Hsin-Chu,Taiwan
Povacoat affecting solid-state polymorphic changes of indomethacin after co-evaporation from different types of solvents via conventional and microwave drying techniques
Shan-Yang Lin*,Hong-Liang Lin,Ying-Ting Chi,Ru-Ying Hung, Yu-Ting Huang,Chi-Yu Kao,Wei-Hsien Hsieh
Department of Biotechnology and Pharmaceutical Technology,Yuanpei University of Medical Technology,Hsin-Chu,Taiwan
A R T I C L EI N F O
Article history:
Received 20 July 2015
Received in revised form 12
September 2015
Accepted 14 September 2015
Available online 1 October 2015
Povacoat
Indomethacin(IMC)
Air-drying
Microwave-drying
α-IMC
Amorphous IMC
The effect of Povacoat on the solid state characteristics and polymorphic transformation of γ-form of indomethacin(γ-IMC)after solvent co-evaporation via two drying methods was investigated by thermoanalytical and FTIR spectroscopic studies.The types of solvents(water, ethanol,and water:ethanol(1:9~2:8,v/v)),weight ratios of Povacoat:IMC(4:1~4:10,w/w), and drying methods(air-drying and microwave-drying)were selected as variables in this study.A physical mixture of Povacoat and γ-IMC was also carried out by neat grinding process. The results indicate that there was no interaction between Povacoat and γ-IMC or no polymorphic change of γ-IMC after the neat co-grinding process.Since Povacoat was only soluble in water or<30%ethanol aqueous solution but γ-IMC was only soluble in organic solvents, different solvents and drying methods might signifcantly infuence the solid-state characteristics of IMC formed in the presence of Povacoat.It was found that the amorphous IMC was formed in the Povacoat/IMC evaporates after dissolving both Povacoat and γ-IMC in water:ethanol(mixed ratio=1:9 and 2:8,v/v)and co-evaporation via microwave-drying,whereas the α-IMC was formed in the Povacoat/IMC evaporates via the air-drying method.In addition,the predominate α-IMC formation was only found in all the Povacoat/IMC evaporates with different weight ratios prepared by previously dissolving in water:ethanol(1:9,v/v)and co-evaporation via air-drying,but the amorphous IMC formation after co-evaporation via microwave-drying was obtained except the 4:10(w/w)weight ratio.The thermal effect of microwave irradiation and the presence of Povacoat might be responsible for the amorphous IMC formation in the Povacoat/IMC evaporates.
©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
In recent years,over 40%of the currently marketed active pharmaceutical ingredients(APIs)have been reported to have poor water solubility problems,leading to a limited and variable bioavailability of APIs[1,2].In order to improve the solubility and dissolution of these poorly water-soluble APIs,numerous solubility improvement approaches have been developed and commonly categorized into physical modifcations,chemical modifcations,and other techniques[3–5].Solid dispersion is one of the well-known physical modifcation approaches to improve the dissolution rate and bioavailability of the poorly water-soluble drugs[6–8],in which the drug’s particle size might be markedly reduced,molecularly dispersed or completely dissolved within the high molecular weight water-soluble polymers.Once the solid dispersion is exposed to aqueous media,a supersaturated state may be achieved during dissolution.The high molecular weight water-soluble polymers not only have an ability to prevent precipitation or crystallization of the drug from the supersaturated state but also form a drug-polymer composite or assembly to retard the nucleation and crystal growth[9–11].Therefore,the selection of the appropriate high molecular weight water-soluble polymer plays a more critical role in affecting the physical stability and bioavailability of drugs embedded in the solid dispersion system duringin vitroandin vivoconditions[12–14].
Untilnow,severalpharmaceuticalpolymerssuch as polyethylene glycol(PEG),polyvinylpyrrolidone(PVP), polyvinylpyrrolidone/vinyl acetate copolymer(PVP/VA), hydroxypropylmethyl cellulose(HPMC),hydroxypropylmethyl cellulose acetate/succinate(HPMCAS),hydroxypropyl cellulose(HPC),Eudragits and Soluplus used for solid dispersions have been reported[7,15–18].Recently,a novel pharmaceutical polymer named as Povacoat,produced by co-polymerizing acrylic acid(AA)and methyl methacrylate(MMA)copolymer with polyvinyl alcohol(PVA),has been successfully developed as a capsule shell for flling the liquid formulations to enhance the bioavailability of water-insoluble drugs[19–21]. The chemical structure of Povacoat is indicated in Fig.1a[22]. In addition,Povacoat has also been reported to act as a binder or flm-coating material for pharmaceutical applications[23–25]. Particularly,the oxygen-blocking and light protective abilities of Povacoat flm have increased its application in the pharmaceutical industry.
Povacoat belonging to a thermoplastic polymer has been supplied as Type R with the molecular weight of 200,000 and Type F with a molecular weight of 40,000.Type R has been used as a hard capsule material,but Type F is being applied to act as a binder for wet granulation or as a flm coating polymer [21–25].In our previous study,Lin et al.had investigated the thermal stability of Povacoat and found that Povacoat could able to gradually form six-membered cyclic anhydrides via intramolecular ester condensation after the heating temperature>167°C[26].More recently,Povacoat had been reported to not only prevent the aggregation of nanoparticles more effectively than the other polymers but also provide a rapid onset of action and enhanced bioavailability of a nanocrystal formulation of meloxicam after oral administration[27,28].On the other hand,Xu et al.had indicated that Povacoat was a valuable and promising excipient for the formulation of solid dispersions prepared by hot-melt extrusion method to improve the dissolution of a poorly water-soluble drug,bifendate[29].
Whether Povacoat could infuence the solid state characteristics of drugs in the pharmaceutical processing operations is quite an interesting topic.
In the present study,indomethacin(IMC,Fig.1b)belonging to a typical BCS class II drug with poorly water-soluble property has been chosen as a model drug,which had been extensively examined in many polymorphic studies[30–33].It is well known that IMC exists in several polymorphic forms and an amorphous form[34–37].IMC can exist as the metastable α form and thermodynamically favored γ form.The polymorphic transformation of IMC is easily occurred after different processing[30,38–41].The amorphous IMC is unstable and may be converted to α and/or γ forms of IMC after aging or various treatments[30,31,42,43].This suggests that IMC is highly sensitive to different pharmaceutical processing to cause the polymorphic transformation.
Since Povacoat is only soluble in water but IMC is only soluble in organic solvents,different types of solvents were selected as a solvent in this study.The effect of Povacoat on the solid-state characteristics and physical transformation of IMC after solvent co-evaporation via two drying methods was investigated by thermoanalytical and FTIR spectroscopic studies.
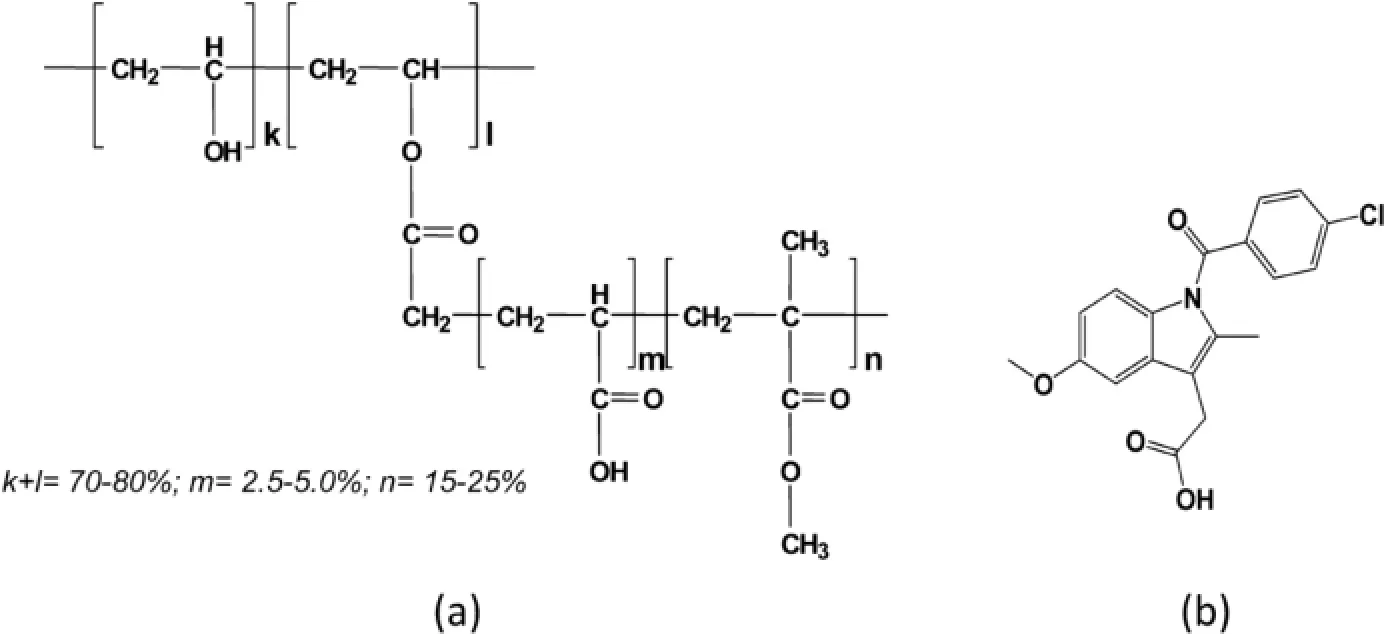
Fig.1–Chemical structures of Povacoat(a)and indomethacin(b).
2.Materials and methods
2.1.Materials
Indomethacin(IMC,γ-form)was purchased from Sigma-Aldrich Chemical Co.(St.Louis,Missouri,USA),which was confrmed by FTIR microspectroscopy and directly used without further treatment.Povacoat(Type F,Mw=40 000)was kindly supplied by Daido Chemical Co.(Osaka,Japan).All organic solvents used were reagent grade.Potassium bromide(KBr)crystals were bought from Jasco Co.(Tokyo,Japan).
2.2.Preparation of different IMC polymorphs
The α-form of IMC(α-IMC)was prepared by dissolving raw material of γ-IMC in the heated absolute ethanol,followed by precipitation after adding Milli-Q water(antisolvent)[33,44]. Amorphous IMC was prepared by melting the γ-IMC at 165°C under nitrogen atmosphere using differential scanning calorimetry(DSC,Q 20,TA Instruments,Inc.,New Castle,DE,USA) and then rapidly quenching the molten sample in liquid nitrogen[31,33,44].All the samples were vacuum-dried and stored in a desiccator flled with anhydrous calcium chloride.
2.3.Preparation of Povacoat/IMC ground mixture or evaporates
A ground mixture of Povacoat and γ-IMC was prepared in a 4:1 (w/w)weight ratio by co-grinding both components in a mortar with pestle for 10 min at room temperature.In addition,each physical mixture of Povacoat and γ-IMC(weight ratio=4:1,w/w) was respectively prepared,and then completely dissolved or dispersed in ethanol,water,or water:ethanol(1:9~2:8,v/v)by ultrasonication for 3 minutes,and then co-evaporated under a hood at ambient temperature(defned as air-drying)or microwave irradiation(defned as microwave-drying).The domestic microwave oven(TMO-202,Tatung Co.,Taipei,ROC)used in this study had full power of 800 W to heat the sample to approximately 78°C in the 3 min course of irradiation[45].After the solvent had been completely evaporated,each Povacoat/IMC evaporate was vacuum-dried for 24 h and stored at 25°C. Povacoat alone was also dissolved or dispersed in each above solvent,and carried out by the same procedure.Furthermore,different physical mixtures of Povacoat and γ-IMC with various weight ratios(4:1~4:10,w/w)were also prepared by dissolving in the solvent of water:ethanol(1:9,v/v)by ultrasonication for 3 minutes,and then co-evaporated via airdrying or microwave-drying method.All the samples were vacuum-dried for 24 h and stored at 25°C for further examinations.Fig.2 shows the fow chart for preparing Povacoat/ IMC ground mixture or evaporates.
2.4.Identifcation and characterization of different samples
Each sample was respectively analyzed by DSC(DSC,Q 20,TA Instruments,Inc.,New Castle,DE,USA)from 30°C to 250°C at 3°C/min with an open pan system in a stream of N2gas. The instrument was calibrated for temperature and heat fow using indium as a standard.Moreover,a trace amount of sample was sealed inside two KBr pellets(without any grinding process with KBr powders)by direct compression with an IR spectrophotometric hydraulic press(Riken Seiki Co.,Tokyo,Japan)at 400 kg/cm2for 15 s.The compressed KBr disc was examined by transmission FTIR microspectroscopy(IRT-5000-16/FTIR-6200,Jasco Co.,Tokyo,Japan)with a mercury-cadmium telluride (MCT)detector.All FTIR spectra were generated by compiling a series of 256 interferograms collected at 4 cm−1resolution and at 100 scans[26,33,45].All analyses were performed in triplicate.
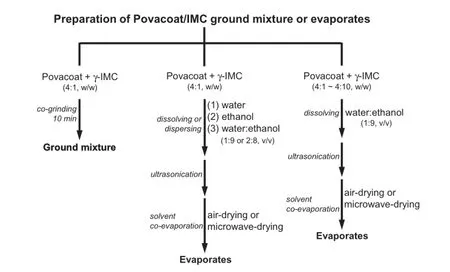
Fig.2–Flow chart for preparing Povacoat/IMC ground mixture or evaporates.
3.Results and discussion
3.1.Identifcation of different samples
Fig.3 shows the DSC curves and FTIR spectra of γ-IMC(a),α-IMC (b),amorphous IMC(c),and Povacoat(d).The DSC curve of γ-IMC exhibited one endothermic peak at 161°C,while the α-IMC prepared showed one endothermic peak at 156°C(Fig.3A-a and b).Both endothermic peaks at 161 and 156°C corresponded to the fusion of γ-IMC and α-IMC,respectively,which were in agreement with previously reported values[30,33,44,45].However, the amorphous IMC showed a different DSC profle from that of DSC curves of γ-and α-forms of IMC.Obviously,one exothermic peak at 123°C and three endothermic peaks at 44,156 and 161°C were observed in the DSC curve of the amorphous IMC(Fig.3A-c).The exothermic peak at 123°C was attributed to the recrystallization of the amorphous IMC after passing through the glass transition temperature at 44°C[33,45] and another two endothermic peaks at 156°C and 161°C were respectively corresponded to the fusion of α-IMC and γ-IMC. This indicates that the amorphous IMC frst exhibited an endothermic relaxation peak near at 44°C,and then accompanied by exothermic recrystallization,and followed by transformation into α-IMC and less often into γ-IMC.While Povacoat exhibited two small broad endothermic peaks nearly at 82 and 197°C(Fig.3A-d),the former was due to the glass transition temperature of Povacoat[21]and the latter might be attributed to the cyclic anhydride formation via intramolecular ester condensation in the Povacoat structure by DSC heating process [26].
The FTIR spectra of γ-IMC(a),α-IMC(b),amorphous IMC(c), and Povacoat(d)are also displayed in Fig.3B.Several unique IR absorption bands and their assignments of γ-IMC and α-IMC are listed as follows(Fig.3B-a and b):1717 cm−1[ν(C–O)of carboxylic acid dimer],1691 cm−1[benzoyl ν(C–O)],1625–1570 and 1480 cm−1(C–C of aromatic rings),1307 cm−1(C–O of acidic group),1270–1200 cm−1(–C–O stretching,ether group), 1067 cm−1(C–Cl)for γ-IMC[33,44–47];1734 cm−1[non-hydrogen bonded acid ν(C–O)],1692 cm−1[benzoyl v(C–O)],1688 and 1649 cm−1[hydrogen bonded acid v(C–O)],1478 cm−1(C–C of aromatic rings),1320 cm−1(C–O of acidic group),1224 cm−1(–C–O stretching,ether group),and 1074 cm−1(C–Cl)for α-IMC, respectively[33,44–47].On the other hand,the amorphous IMC exhibits a unique FTIR spectrum,including a shoulder at 1735 cm−1[non-hydrogen bonded acid ν(C–O)],1710 cm−1[asymmetric acid ν(C–O)of a cyclic dimer],1683 cm−1[benzoyl v(C–O)],1591 cm−1[ring vibration of indole](Fig.3B-c),which was in agreement with the reported IR spectrum of amorphous IMC[33,44–47].It is also found that the band at 1717 cm−1due to asymmetric acid ν(C–O)of a cyclic dimer for crystalline γ-IMC was shifted to a lower frequency of about 1710 cm−1, implying that the dimers formation was also presented in the amorphous state of IMC[44,47].On the other hand,the FTIR spectrum(Fig.3B-d)of Povacoat indicated the peaks at 1729 cm−1(C–O stretching),1436 cm−1(C–H bending),1265 and 1245 cm−1(C–O stretching),1100–1000 cm−1(C–O stretching in C–O–Hgroups and COC groups)and 842 cm−1(C–H rocking mode) [26,48,49].

Fig.3–DSC curves and FTIR spectra of γ-IMC(a),α-IMC(b),amorphous IMC(c),and Povacoat(d).
3.2.Grinding effect on the Povacoat/IMC physical mixture
Fig.4a shows the DSC curve and FTIR spectrum of the ground mixture of Povacoat and γ-IMC with a 4:1(w/w)weight ratio after co-grinding both components in a mortar with pestle for 10 min.It clearly indicates that the DSC curve exhibited two broad endothermic peaks at 76 and 195°C,and a sharp endothermic peak at 160°C,respectively.The frst endothermic peak at 76°C corresponded to the glass transition temperature of Povacoat[21]and the latter peak at 195°C was due to the cyclic anhydride formation via intramolecular ester condensation in the Povacoat structure by DSC heating process [26],whereas the sharp endothermic peak at 160°C was attributed to the fusion of γ-IMC.The FTIR spectrum of this ground mixture seemed to be superimposed by the FTIR spectra of Povacoat(1727,1436,1373 and 1243 cm−1)and γ-IMC(1691,1479, 1453,1308,1087,1068 and 841 cm−1).This suggests that the neat grinding process did not induce any interaction between Povacoat and γ-IMC or failed to cause a polymorphic change of γ-IMC after co-grinding with Povacoat.
3.3.Effect of solvent types on the polymorphic changes of IMC in various Povacoat/IMC evaporates via two drying
methods
The DSC curves and FTIR spectra of IMC-free Povacoat evaporates prepared by different solvents via air-drying or microwavedrying process exhibited similar plots to that of the raw material of Povacoat(data not shown),indicating that the types of solvent and drying method did not infuence the solid-state property of Povacoat.
Due to the solubility problems of Povacoat(only soluble in water or<30%ethanol aqueous solution)and γ-IMC(only soluble in organic solvents),different solvents and/or different preparation methods seem to signifcantly infuence the solidstate characteristics of IMC formed in various Povacoat/IMC evaporates[47,50,51].Fig.4 displays various DSC curves and FTIR spectra of the Povacoat/IMC evaporates prepared by dissolving and/or dispersing both components in different solvents and then co-evaporation via air-drying or microwave-drying method.

Fig.4–DSC curve and FTIR spectra of the Povacoat/γ-IMC ground mixture(a)and different Povacoat/IMC evaporates(b~g).Key:solvent:b,ethanol via microwave drying;c,water via microwave drying;d-e,water:ethanol(1:9,v/v)via air-drying and microwave-drying;f-g,water:ethanol(2:8,v/v)via air-drying and microwave-drying.
When ethanol alone was used as a solvent,the DSC curve of the Povacoat/IMC evaporates prepared by microwavedrying method revealed two broad endothermic peaks at 93°C(glass transition temperature of Povacoat)and 196°C(cyclic anhydride formation of Povacoat)(Fig.4A-b).On the other hand, two FTIR spectral peaks at 1479,1375 and 1092 cm−1appeared in the FTIR spectra of this sample,indicating that α-IMC and/ or amorphous IMC might be dispersed in the polymeric matrix of Povacoat(Fig.4B-b).However,when water was used as the onlysolvent,theDSCcurveofthePovacoat/IMCevaporatesprepared by microwave-drying method illustrated an interested DSC curve,including two broad endothermic peaks at 75 and 194°C and two very small endothermic peaks at 152 and 160°C (Fig.4A-c).The former two broad endothermic peaks were attributedtotheglasstransitiontemperatureandcyclicanhydride formation of Povacoat,whereas the latter two small endothermic peaks corresponded to the fusion of α-IMC and γ-IMC.The appearance of FTIR peaks at 1477,1373 and 1323 cm−1assigned to α-IMC and at 1088 and 841 cm−1that corresponded to γ-IMC was also found in the FTIR spectrum of this sample (Fig.4B-c).Although Povacoat could dissolve in water but γ-IMC did not,the thermal effect of microwave irradiation might initially cause the dissolution of a partial γ-IMC in the water and then transited to α-IMC after drying.The DSC curve and FTIR spectra of this sample confrmed the co-existence of α-IMC and γ-IMC dispersed in the polymeric matrix of Povacoat(Figs.4A-c and 3B-c).
When the water:ethanol(1:9,v/v)was used as a solvent,the DSC curve of Povacoat/IMC evaporates prepared by air-drying process is displayed in Fig.4A-d.It clearly indicates that except 84 and 195°C due to glass transition temperature and cyclic anhydride formation of Povacoat,a marked endothermic peak at 151°C attributed to the fusion of α-IMC was observed.The FTIR spectrum as shown in Fig.4B-d seemed to be superimposed by the FTIR spectra of Povacoat(1729 and 1436 cm−1)and α-IMC(1692,1650,1608,1477,1455,1091,1018 and 931 cm−1). Since the water:ethanol(1:9,v/v)was used,the γ-IMC was completelydissolvedandalargeproportionofγ-IMCwastransformed to α-IMC by slow evaporation under a hood at ambient temperature(air-drying),leading to the obvious peaks of α-IMC appearedinDSCcurveandFTIRspectra.TheeffectofmicrowavedryingprocessontheDSCcurveandFTIRspectrumofPovacoat/ IMC evaporates was markedly shown in Fig.4e,when the water:ethanol(1:9,v/v)was used as a solvent.Only two small broad endothermic peaks at 82 and 198°C were found in the DSC curve,due to the glass transition temperature and cyclic anhydride formation of Povacoat.However,several characteristic FTIR peaks at 1685,1592,1477,1358,1090 and 1068 cm−1that corresponded to amorphous IMC were clearly observed in the FTIR spectrum of this Povacoat/IMC evaporates,as compared with the FTIR spectrum of amorphous IMC(Fig.3B-c).Here,the physical mixture of Povacoat and IMC was completely dissolved in water:ethanol(1:9,v/v)through ultrasonication for 3 min.In the process of solvent evaporation via microwave-drying,the thermal effect of microwave irradiation might induce the formation of amorphous IMC in the presence of Povacoat.The same results were also observed in the DSC curves and FTIR spectra of the Povacoat/ IMC evaporates prepared by dissolving both components in the water:ethanol(2:8,v/v)solvent and then evaporation via air-drying or microwave-drying method,as shown in Fig.4f and g.
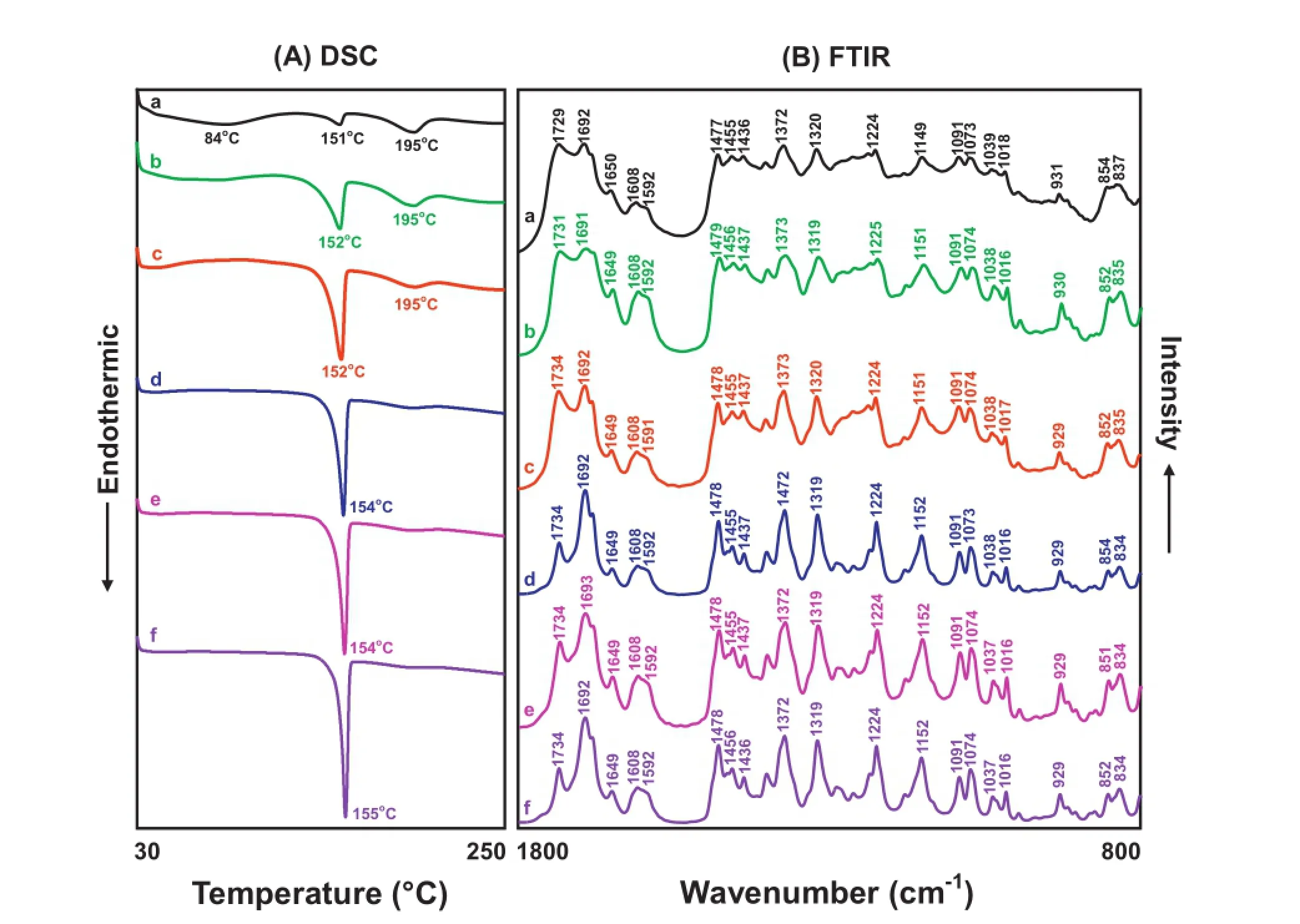
Fig.5–DSC curves and FTIR spectra of different Povacoat/IMC evaporates with various weight ratios of Povacoat and γ-IMC after dissolving or dispersing in water:ethanol(1:9,v/v)and solvent evaporation via air-drying method.Key:weight ratio(w/w)of Povacoat:γ-IMC:a,4:1;b,4:2,c,4:4,d,4:6;e,4:8;f,4:10.
It should be noted that in the absence of Povacoat the uncertain polymorphic forms of IMC(α,γ,amorphous or its mixture)could be obtained after previously dissolving IMC in the water:ethanol mixed solution and then microwavedrying(data not shown).The solubility of IMC in the water:ethanol mixed solution and the evaporation speed induced by thermal effect of microwave-drying might be responsible for different polymorphic formation of IMC.Once Povacoat was incorporated into the water:ethanol mixed solution and then microwave-drying,only the amorphous IMC was formed.This implies that Povacoat might improve and contribute to the amorphous formation of IMC in this system via microwave-drying process.
3.4.Effect of Povacoat:IMC weight ratios on the polymorphic changes of IMC in the various Povacoat/IMC evaporates via two drying methods
Fig.5displays the DSC curves and FTIR spectra of different Povacoat/IMC evaporates with various weight ratios of Povacoat and γ-IMC after dissolving or dispersing in water:ethanol(1:9, v/v)and solvent evaporation via air-drying method.
Three endothermic peaks at 84,151 and 195°C were observed in the DSC curve of the Povacoat/IMC evaporate prepared by mixing Povacoat and γ-IMC in weight ratio of 4:1(w/w),both endothermic peaks at 84 and 195°C were attributed to the glass transition temperature and cyclic anhydride formation of Povacoat[21,26],but an endothermic peak at 151°C corresponded to the α-IMC.On the other hand,the FTIR spectrum of this Povacoat/IMC evaporate with a 4:1(w/w)weight ratio was superimposed by the IR spectra of Povacoat and α-IMC. It is evident that the endothermic peak at 151°C due to the α-IMC became sharper and shifted to higher temperatures with the increase of the amount of γ-IMC added.The FTIR spectra of these Povacoat/IMC evaporates clearly indicate that the IR peaks at 1731(1734),1692(1691~1693)and 1649 cm−1assigned to α-IMC became more predominate,suggesting that the α-IMC played an important role in this system afterpreparation with air-drying method at ambient temperature. Particularly,the γ-IMC completely dissolved in the water:ethanol (1:9,v/v)solvent might result in a large amount of α-IMC formation after slow evaporation via air-drying method.
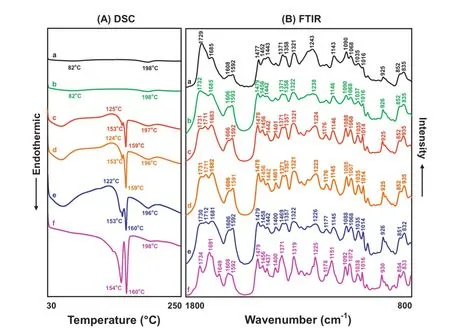
Fig.6–DSC curves and FTIR spectra of different Povacoat/IMC evaporates with various weight ratios of Povacoat and γ-IMC after dissolving or dispersing in water:ethanol(1:9,v/v)and solvent evaporation via microwave-drying method.Key:weight ratio(w/w)of Povacoat:γ-IMC:a,4:1;b,4:2,c,4:4,d,4:6;e,4:8;f,4:10.
However,the Povacoat/IMC evaporates prepared by microwave-drying method exhibited different thermal behaviors and FTIR spectral patterns,as shown in Fig.6.When the weight ratios of Povacoat:γ-IMC were 4:1 and 4:2(w/w),similar DSC curves and FTIR spectra for both Povacoat/IMC evaporates were obtained.The broad endothermic peaks at 82 and 198°C in the DSC curve might be attributed to the glass transition temperature and cyclic anhydride formation of Povacoat [21,26],respectively.In addition,several unique FTIR peaks at 1685,1608(1606),1592(1593),1477(1479),1358(1360),1089(1090) and 1068(1066)cm−1that corresponded to amorphous IMC were observed(Fig.6a and b)because the Povacoat/IMC physical mixture was completely dissolved in water:ethanol(1:9,v/v) by ultrasonication for 3 minutes.In the microwave-drying process,the thermal energy of microwave irradiation might induce the formation of amorphous IMC in the presence of Povacoat.The amorphous IMC formed might be embedded in the polymeric matrix of Povacoat,leading to the disappearance of both exothermic and endothermic peaks of amorphous IMC in the DSC curves,as compared with the DSC curve of amorphous IMC(Fig.3A-c.By increasing the amount of γ-IMC, the thermal effect of microwave irradiation was able to induce more amounts of amorphous IMC formation in the presence of Povacoat,resulting in the appearance of the exothermic peak at 125(124 and 122)°C and two endothermic peaks at 153(154) and 159(160)°C(Fig.6c~e),as compared with the DSC curve of amorphous IMC(Fig.3c).Once the amount of γ-IMC was over added,however,only the α-IMC was formed in the Povacoat/ IMC evaporates(Fig.6f).This clearly implies that the weight ratios of Povacoat/IMC and drying methods could markedly infuence the solid-state polymorphic transformation of IMC polymorphs in the presence of Povacoat.
4.Conclusion
In the present study,the types of solvent,weight ratios of Povacoat and γ-IMC,and drying methods might induce different polymorphic changes of IMC in the Povacoat/IMC evaporates.The thermal effect of microwave-drying method might induce the amorphous IMC formation in the presence of Povacoat.
Acknowledgements
This work was supported by Ministry of Science and Technology,Taiwan,ROC(MOST 103-2320-B-264-002-MY2).
R E F E R E N C E S
[1]Avdeef A.Absorption and drug development:solubility, permeability,and charge state.2nd ed.Hoboken(NJ):John Wiley&Sons;2003.
[2]Kerns EH.High throughput physicochemical profling for drug discovery.J Pharm Sci 2001;90:1838–1858.
[3]Savjani KT,Gajjar AK,Savjani JK.Drug solubility:importance and enhancement techniques.ISRN Pharm 2012;2012:Article ID 195727.
[4]Kumar S,Bhargava D,Thakkar A,et al.Drug carrier systems for solubility enhancement of BCS class II drugs:a critical review.Crit Rev Ther Drug Carrier Syst 2013;30:217–256.
[5]Kawabata Y,Wada K,Nakatani M,et al.Formulation design for poorly water-soluble drugs based on biopharmaceutics classifcation system:basic approaches and practical applications.Int J Pharm 2011;420:1–10.
[6]Kumar S,Gupta SK.Pharmaceutical solid dispersion technology:a strategy to improve dissolution of poorly water-soluble drugs.Recent Pat Drug Deliv Formul 2013;7:111–121.
[7]Alam MA,Ali R,Al-Jenoobi FI,et al.Solid dispersions:a strategy for poorly aqueous soluble drugs and technology updates.Expert Opin Drug Deliv 2012;9:1419–1440.
[8]Bhatnagar P,Dhote V,Chandra S,et al.Solid dispersion in pharmaceutical drug development:from basics to clinical applications.Curr Drug Deliv 2014;11:155–171.
[9]Sapkal1 S,Babhulkar M,Rathi A,et al.An overview on the mechanisms of solubility and dissolution rate enhancement in solid dispersion.Int J PharmTech Res 2013;5:31–39.
[10]Kumar P,Singh C.A study on solubility enhancement methods for poorly water soluble drugs.Am J Pharmacol Sci 2013;1:67–73.
[11]Williams HD,Trevaskis NL,Charman SA,et al.Strategies to address low drug solubility in discovery and development. Pharmacol Rev 2013;65:315–499.
[12]Wegiel LA,Mauer LJ,Edgar KJ,et al.Mid-infrared spectroscopy as a polymer selection tool for formulating amorphous solid dispersions.J Pharm Pharmacol 2014;66:244–255.
[13]Van Eerdenbrugh B,Taylor LS.Small scale screening to determine the ability of different polymers to inhibit drug crystallization upon rapid solvent evaporation.Mol Pharm 2010;7:1328–1337.
[14]Chauhan H,Kuldipkumar A,Barder T,et al.Correlation of inhibitory effects of polymers on indomethacin precipitation in solution and amorphous solid crystallization based on molecular interaction.Pharm Res 2014;31:500–515.
[15]Ali S,Kolter K.Challenges and opportunities in oral formulation development.Am Pharm Rev 2012;15(7).
[16]Kou X,Zhou L.Stability of amorphous solid dispersion.In: Shah N,Sandhu H,Choi DS,et al.,editors.Amorphous solid dispersions:theory and practice.New York:Springer-Verlag; 2014.
[17]Newman A,Knipp G,ZografG.Assessing the performance of amorphous solid dispersions.J Pharm Sci 2012;101:1355–1377.
[18]Reintjes T.Solubility enhancement with BASF pharma polymers: Solubilizer compendium.BASF SE Pharma Ingredients& Services,Germany,October,2011.
[19]Hoshi N,Ogura T,Shimamoto T,et al.Development of PVA copolymer capsules.Pharm Tech Japan 2003;19:1–12.
[20]Hoshi N,Ogura T,Shimamoto T,et al.Development of PVA copolymer capsules.Pharm Tech Eur 2004;16:37–46.
[21]Hoshi N,Kida A,Hayashi T,et al.Creating PVA copolymer capsules.Pharm Tech Eur 2008;20:17–25.
[22]Daido Chemical Corporation.The characteristics of Povacoat and its applications,2010.
[23]Fujii T,Noami M,Tomita K,et al.Development of a new coating agent,PVA copolymer(I).Pharm Tech Japan 2005;21:257–261.
[24]Fujii T,Noami M,Tomita K,et al.PVA copolymer:the new coating agent.Pharm Tech Eur 2008;20:32–39.
[25]Kojo A,Noami M,Funaki T.Development of a new coating agent,PVA copolymer(II).Pharm Tech Japan 2009;25:369–374.
[26]Lin SY,Cheng WT,Wei YS,et al.DSC-FTIR microspectroscopy used to investigate the thermal-induced intramolecular cyclic anhydride formation between Eudragit E and PVA copolymer.Polym J 2011;43:577–580.
[27]Yuminoki K,Seko F,Horii S,et al.Preparation and evaluation of high dispersion stable nanocrystal formulation of poorly water-soluble compounds by using Povacoat.J Pharm Sci 2014;103:3772–3781.
[28]Ochi M,Kawachi T,Toita E,et al.Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors.Int J Pharm 2014;474:151–156.
[29]Xu M,Zhang C,Luo Y,et al.Application and functional characterization of POVACOAT,a hydrophilic co-polymer poly(vinyl alcohol/acrylic acid/methyl methacrylate)as a hot-melt extrusion carrier.Drug Dev Ind Pharm 2014;40:126–135.
[30]Okumura T,Ishida M,Takayama K,et al.Polymorphic transformation of indomethacin under high pressures. J Pharm Sci 2006;95:689–700.
[31]Greco K,Bogner R.Crystallization of amorphous indomethacin during dissolution:effect of processing and annealing.Mol Pharm 2010;7:1406–1418.
[32]Dubbini A,Censi R,Martena V,et al.Infuence of pH and method of crystallization on the solid physical form of indomethacin.Int J Pharm 2014;473:536–544.
[33]Kao CY,Huang HH,Huang YT,et al.Thermoanalytical and spectroscopic studies on amorphization and phase transition of amorphous indomethacin prepared by two melt-cooling processes.Sci Lett 2015;4:148.
[34]Surwase SA,Boetker JP,Saville D,et al.Indomethacin:new polymorphs of an old drug.Mol Pharm 2013;10:4472–4480.
[35]Kaneniwa N,Otsuka M,Hayashi T.Physicochemical characterization of indomethacin polymorphs and the transformation kinetics in ethanol.Chem Pharm Bull (Tokyo)1985;33:3447–3455.
[36]Lin SY.Isolation and solid-state characteristics of a new crystal form of indomethacin.J Pharm Sci 1992;81:572–576.
[37]Crowley KJ,ZografG.Cryogenic grinding of indomethacin polymorphs and solvates:assessment of amorphous phase formation and amorphous phase physical stability.J Pharm Sci 2002;91:492–507.
[38]Lin SY,Cherng JY.Polymorphic transformation of indomethacin in precirol solid dispersion system.J Therm Anal 1995;45:1565–1577.
[39]Trotta M.Infuence of phase transformation on indomethacin release from microemulsions.J Control Release 1999;60:399–405.
[40]Otsuka M,Matsumoto T,Kaneniwa N.Effect of environmental temperature on polymorphic solid-state transformation of indomethacin during grinding.Chem Pharm Bull(Tokyo)1986;34:1784–1793.
[41]Guo Z,Ma M,Wang T,et al.A kinetic study of the polymorphic transformation of nimodipine and indomethacin during high shear granulation.AAPS PharmSciTech 2011;12:610–619.
[42]Vyazovkin S,Dranca I.Effect of physical aging on nucleation of amorphous indomethacin.J Phys Chem B 2007;111:7283–7287.
[43]Karmwar P,Boetker JP,Graeser KA,et al.Investigations on the effect of different cooling rates on the stability of amorphous indomethacin.Eur J Pharm Sci 2011;44: 341–350.
[44]Taylor LS,ZografG.Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions.Pharm Res 1997;14:1691–1698.
[45]Lin HL,Zhang GC,Huang YT,et al.An investigation of indomethacin-nicotinamide cocrystal formation induced by thermal stress in the solid or liquid state.J Pharm Sci 2014;103:2386–2395.
[46]Terife G,Wang P,Faridi N,et al.Hot melt mixing and foaming of Soluplus®and Indomethacin.Polym Eng Sci 2012;52:1629–1639.
[47]Karmwar P,Graeser K,Gordon KC,et al.Investigation of properties and recrystallisation behaviour of amorphous indomethacin samples prepared by different methods.Int J Pharm 2011;417:94–100.
[48]Arndt KF,Richter A,Ludwig S,et al.Poly(vinyl alcohol)/ poly(acrylic acid)hydrogels:FT-IR spectroscopic characterization of crosslinking reaction and work at transition point.Acta Polymer 2000;50:383–390.
[49]Mansur HS,Sadahira CM,Souza AN,et al.FTIR spectroscopy characterization of poly(vinyl alcohol)hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde.Mater Sci Eng C 2008;28:539–548.
[50]Andronis V,Yoshioka M,ZografG.Effects of sorbed water on the crystallization of indomethacin from the amorphous state.J Pharm Sci 1997;86:346–351.
[51]Giulietti M,Bernardo A.Crystallization by antisolvent addition and cooling.In:Andreeta MRB,editor. Crystallization-science and technology.INTECH Open Access Publisher;2012.p.379–396.
*< class="emphasis_italic">Corresponding author.
.Department of Biotechnology and PharmaceuticalTechnology,Yuanpei University of MedicalTechnology,No.306, Hsin-Chu 30015,Taiwan.Tel.:+886 3 6102439;fax:+886 3 6102328.
E-mail address:sylin@mail.ypu.edu.tw(S.-Y.Lin).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.09.005
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- PEGylation in anti-cancer therapy:An overview
- Near-infrared light-responsive inorganic nanomaterials for photothermal therapy
- Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole
- Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying:Physicochemical and in silico studies
- Preparation and evaluation of PEGylated phospholipid membrane coated layered double hydroxide nanoparticles
- Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study
