Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying:Physicochemical and in silico studies
2017-01-19
Department of Pharmaceutical Sciences and Technology,Institute of Chemical Technology,Nathalal Parekh Marg,Matunga,Mumbai,Maharashtra,India
Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying:Physicochemical and in silico studies
Jaywant N.Pawar*,Rahul T.Shete,Avinash B.Gangurde, Kailas K.Moravkar,Sharadchandra D.Javeer,Divakar R.Jaiswar, Purnima D.Amin
Department of Pharmaceutical Sciences and Technology,Institute of Chemical Technology,Nathalal Parekh Marg,Matunga,Mumbai,Maharashtra,India
A R T I C L EI N F O
Article history:
Received 26 June 2015
Received in revised form 16 August 2015
Accepted 25 August 2015
Available online 1 October 2015
Artemether
Spray drying
Solid dispersion
In silicostudy
Docking
Artemether(ARM)is a poorly water soluble and poorly permeable drug effective against acute and severe falciparum malaria,hence there is a strong need to improve its solubility.The objective of the study was to enhance the solubility and dissolution rate of ARM by preparation of solid dispersions using spray-drying technique.Solid dispersions of ARM were prepared with Soluplus,Kollidon VA 64,HPMC and Eudragit EPO at weight ratios of 1:1,1:2, 1:3 using spray drying technology,and characterized by Fourier transform infrared spectroscopy,differential scanning calorimetry(DSC),and X-ray powder diffraction(XRD)to identify the physicochemical interaction between drug and carrier,as well as effect on dissolution. The prepared solid dispersion of ARM with polymers showed reduced crystallinity as compared to neat ARM,which was confrmed by DSC and XRD.Drug/polymer interactions were studiedin-silicoby docking and molecular dynamics which indicated formation of van der Waals type of interactions ofARM with the polymers.Based on solubility studies,the optimum drug/Soluplus ratio was found to be 1:3.The dissolution studies of formulation SD3 showed highest drug release up to 82%compared to neat ARM giving only 20%at 60 minutes.The spray-dried products were free of crystalline ARM;possessed higher dissolution rates,and were stable over a period according to ICH guidelines.These fndings suggest that an amorphous solid dispersion of ARM could be a viable option for enhancing the dissolution rate of ARM.
©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Artemisinin is an important class of antimalarial drugs,structurally characterized by incidence of a sesquiterpene lactone with a peroxide bridge and is the active constituent of the Chinese medicinal herbArtemisia annua[1–3].Different types of active metabolites of artemisinin have been synthesized,viz. artemether,artesunate,and arteether are currently in use or under clinical trials[4].
β-Artemether[5]is one of the artemisinin derivatives which has been proven to be effective against acute uncomplicated and severe falciparum malaria[6].ARM is active againstPlasmodium vivaxas well as chloroquine-sensitive and chloroquineresistant strains ofPlasmodium falciparum.ARM is also indicated in the treatment of cerebral malaria.ARM,having the chemical structure as shown in Fig.1,shows rapid onset of schizontocidal action and is metabolized in the liver to a demethylated derivative,dihydroartemisinin.However,the therapeutic potential of ARM is markedly delayed due to its low oral bioavailability.The low bioavailability of ARM results from its poor aqueous solubility[5],resulting in poor absorption upon oral administration.This is due to a large fraction of the drug that remained undissolved to reach absorption site. Under such conditions,the bioavailability can be increased by using a water-soluble formulation[6].
IUPAC name:[7]-[8a-beta,9-alpha,12-beta,12aR]-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[9]-1,2-benzodioxepin.
Formulation scientists are working on different approaches to enhance the dissolution rate of poorly soluble drugs includes,solid dispersions prepared by spray-drying[7,8],freezedrying[9],mechanical milling[10],hot melt extrusion[11,12], supercritical fuid extraction[13,14],co-crystal formation[15,16], inclusion complexation using cyclodextrins[17],microencapsulation[18],and particle size reduction[19,20].However,most of these approaches face demerits of scale up issues or economic challenge.
Literature reports that solid dispersion[21]using watersoluble polymers showed good improvement in dissolution rate and bioavailability.Researchers have established various approaches for dissolution rate enhancement of ARM with PEG 6000 and PVP by solvent evaporation and lyophilization method [22],ARM with PEG 4000 and PVP K25 by freeze-drying and melt extrusion method[23],and ARM inclusion complexes with hydroxypropyl β-cyclodextrin[2]microencapsulation[18].In addition,spray drying technology has also explored for solubility enhancement ARM using polyvinylpyrrolidone as a carrier[21], also by making microparticles of ARM[24]and using polyethylene glycol as hydrophilic polymer[25].The ARM–Soluplus SD system has been developed using hot melt extrusion technology for improving the dissolution rate of ARM[5].
Spray drying is known to produce predominately-amorphous material due to the instantaneous transition between liquid and solid phases.In spray drying,the drug–polymer solution is atomized and dispersed into hot gas,which causes the solvent to evaporate and leads to the generation of spherical solid particles[26].Spray drying is the most universally used industrial process as the product from spray drying process meets the highest quality standards with reference to the particle size,shape,homogeneity,and uniform distribution of products.Different types of particles can be produced by this technology[27,28].The wide variety of processing parameters[25]makes it a powerful technique to tune the physical state[29]and the particle morphology of pharmaceutical systems[30].
The present study aims to elucidate the potential of enhancing the solubility and dissolution rate of ARM using hydrophilic polymers like Soluplus,KollidonVA 64,HPMC and Eudragit EPO by spray-drying technology.Kollidon VA 64 is a water-soluble vinylpyrrolidone–vinyl acetate copolymer.Low viscosity HPMC is an odorless and tasteless,white or creamywhite fbrous or granular powder.It is partly O-methylated and O-cellulose[31].Soluplus is a polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer,a new polymer with amphiphilic properties[5].Eudragit EPO is a cationic copolymer based on dimethylaminoethyl methacrylate,butyl methacrylate,and methyl methacrylate.It is a white powder with a characteristic amine-like odor.
The chemical interaction of ARM with the polymers triggering enhancement in solubility of ARM was simulatedin silicoby means of docking and molecular dynamics[18].The docking analysis mimics the drug binding to proteins to understand the interactions occurring at the molecular level.This approach has found relevant applications in material science, particularly in the area of drug–polymer interactions[32].Some authors have exploitedin silicostudies to predict and rationalize the design of drug delivery systems as well[33–35].In the present study,we attempted to study the interactions between ARM and the respective polymers viz.Soluplus, KollidonVA 64,HPMC and Eudragit EPO by combining docking and molecular dynamics study[18].The analysis of the molecular phenomena involved in the recognition capability of polymeric complex represents clearly an intricate but fascinating research topic,which has been implicated from the results.
In the present study,we attempted to simulate the interactions between ARM and the respective hydrophilic polymers combining several computational techniques.In particular,the polymers were simulated as functional oligomers simplifying the whole composition and structures and therefore maintaining the recognition properties.
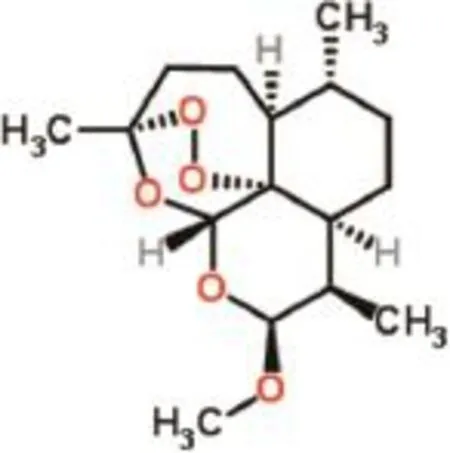
Fig.1–Chemical structure of ARM.
ARM solid dispersions were prepared by spray drying technology from various ratios of Soluplus,Kollidon VA 64,HPMC and Eudragit EPO as hydrophilic polymeric carriers.Furthermore,the study undertakes to investigate solid-state characterization using differential scanning calorimetry(DSC),powder X-ray diffractometry(XRD)and attenuated total refectance–Fourier transform infrared(ATR-FTIR)spectroscopy,and morphological characterization using scanning electron microscopy(SEM)and studied their dissolution properties along within silicostudies to predict possible mechanism for improved dissolution rate with the respective polymers.
2.Materials and methods
2.1.Materials
ARM was obtained as a generous gift from Mangalam Drugs and Organics Ltd.Mumbai,India.The polymers,viz.Soluplus and Kollidon VA 64,were gifted from BASF AG(Germany). Eudragit EPO and HPMC were obtained as gift samples from Evonik industries(Germany)and Dow Chemicals(India)respectively.All other chemicals and solvents used were of analytical grade and were procured from Merck India Ltd.All the materials were used as received.
2.2.Method
2.2.1.Preparation of ARM solid dispersions by spray drying technique
ARM SDs with the different hydrophilic polymers were obtained by spray drying(water:acetone,20:80)solutions of ARM with other hydrophilic polymers.The spray drying was carried out using the instrument Spray dryer SD-1100(JISL,Mumbai, India).ARM(5 gm/batch)was dissolved in 10 mL of acetone. The other hydrophilic polymers were dispersed in 160 mL of distilled water in different ratios as shown in Table 1.
The organic phase was then slowly added to aqueous phase on magnetic stirrer with continuous stirring for a period of 30 min.The solid concentration was varied depending upon the different batch ratios.The SDs were prepared using a spray dryer with the following conditions:inlet temperature of 70–72°C,feed rate of 3 ml/min,outlet temperature of 42–44°C, and aspirator set to at 40 m3/h;the spray dryer had a nozzle diameter of 1 mm.The spray-dried particles were collected into paraflm-sealed scintillation vials,and the closed vials were stored at room temperature prior to analysis.A physical mixture of ARM with Soluplus,KollidonVA 64,HPMC and Eudragit EPO was also prepared by carefully mixing the two substances in a glass vial[36].
2.3.Apparent solubility study
Solubility study was carried out for neat ARM and ARM SDs in 20 ml of distilled water containing 1%SLS and phosphate buffer of pH 7.2 containing 1%SLS to check out the maximum solubility of ARM and ARM SDs in respective dissolution media after 72 hrs.An excess amount of ARM and ARM SDs made up of Soluplus,KollidonVA 64,HPMC and Eudragit EPO in the ratios of 1:1,1:2,and 1:3 was added to 10 ml of freshly prepared distilled water containing 1%SLS and phosphate buffer of pH 7.2 containing 1%SLS in clean test tubes and sealed.The samples were then sonicated for 15 min at room temperature.Thereafter,the test tubes were shaken for 72 hrs at 37±0.5°C at a speed of 75 rpm on an orbital shaking thermostable incubator(Boekel Scientifc,Germany).The samples were centrifuged at 10,000 rpm for 10 min and fltered through a 0.45 μm Millipore membrane flter.The frst 1 mL of the fltrate was discarded. Samples were then suitably diluted with the respective dissolutionmediumandanalyzedat211 nmonUV-spectrophotometer(UV-1601 PC,Shimadzu,Japan).All solubility measurements were performed in triplicates.
2.4.Structural analysis by FTIR
FTIR analysis was done to investigate the molecular structures of ARM and Soluplus,KollidonVA 64,HPMC and Eudragit EPO and their SD systems.The samples were analyzed for their functional groups using Shimadzu MIRACLE IR Affnity-1 FTIR spectrophotometer.The samples were premixed with KBr using mortar and pestle,and KBr disks were prepared by means of a hydraulic press.The scanning range was 4000–400 cm−1resolution of 4 cm−1.
2.5.Thermal analysis(DSC)
DSC analysis was performed to check the physical state of SDs with respect to neat ARM and Soluplus,Kollidon VA 64,HPMC and Eudragit EPO polymers,respectively,using Pyris-6 DSC Perkin Elmer(USA).Approximately 3–4 mg of sample was placedin aluminum pan and crimped using a press.An empty aluminum pan was used as a blank.Samples were heated at the rate of 10°C/minute from 30°C to 300°C under 17 ml/min of nitrogen fow to obtain the endothermic peaks.
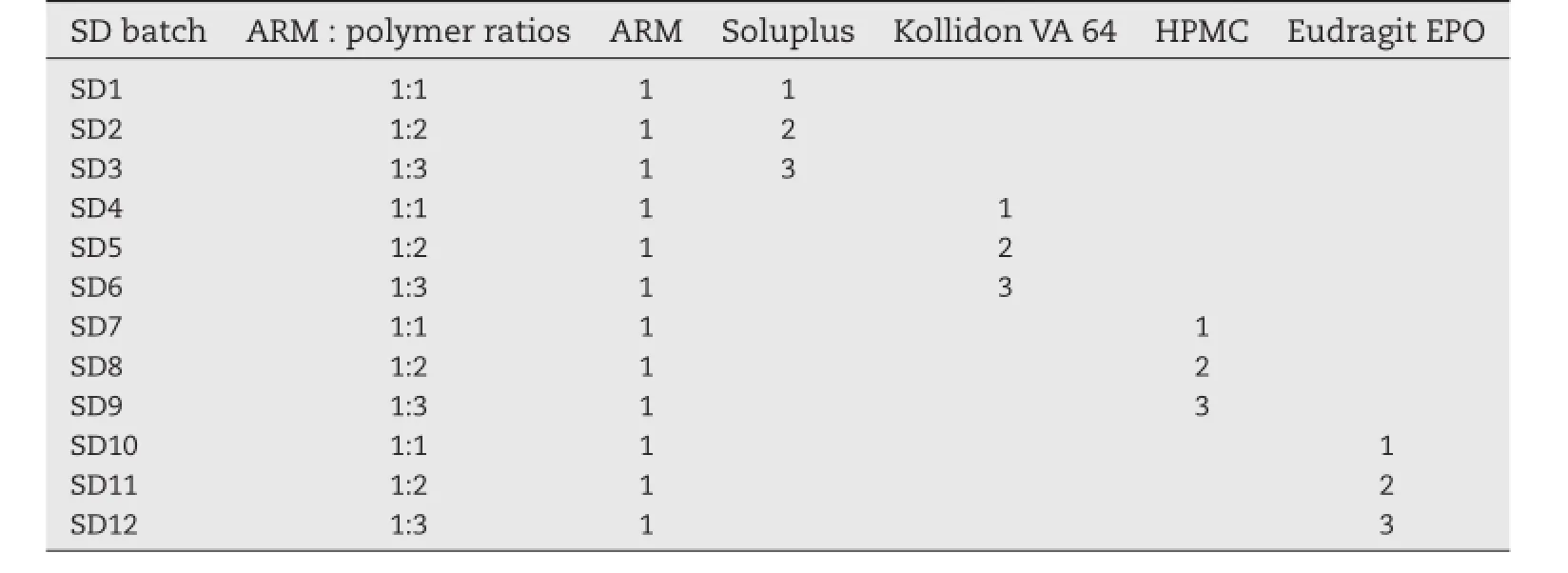
Table 1–Different solid dispersion batches containing ARM and polymers in ratios.
2.6.Crystallinity studies(PXRD)
X-ray diffraction studies were done for physical characterization of SDs.X-ray diffraction patterns were obtained by ADVANCE D8 system with CuKα radiation(Bruker,USA).The recording spectral range was set at 10°–50°(2θ)using the Cutarget X-ray tube and Xe-flled detector.The voltage 40 kV with current 20 mA was set.The samples were placed in a zero background sample holder and incorporated on a spinner stage. Soller slits(0.04 rad)were used in the incident and diffracted beam path.
2.7.Confrmation of surface morphology by SEM
The particulate morphologies of the ARM powder and ARM SD were examined using a scanning electron microscope(XL 30 Model JEOL 6800 SEMTokyo,Japan).The powder samples ARM and ARM SD were spread on a carbon fber tape,and sample coated by platinum–palladium during 100 s under argon gas. The samples were observed on 15.0 kV.
2.8.Encapsulation effciency study
An amount of the SD sample containing about 40 mg of ARM was weighed and dissolved in suffcient methanol to produce 100 ml.The resulting solution was fltered using a sintered glass crucible,discarding the frst 10 ml.2 ml of the resulting solutions was pipetted into a quick-ft test tube and 2 ml of concentrated HCl was added.The test tubes were stoppered and allowed to stand in a water bath at room temperature for 25 minutes.The resulting solutions were diluted with suffcient methanol to 50 ml.The absorbance reading at a maximum of 211 nm was taken against a blank solution made up of 2 ml of HCl made up to 50 ml with methanol.The contents of ARM were calculated from the calibration curve.Encapsulation effciency was defned by the actual amounts of ARM in the prepared SDs according to Eq.(1)[35].
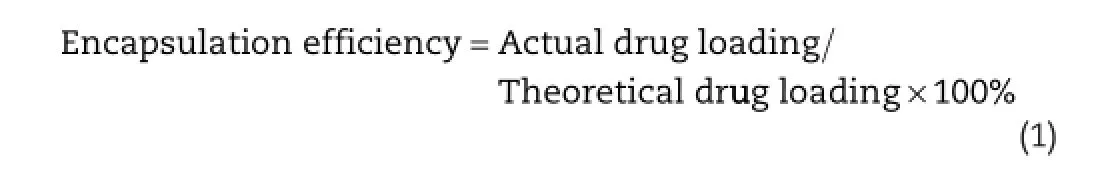
2.9.In vitro dissolution study
A dissolution study of ARM and SDs was performed using USP basket method by dissolution apparatus(Electrolab Pvt.Ltd. India)at 37±0.2°C.Samples equivalent to 40 mg of ARM SDs were flled in hard gelatin capsules and were added to dissolution media containing 1000 ml phosphate buffer of pH 7.2 with 1%SLS(sodium lauryl sulfate)at a temperature of 37±0.2°C.The solutions were stirred with a rotating basket at 100 rpm.Samples(5 ml)were withdrawn from each vessel at predetermined time intervals(10,20,30,40,50,60,and 120 min),fltered over a cellulose acetate flter of 0.45 μm.At each time point,the same volume of fresh medium maintained at the same temperature was added to dissolution media to maintain the sink conditions.The concentration ofARM in each sampled aliquot was determined using an UV-visible spectrophotometer at 211 nm and a standard calibration curve that was linear over the UV absorbance range.
2.10.In silico molecular modeling studies
All modeling studies were carried out using Schrödinger’s Maestro software[37]installed on Fujitsu Celsius workstations.Structures of ARM,Soluplus,Kollidon VA 64,HPMC and Eudragit EPO were built using 2D sketcher utility based on literature reports and further prepared using Ligprep[38].Docking analysis was carried out using fexible docking option in Glide module[39].The,ARM–HPMC,ARM–Eudragit EPO and ARM–Soluplus complexes were generated by docking.The ARM–Kollidon VA 64 complex was generated and subjected to Macromodel minimization[40].
The lowest energy complexes obtained from docking were subjected to system builder panel and further to the default protocol and fnally to 10 ns MD simulations in vacuum using Desmond[41].The lowest energy complexes obtained from these MD simulation were used to analyze the interaction of ARM with the different polymers.The simulations were performed using NVT ensemble with OPLS_2005 force feld[42].
The Nose–Hoover thermostat[43]was used to maintain temperature at 300 K.periodic boundary conditions were applied throughout and equations of motion were integrated using the multistep RESPA integrator[44]with inner time step of 2.0 fs for bounded interactions and non-bonded interactions within the short range cutoff.An outer time step of 6.0 fs was used for non-bonded interactions beyond the cutoff.The outputs of the simulation were collected every 10 ps over the trajectory during the production runs and analyzed using the different tools in Desmond.
3.Results and discussion
3.1.Drug solubility study
The infuence of different hydrophilic polymers on apparent solubility of the ARM was calculated.As presented in Table 2, it can be seen that the solubility study of SDs made by spray drying showed an increase in the drug solubility with an increase in the ratio of hydrophilic polymers.The solubility of pure ARM in distilled water containing 1%SLS was found to be 0.0180 μg/mL,indicating its poor solubility.Table 2 shows the solubility data of the spray dried SDs of ARM with different polymers marked a difference compared to neat ARM.
A linear relationship was observed with respect to increase in solubility of ARM to increasing ratio of Soluplus, Kollidon VA 64,HPMC and Eudragit EPO.The spray drying process causes the decrease in particle size of SDs.According to the Noyes–Whitney equation[1,45],the dissolution rate and saturation solubility of drugs can be increased by reducing the particle size,causing an increase in particle surface area[46]. This implies the solubilizing properties of hydrophilic polymersfor ARM using spray-drying technology.1:1,1:2 and 1:3 ratios of ARM with Soluplus,KollidonVA 64,HPMC,and Eudragit EPO enhanced the solubility of ARM in water up to an extent 80.22, 73.29,67.39 and 72.24 μg/ml,respectively.
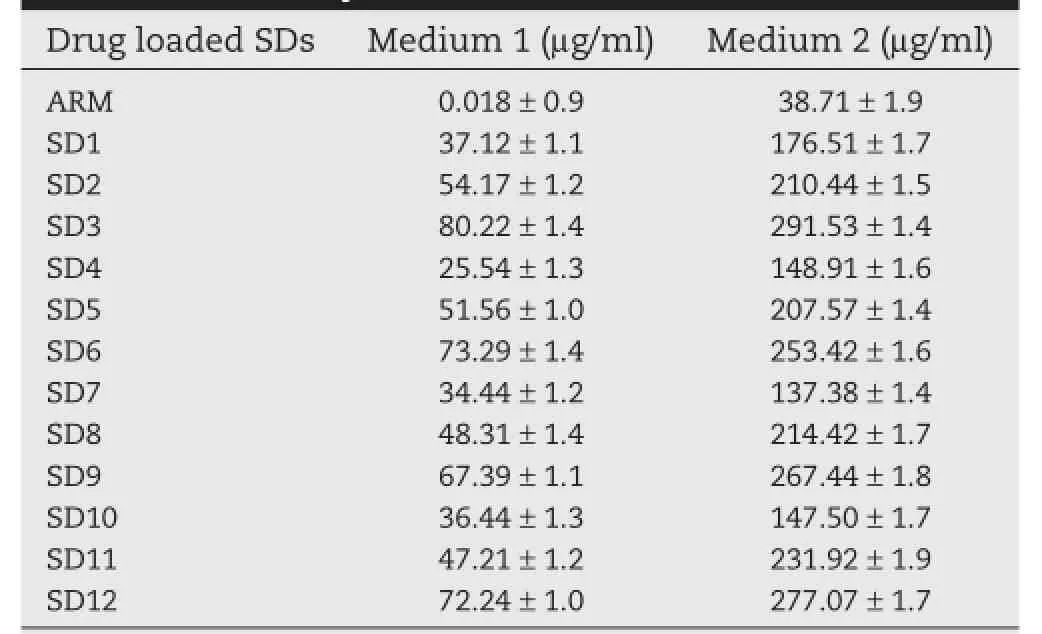
Table 2–Solubility of ARM and SDs.
The solubility of pure ARM and SDs was higher in medium 2 as compared to medium 1.The hydrophilic polymers like Soluplus by hot melt extrusion technology[5]and using polyvinylpyrrolidone[46]have been reported to increase the solubility of ARM.The increase in solubility in both dissolution media could probably be elucidated by hydrophilic environment created by the carrier around the drug resulting in a decrease in particle size of SDs and increased wettability of ARM.The increase in solubility could also be because of a decrease in the interfacial tension between the drug and the dissolution medium.
3.2.Fourier transform infrared spectroscopy(FTIR)
The FTIR spectra of neat ARM,Soluplus,SD3 and Kollidon VA 64 and SD6 are shown in Fig.2a,and ARM,HPMC,SD9,Eudragit EPO and SD12 are shown in Fig.2b.The FTIR spectra of ARM shows different peaks in fngerprint region like,peak at 1250 cm−1for C—O,peak at 1450 cm−1is found for C—C bending, a peak at 1138 cm−1can be assigned to C—H stretching vibration in C—O—C component,respectively representing 1,2,4-trioxane ring.The functional group region peak at 3129 cm−1is observed for C—H stretching in-CH3.
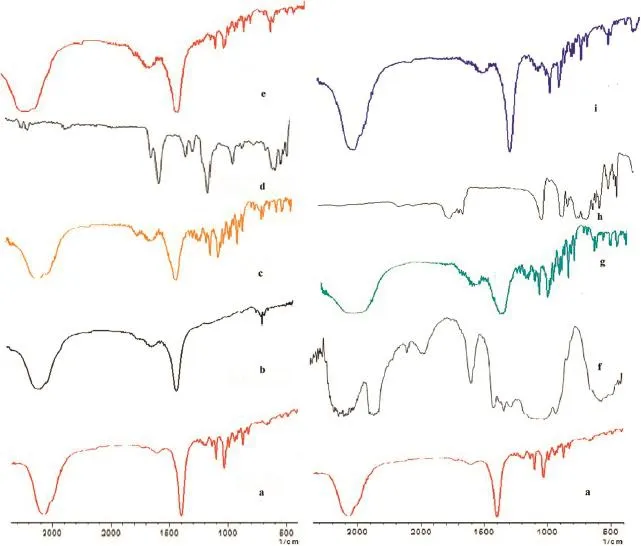
Fig.2–FTIR spectra’s of ARM(a),Soluplus(b),SD 3(c),Kollidon VA64(d),SD 6(e),HPMC(f),SD9(g),Eudragit EPO(h)and SD 12(i).
In case of Soluplus and Kollidon VA 64,HPMC and Eugragit EPO all these hydrophilic polymers showed that a peak at 1450–1480 cm−1is found for C—C binding.It also showed a broad band at 3000–3500 cm−1,which was attributed to the presence of more numbers of-OH stretching groups.
Soluplus,KollidonVA64 and HPMC showed a very broad band at 3430 cm−1which was attributed to the presence of water as both of these polymers are hygroscopic in nature and absorbs moisture from the environment.All the polymers have a hydrophilic surface with lots of hydrophilic groups,resulting into diffusion of dissolution medium and accelerated release ofARM [47].
The shifting of O—H and C—H stretching vibrations in FTIR spectra revealed red and blue shifting of the carbonyl group in the fngerprint region,indicating an interaction betweenARM and the respective hydrophilic polymers.The extent of interaction varied depending on the nature and molecular weight of the carriers and on the drug–polymer ratio.An increase in the ratio of polymer to the ARM indicated that high polymer binding to the drug improves the pharmaceutical effciency of SDs.
3.3.Differential scanning calorimetry(DSC)studies
The DSC thermograms of neat ARM,Soluplus,Kollidon VA 64, HPMC and Eudragit EPO and spray dried SDs are shown in Fig.3. Pure ARM started melting at 84.86°C with a sharp endothermic peak at 86.64°C and an exothermic peak at 172°C with enthalpy change of 56.68 J/g,whereas all pure hydrophilic polymers did not show a melting endotherm because of its amorphous nature.SDs of ARM showed a slight decrease in ΔH and peak height,in accordance with XRD patterns.The SD3, SD9 and SD12 showed a very small endothermic peak at a lower temperature compared to the neat ARM,indicating some crystallinity;the results are in resemblance with the XRD data,as SD6 has not shown any peak in DSC thermogram as well as XRD diffractogram.
The heat of fusion of the pure ARM was higher than that of spray dried SDs.The heat of fusion of SDs depended on the ratio of polymer to pure ARM and spray drying conditions such as inlet temperature and feed rate.The heat of fusion decreased with the increase in polymer ratio.The heat of fusion is proportional to the amount of crystallinity of the samples. These results suggest that the crystalline nature of ARM was diminished when they were processed by spray drying technique-forming SDs.Therefore,it seems that amorphous systems can be formed using spray drying technology.
3.4.X-ray diffraction studies
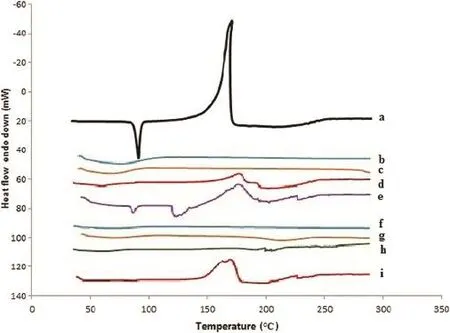
Fig.3–DSC thermograms of ARM(a),Soluplus(b),Kollidon VA 64(c),SD 3(d),SD 6(e),HPMC(f),Eudragit EPO(g),SD 9(h)SD 12(i).
The representative X-ray diffarctograms of ARM and optimized SDs are shown in Fig.4,which illustrates the changes in drug crystal structure.X-ray diffraction studies were performed to elucidate the physical state of the pure ARM in the SDs.The X-ray patterns of ARM presented numerous distinct peaks at 2θvalues of 7.29°,10.04°,18.04°19.68°and 22.1°,indicating the crystalline nature of the drug;data resembled those in previous literature[21,25].Soluplus,KollidonVA 64,Eudragit EPO and HPMC polymers are amorphous in nature so the XRD analysis has not been carried out.The spray dried ARM particles showed a similar diffraction pattern as of pure ARM,but with lower peak intensity,inferring that the crystallinity of spray-dried SDs of SD3 and SD6 decreased during the spray drying process.The high peak intensity signal of ARM drug at 2θvalue of 10.04°was found to be absent in the XRD of SDs of ARM indicating stronger interactions with all hydrophilicpolymers.The X-ray patterns of the SDs were changed due to the polymer to drug ratios and also the spray drying conditions like feed fow rate and temperature.From the X-ray patterns of SD9 and SD12,we can conclude that the crystalline nature of ARM was still maintained due to excess drug that remained in the spray drying process,so some peaks with reduced intensity were still observed,indicating partial crystallinity.The diffractograms of SD3 and SD6 were found completely amorphous in nature absence of any peak.The new peaks observed in case of SD9 and SD 12 suggested some physical interaction between the drug and the polymer,which lead to changes in the crystal structure.The results are in resemblance with the FTIR data as well asin-silicostudies.From the XRD observations of SD9 and SD12,we can conclude that ARM was still available in its crystalline nature,but the relative drop in the diffraction intensity of ARM in SDs suggests that some crystals remained in SDs.
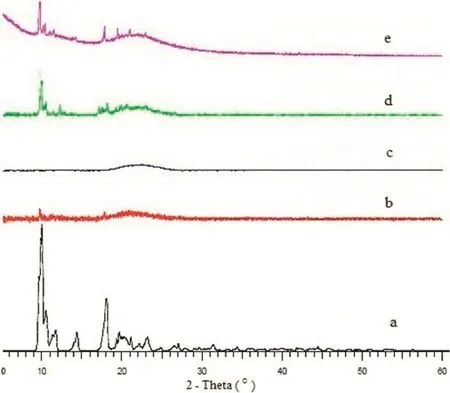
Fig.4–PXRD patterns of pure ARM(a),SD 3(b),SD 6(c),SD 9(d),SD 12(e).
3.5.Scanning electron microscopy(SEM)studies
SEM micrographs of pure ARM,Soluplus and ARM SDs are shown in Fig.5.From the SEM micrograph,it was evident that spray drying of ARM resulted in a signifcant particle size reduction of ARM.SEM micrographs of pure ARM in Fig.5a revealed large crystalline blocks,the particles were lacking uniformity in size and comparatively larger than the spray dried SDs,whereas ARM SD3,SD6,SD9 and SD12 were found to be without sharp edges and uniform particles.Extensive deposition of the ARM was observed on Soluplus in case of SD3. This may be due to the large surface area imparted by the hydrophilic nature of the polymers.The other ARM SD6,SD9 and SD12,appeared to be agglomerated and offered a smooth surface owing to the presence of hydrophilic polymers.The spray-dried particles showed more homogeneity,which was more noticeable at the higher inlet temperature of the spray drying process.The higher feed concentration and outlet temperature did not infuence the morphology of ARM during the spray drying process.
3.6.Encapsulation effciency of prepared solid dispersions
The encapsulation effciency of the prepared SDs in various ratios of polymers is shown in Table 3.Solid dispersions with the low encapsulation effciency were due to the nonencapsulated drug in the polymer,whereas solid dispersions with the high encapsulation effciency were greatly encapsulated in the polymer.The encapsulation effciency was enhanced by increasing the ratios of polymers.Additionally, it is viewed that SDs with the relatively low encapsulation effciency were because of the well non-bondingARM in polymers.

Table 3–Percentage encapsulation of SDs.
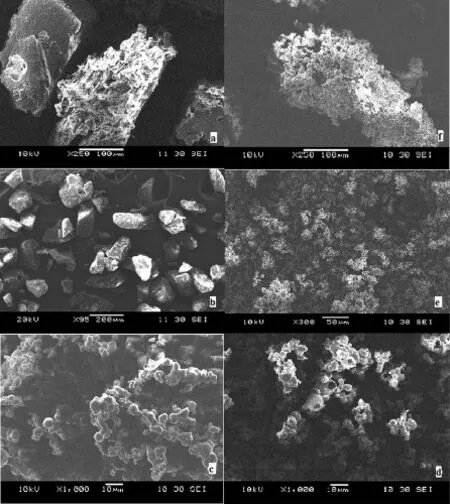
Fig.5–SEM images of pure ARM(a),Soluplus(b),SD 3(c),SD 6(d),SD 9(e),and SD 12(f).
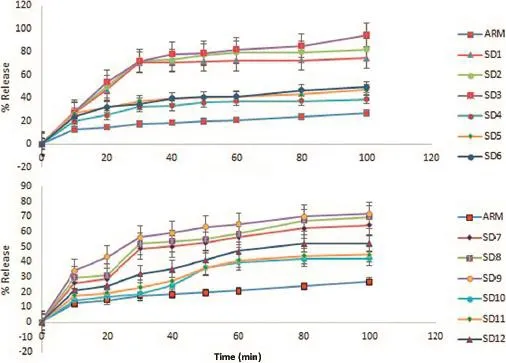
Fig.6–In vitrorelease of formulation batches from SD1 to SD12 in phosphate buffer pH 7.2 containing 1%SLS(mean±SD).
3.7.Dissolution studies of ARM SDs
For the assessment of dissolution rate improvement,dissolution studies were conducted.Thein vitrodissolution profle of pure ARM and SDs of ARM are shown in Fig.6,respectively.It is seen that more than 82%of ARM was dissolved from the SD3 of ARM:Soluplus compared to pure ARM showing only 20%drug release after 60 min.The dissolution profle of theSD formulation of ARM with Soluplus,Kollidon VA 64,HPMC and Eudragit EPO showed drug release(SD3=81.99%, SD6=40.58%,SD9=64.71%and SD12=47.32%,respectively,at the end of t60minutes)compared to 20.77%of pure ARM.The drug dissolution increased with increasing the amount of the drug carrier.However,by observing the relative standard deviation values(represented by error bars in Fig.6),it was concluded that only the spray-dried SDs showed a very rapid and uniform dissolution,which is a signifcant characteristic of the dissolution profle[29].Therefore,the results obtained in this study suggested that the complexation ofARM with polymers could be used as a formulation strategy for solubility and dissolution rate enhancement.
The increase in the dissolution rate in the case of the SD formulation is attributed to the amorphous state of the ARM that offers a lower thermodynamic barrier to dissolution and the formation dispersion where the drug is dispersed inside the hydrophilic polymers.An amorphous formulation system will dissolve at a faster rate because of its higher internal energy and superior molecular motion[48].Our spray dried ARM formulations with the increased dissolution rate could be deciphered into an enhanced bioavailability formulation upon oral administration.
3.8.In silico studies of ARM with hydrophilic polymers
Molecular modeling studies have shown that the drug forms complexes of ARM with the polymers.ARM was seen to form strong van der Waals interaction with the polymers.In case of Soluplus,good hydrophobic contacts were seen between the CH2group of oxirane ring of the ARM and CH2group of polymer and CH3of the same ring with the CH2group of polymer.O (oxygen)of the oxirane ring shows hydrogen bond(distance 1.92 Å)with the OH group of Soluplus as shown in Fig.7.
In silicostudies on HPMC,shown in Fig.8,infer that it formed good hydrophobic contacts such as interactions of the CH3group attached to the oxirane ring ofARM with the CH2group of HPMC and phenyl ring of ARM interacting with the CH2groups of the polymer.
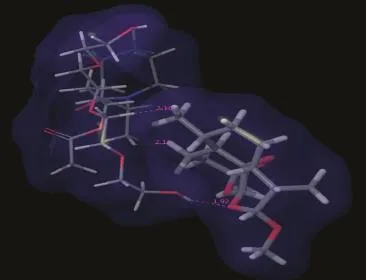
Fig.7–ARM:Soluplus SD showing formation of hydrogen bonding along with good van der Waals interactions.
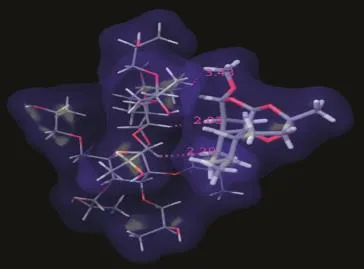
Fig.8–ARM:HPMC SD showing formation of good van der Waals contacts between drug and polymer.
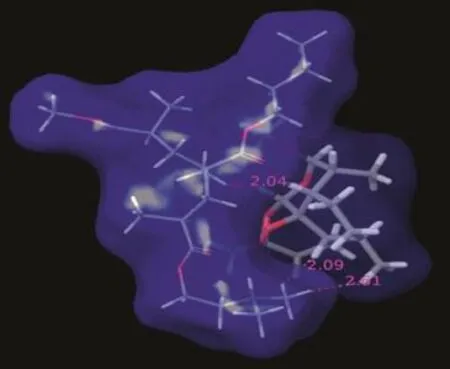
Fig.9–ARM:Eudragit EPO SD showing formation of good van der Waals contacts between drug and polymer.
Eudragit EPO showed vdW interactions between the phenyl ring and CH3group of the ARM as shown in Fig.9.
Modeling studies on Kollidon VA64 and ARM showed formation of vdW interactions between the polymer and ARM in the lowest energy pose obtained from MD simulations as shown in Fig.10.
4.Conclusion
The study demonstrated that SDs of ARM formed by spray drying technique using hydrophilic polymers like Soluplus, Kollidon VA 64,HPMC and Eudragit EPO show a signifcantly higher dissolution rate.In-silicostudies indicated that ARM primarily forms van der Waals interactions with the hydrophilic polymers,in addition to hydrogen bonding as seen in case of Soluplus.Thus,we can conclude that spray-drying techniqueproduces an amorphous spherical-shaped ARM,which interacts with the hydrophilic polymers primarily via van derWaals type of interaction,and this in combination with the effect of hydrophilic polymers increases the dissolution rate of the poorly water-soluble drug ARM,which could translate into increased bioavailability upon oral administration.This hypothesis can be explored in further studies.

Fig.10–ARM:Kollidon VA64 showing formation of good van der Waals contacts between drug and polymer.
Acknowledgment
The authors would like to thank University Grants Commission,Government of India,for their fnancial support during research work.The authors also thankful to Prof.M.S.Degani and Mr.Harish S.Kundaikar;from ICT Mumbai,for their help in In-Silico study.
R E F E R E N C E S
[1]Müller R,Jacobs C,Kayser O,et al.Nanosuspensions as particulate drug formulations in therapy:rationale for development and what we can expect for the future.Adv Drug Deliv Rev 2001;47:3–19.
[2]Yang B,Lin J,Chen Y,et al.Artemether/hydroxypropyl-βcyclodextrin host–guest system:characterization, phase-solubility and inclusion mode.Bioorg Med Chem 2009;17:6311–6317.
[3]Meshnick SR,et al.Artemisinin and its derivatives.In: Antimalarial chemotherapy.Springer;2001.p.191–201.
[4]Na-Bangchang K,Karbwang J.Current status of malaria chemotherapy and the role of pharmacology in antimalarial drug research and development.Fundam Clin Pharmacol 2009;23:387–409.
[5]Fule RA,Meer TS,Sav AR,et al.Artemether-Soluplus hot-melt extrudate solid dispersion systems for solubility and dissolution rate enhancement with amorphous state characteristics.J Pharm 2013;2013.
[6]Shah PP,Mashru RC.Development and evaluation of artemether taste masked rapid disintegrating tablets with improved dissolution using solid dispersion technique. AAPS PharmSciTech 2008;9:494–500.
[7]Janssens S,Anné M,Rombaut P,et al.Spray drying from complex solvent systems broadens the applicability of Kollicoat IR as a carrier in the formulation of solid dispersions.Eur J Pharm Sci 2009;37:241–248.
[8]Brinkmann-Trettenes U,Bauer-Brandl A.Solid phospholipid nano-particles:investigations into formulation and dissolution properties of griseofulvin.Int J Pharm 2014;467:42–47.
[9]Mohammadi G,Hemati V,Nikbakht M-R,et al.In vitro and in vivo evaluation of clarithromycin–urea solid dispersions prepared by solvent evaporation,electrospraying and freeze drying methods.Powder Technol 2014;257:168–174.
[10]Peltonen L,Hirvonen J.Pharmaceutical nanocrystals by nanomilling:critical process parameters,particle fracturing and stabilization methods.J Pharm Pharmacol 2010;62:1569–1579.
[11]Yun F,Kang A,Shan J,et al.Preparation of osthole-polymer solid dispersions by hot-melt extrusion for dissolution and bioavailability enhancement.Int J Pharm 2014;465:436–443.
[12]Zhang Y,Luo R,Chen Y,et al.Application of carrier and plasticizer to improve the dissolution and bioavailability of poorly water-soluble baicalein by hot melt extrusion.AAPS PharmSciTech 2014;15:560–568.
[13]Bouchard A,Jovanovic´N,Hofand GW,et al.Supercritical fuid drying of carbohydrates:selection of suitable excipients and process conditions.Eur J Pharm Biopharm 2008;68:781–794.
[14]Huang Z,Guo Y,Miao H,et al.Solubility of progesterone in supercritical carbon dioxide and its micronization through RESS.Powder Technol 2014;258:66–77.
[15]Suresh S,Nangia A.Lornoxicam salts:crystal structures, conformations,and solubility.Cryst Growth Des 2014;14:2945–2953.
[16]Moradiya HG,Islam MT,Halsey S,et al.Continuous cocrystallisation of carbamazepine and trans-cinnamic acid via melt extrusion processing.CrystEngComm 2014;16:3573–3583.
[17]Vyas A,Saraf S,Saraf S,et al.Cyclodextrin based novel drug delivery systems.J Incl Phenom Macrocycl Chem 2008;62:23–42.
[18]Gangurde AB,Fule RA,Pawar JN,et al.Microencapsulation using aqueous dispersion of lipid matrix by fuidized bed processing technique for stabilization of choline salt. J Pharm Invest 2015;45:209–221.
[19]Olusanmi D,Jayawickrama D,Bu D,et al.A control strategy for bioavailability enhancement by size reduction:effect of micronization conditions on the bulk,surface and blending characteristics of an active pharmaceutical ingredient. Powder Technol 2014;258:222–233.
[20]Onoue S,Terasawa N,Nakamura T,et al.Biopharmaceutical characterization of nanocrystalline solid dispersion of coenzyme Q 10 prepared with cold wet-milling system.Eur J Pharm Sci 2014;53:118–125.
[21]Mistry AK,Nagda CD,Nagda DC,et al.Formulation and in vitro evaluation of ofoxacin tablets using natural gums as binders.Sci Pharm 2014;82:441.
[22]Ansari MT,Haneef M,Murtaza G,et al.Solid dispersions of artemisinin in polyvinyl pyrrolidone and polyethylene glycol.Adv Clin Exp Med 2010;19:745–754.
[23]Ansari MT,Karim S,Ranjha NM,et al.Physicochemical characterization of artemether solid dispersions with hydrophilic carriers by freeze dried and melt methods.Arch Pharm Res 2010;33:901–910.
[24]Sahoo NG,Kakran M,Abbas A,et al.Preparation, characterization and dissolution behavior of artemisinin microparticles.Adv Powder Technol 2011;22:458–463.
[25]Irene B,Veronica A,Laura A,et al.A hyperbranched polyester as antinucleating agent for artemisinin in electrospun nanofbers.Eur Polym J 2014;60:145–152.
[26]Janssens S,Van den Mooter G.Review:physical chemistry of solid dispersions.J Pharm Pharmacol 2009;61:1571–1586.
[27]Takeuchi H,Nagira S,Yamamoto H,et al.Solid dispersion particles of amorphous indomethacin with fne porous silica particles by using spray-drying method.Int J Pharm 2005;293:155–164.
[28]Sahoo NG,Kakran M,Li L,et al.Fabrication of composite microparticles of artemisinin for dissolution enhancement. Powder Technol 2010;203:277–287.
[29]Mauludin R,Müller RH,Keck CM,et al.Development of an oral rutin nanocrystal formulation.Int J Pharm 2009;370:202–209.
[30]Caron V,Tajber L,Corrigan OI,et al.A comparison of spray drying and milling in the production of amorphous dispersions of sulfathiazole/polyvinylpyrrolidone and sulfadimidine/polyvinylpyrrolidone.Mol Pharm 2011;8:532–542.
[31]Torres FG,Boccaccini AR,Troncoso OP,et al.Microwave processing of starch-based porous structures for tissue engineering scaffolds.J Appl Polym Sci 2007;103:1332–1339.
[32]Monti S,Cappelli C,Bronco S,et al.Towards the design of highly selective recognition sites into molecular imprinting polymers:a computational approach.Biosens Bioelectron 2006;22:153–163.
[33]Machácˇková M,Tokarský J,Cˇapková P,et al.A simple molecular modeling method for the characterization of polymeric drug carriers.Eur J Pharm Sci 2013;48:316–322.
[34]Fule R,Amin P.Development and evaluation of lafutidine solid dispersion via hot melt extrusion:investigating drugpolymer miscibility with advanced characterisation.Asian J Pharm Sci 2013;doi:10.1016/j.ajps.2013.12.004.
[35]Cilurzo F,Selmin F,Vistoli G,et al.Binary polymeric blends to microencapsulate nitrofurbiprofen:physicochemical and in silico studies.Eur J Pharm Sci 2007;31:202–210.
[36]Balducci AG,Magosso E,Colombo G,et al.Agglomerated oral dosage forms of artemisinin/β-cyclodextrin spray-dried primary microparticles showing increased dissolution rate and bioavailability.AAPS PharmSciTech 2013;14:911–918.
[37]Maestro version 9.4,Schrödinger,LLC,New York,NY;2013.
[38]LigPrep version 2.6,Schrödinger,LLC,New York,NY;2013.
[39]Glide version 5.9,Schrödinger,LLC,New York,NY;2013.
[40]MacroModel 10.0,Schrödinger,LLC,New York,NY;2013.
[41]Desmond Molecular Dynamics System 3.4,D.E.Shaw Research,New York,NY,2013.Maestro-Desmond Interoperability Tools 3.4,Schrödinger,New York,NY;2013.
[42]Banks JL,Beard HS,Cao Y,et al.J Comput Chem 2005;26:1752.
[43]Hoover WG.Phys Rev A 1985;31:1695.
[44]Humphreys DD,Friesner RA,Berne BJ.J Phys Chem 1994;98:6885.
[45]Dokoumetzidis A,Macheras P.A century of dissolution research:from Noyes and Whitney to the biopharmaceutics classifcation system.Int J Pharm 2006;321:1–11.
[46]Sahoo NG,Abbas A,Li CM,et al.Micro/nanoparticle design and fabrication for pharmaceutical drug preparation and delivery applications.Curr Drug Ther 2008;3:78–97.
[47]Zhang Y,Wang J,Bai X,et al.Mesoporous silica nanoparticles for increasing the oral bioavailability and permeation of poorly water soluble drugs.Mol Pharm 2012;9:505–513.
[48]Subramaniam B,Rajewski RA,Snavely K,et al. Pharmaceutical processing with supercritical carbon dioxide.J Pharm Sci 1997;86:885–890.
Abbreviations:ARM,artemether;SDs,solid dispersions.
*< class="emphasis_italic">Corresponding author.
.Department of Pharmaceutical Sciences and Technology,Institute of Chemical Technology,Mumbai,India.Tel.: +91 33612211;fax:+91 33611020.
E-mail address:jaywantpawar.ict@gmail.com(J.N.Pawar).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.08.012
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- PEGylation in anti-cancer therapy:An overview
- Near-infrared light-responsive inorganic nanomaterials for photothermal therapy
- Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole
- Povacoat affecting solid-state polymorphic changes of indomethacin after co-evaporation from different types of solvents via conventional and microwave drying techniques
- Preparation and evaluation of PEGylated phospholipid membrane coated layered double hydroxide nanoparticles
- Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study
