Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole
2017-01-19KanokpornBurapapadhHirofumiTakeuhiPornsakSriamornsak
Kanokporn Burapapadh,Hirofumi Takeuhi, Pornsak Sriamornsak*
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
bPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
cLaboratory of Pharmaceutical Engineering,Gifu Pharmaceutical University,1-25-4 Daigaku-Nishi, Gifu 501-1196,Japan
Original Research Paper
Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole
Kanokporn Burapapadha,b,1,Hirofumi Takeuchic, Pornsak Sriamornsaka,b,*
aDepartment of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
bPharmaceutical Biopolymer Group(PBiG),Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand
cLaboratory of Pharmaceutical Engineering,Gifu Pharmaceutical University,1-25-4 Daigaku-Nishi, Gifu 501-1196,Japan
A R T I C L EI N F O
Article history:
Received 10 April 2015
Received in revised form 2 July 2015 Accepted 9 July 2015
Available online 24 August 2015
Pectin
Poorly water-soluble drug
Itraconazole
Nanoparticles
Stability
A simple method to fabricate itraconazole(ITZ)-loaded pectin nanoparticles prepared from nanoemulsion templates is described in this study.Nanoemulsions containing ITZ were prepared by a mechanical homogenization using pectin as emulsifer.After freeze-drying,the morphology,crystallinity state,thermal properties,drug dissolution and stability of the obtained pectin nanoparticles were characterized.The results demonstrated that the morphology of freeze-dried products was different,depending on the type of internal phase;the nanoparticles prepared from chloroform-based nanoemulsions were completely dried and provided a fragile characteristic.The pectin nanoparticles also demonstrated good properties in terms of redispersibility,thermal properties,drug crystallinity and dissolution.The ITZ-loaded pectin nanoparticles showed high percentage of drug dissolved(about 60–80% within 2 h),and maintained their good dissolution properties even after 1-year storage.The results suggested that freeze-dried pectin nanoparticles prepared from nanoemulsions could be used as an effective carrier for enhancement of ITZ dissolution.
©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Pectin is a natural polysaccharide structurally composed by large amounts of poly-D-galacturonic acid bonded via α-1,4-glycosidic linkage.According to its degree of methyl esterifcation,pectin can be classifed as high methoxyl(HM)pectin or low methoxyl (LM)pectin,which yields some differences in its properties[1,2]. Pectin has commonly been used as a gelling agent,a thickening agent and a colloidal stabilizer in the food industry[3]. Its applications in the pharmaceutical industry are increased in the last decade(e.g.[4–6]).The presence of surface-active molecules in pectin offers the emulsifcation properties[7].In addition,pectin is known to be biocompatible and to exhibit very low toxicity,which are mandatory prerequisites for drug delivery application.In the previous study[8],the nanoemulsions containing a poorly water-soluble drug were prepared by using pectin as a polymeric emulsifer.The infuences of type of internal phase,type and concentration of pectin on the droplet size,morphology,and zeta potential of the pectinbased emulsions have been examined.Pectin with high DE offers good emulsion properties because of its high amount of hydrophobic molecules.
Itraconazole(ITZ)is a poorly water-soluble antifungal drug having a broad spectrum of activity against a variety of pathogens causing opportunistic infection in HIV-infected patients [9].ITZ is a weak basic drug(pKa=3.7)which is virtually ionized at low pH,having extremely low water solubility(about 1 ng/mL at neutral pH and about 4–6 μg/mL at pH 1)[10].Over the last decade,a large number of publications dealt with drug delivery strategies for poorly water-soluble and lipophilic drugs, including solid dispersions and nanoparticulate based formulations[11].However,the major disadvantages of solid dispersion are related to their instability(e.g.,physical instability/re-crystallization risks of solid dispersion with the potential of deteriorating drug release and bioavailability).Thus, polymeric nanoparticles have been extensively studied as particulate carriers in the pharmaceutical felds because they show promise as drug delivery systems as a result of their subcellular size and enhanced intestinal absorption of poorly watersoluble drugs[11].In addition,nanoparticles are described to permeate epithelia more easily than microparticles and provide a controlled release of the encapsulated drugs.
In general,high-pressure homogenization(e.g.,microfuidizer)has been used for preparation of nanoparticles according to its advantages of supplying the available energy in a short time and having homogenous fow,which is suitable for the preparation of nanoemulsions[12].The disadvantage of this method,however,is that about 50–100 passages through the microfuidizer are generally required to result in nanosized particles[13,14].Alternatively,other types of homogenizers such as mechanical or ultrasonic homogenizers could be used to reduce processing time and increase batch size[13].Rotor–stator generator type homogenizer is one type of mechanical homogenizer,which is the most economical and the easiest to operate as well as maintain.This homogenizer consists of a rotor that turns within a stationary stator.As the blades rotate, materials are continuously drawn into one end of the mixing head and expelled at high velocity through the openings of the stator.The differential speed and close tolerance between the rotor and stator generate high levels of hydraulic shear,promoting fast mixing and producing small droplets in emulsions [13,15].
The aim of this study was to develop pectin nanoparticles containing a poorly water-soluble drug,ITZ,from nanoemulsion templates.A rotor–stator generator type of homogenizer was used to prepare nanoemulsion templates to avoid highpressure conditions.The effects of homogenization speed,type of internal(oil)phase,type of pectin on the properties of the nanoemulsion templates were also examined.The obtained nanoemulsions were then freeze-dried in order to get the solid nanoparticles.The dried nanoparticles were characterized to investigate the drug properties,including morphology,crystallinity state,thermal properties and dissolution.The stability of nanoparticles in various conditions was also studied.
2.Materials and methods
2.1.Materials
The pectins used in this study were a gift from Herbstreith& Fox KG(Germany),namely,LM pectin(referred to as LMP)with a degree of esterifcation of 38,amidated LM pectin(referred to as ALMP)with a degree of esterifcation of 29 and degree of amidation of 20,and HM pectin(referred to as HMP)with degree of esterifcation of 70.The molecular weight of LMP,ALMP and HMP was 70,150 and 200 kDa,respectively.Caprylic/ capric triglyceride(Miglyol®812)was a gift from Sasol GmbH (Germany)and referred to as CCT.ITZ was from Nosch Labs Private(India).Chloroform was supplied by Carl Roth GmbH (Germany).Deionized water was used as aqueous phase in all preparations.The simulated gastric fuid(SGF)used in this study was prepared based on USP guideline.Briefy,7 mL of hydrochloric acid and 2 g of sodium chloride were dissolved in distilled water before adjusting the solution volume to 1 L;the pH of SGF was adjusted to 1.20±0.05.All other chemicals used in this study were of pharmaceutical grade and used as received without further purifcation.
2.2.Preparation of pectin nonaparticles containing ITZ from nanoemulsion templates by mechanical homogenizer
Oil-in-water or chloroform-in-water emulsions were prepared by using mechanical homogenization.ITZ was dissolved in oil phase(either CCT or chloroform)at different concentrations depending on its solubility(i.e.,0.003%(w/w)in CCT or 3%(w/w)in chloroform)[8].Twenty grams of CCT or chloroform were mixed with pectin solution(80 g)using a rotor–stator type of mechanical homogenizer(Ultra-Turrax®T50 Basic, IKA,Germany).The pectin(HMP)concentration investigated was 0.5,1,1,2 and 3%(w/w).The effect of homogenizing speed on droplet size of emulsions using 3%(w/w)HMP was also determined at varying speeds of 8000,9500,13,500,20,500,and 24,000 rpm.The homogenization was operated for 20 min,in an ice-bath at the controlled temperature of−10°C to avoid overheating.
Consequently,the suitable conditions were chosen for further experiments.Three types of pectin,i.e.,LMP,ALMP and HMP,were used in this study.The obtained nanoemulsions were then dried by a freeze-dryer(Freezone 2.5,Labconco,USA)to prepare the dried particles;the nanoemulsions were pre-frozen by dipping in liquid nitrogen before placing in the freeze-dryer in which solvent was evaporated under 0.29 mbar and−49°C. The dried products were cut into small pieces before further characterization.
2.3.Zeta potential measurement
The zeta potential of the nanoemulsions was measured by a zeta potential analyzer(ZetaPlus,Brookhaven,USA). Nanoemulsions were dispersed in deionized water at a ratio of 1:50(v/v)and the electric feld applied was 1 V.The average and standard deviation of the measurement of three batches of nanoemulsions were reported.
2.4.Morphology examination
The morphology of all nanoemulsions was investigated by a light biological microscope(Motic BA 300,Motic China Group, P.R.China).The nanoemulsions were dropped on a glass slide and a cover slit,then the photos of nanoemulsion droplets were taken and investigated by the Motic Image Plus 2.0 program.
After freeze-drying,the dried samples were also investigated by a scanning electron microscope(Maxim-2000,CamScan Analytical,England).Nanoparticle samples were fxed on SEM stubs with double-sided adhesive tape and then coated in a vacuum with thin gold layer before investigation.
2.6.Redispersibility test
The dried particles were cut into small pieces and then dispersed in a proper amount of distilled water and SGF for 1 h using a magnetic stirrer(C-MAG HS 7,Ikamag,Germany)at a speed of 100 rpm.The median particle size of redispersed particles was determined by a static light scattering method(Laser scattering particle size distribution analyzer LA-950,Horiba, Japan)under continuous stirring and obtained from the measurements of at least three batches.
2.7.Loading capacity and loading effciency of nanoparticles
The loading capacity of the nanoparticles was determined prior to dissolution test.The commercial product of ITZ(Sporal®,lot number B943004,Olic Ltd.,Thailand)and nanoparticles were dispersed in methanol and sonicated for 120 min and then stirred for 3 h to ensure that the pectin wall was broken and the entire ITZ dissolved in the solvent.The methanol solution was then passed through 0.22-μm membrane.The drug amount was investigated by a high performance liquid chromatography,HPLC(Agilent,USA),using Alltima C18 column (5 μm,25 cm×4.6 mm)(Alltech,Italy).The mobile phase consisting of acetonitrile:water(37:63,v/v)was adjusted to pH 2.45 with phosphoric acid,fltered through a membrane flter (0.22 μm),and degassed in sonicator before use.The fow rate was 1.0 mL/min,and the UV detection wavelength was 263 nm. The loading capacity and loading effciency of the nanoparticles were calculated by Equations(1)and(2),respectively.
2.5.Physicochemical characterization
The thermal properties of dried nanoparticles were observed by a Sapphire DSC(Perkin Elmer,Germany).The physical mixtures prepared by mixing ITZ with various pectins at the ITZ to pectin ratio of 1:6,corresponding to the ratio of ITZ to pectin in nanoparticles,were also observed and compared.An accurate amount(2–3 mg)of samples was placed inside standard crimped aluminum pan and heated from 25 to 250°C at a heating rate of 10°C/min under 30 mL/min nitrogen fow.
Powder X-ray diffractometry(PXRD)was used to investigate the crystalline state of ITZ.PXRD patterns of ITZ in nanoparticles and physical mixtures were obtained using the X-ray diffractometer(D8,Bruker,Germany)at 40 kV,40 mA over the range of 5°–45°2θusing a Cu Kα radiation wavelength of 1.5406 Å.
The Fourier transform infrared spectroscopy(FTIR)spectra of all samples were obtained by a Nicolet 4700 FTIR spectrophotometer(Thermo Electron Corporation,USA).ITZ,pectin, physical mixture of ITZ and pectin,and nanoparticle samples were prepared using the KBr disk method.Each sample was blended with KBr powder and compressed to a disk with pressure of 5 tons before placing in the sample holder.The spectral values of the samples were obtained by scanning from 4000 to 400 cm−1at a resolution of 4 cm−1.FTIR spectral parameters of the samples were obtained using a software package (OMNIC FT-IR Software,version 7.2a,Thermo Electron Corporation,USA).
2.8.Dissolution test
Dissolution studies of ITZ were performed in triplicate at 37±0.5°C employing USP apparatus I(basket,100 mesh)with a speed of 100 rpm(DT70,Erweka,Germany).The nanoparticles, which contained an equivalent amount of 3.0±0.1 mg of ITZ, were weighed and transferred to dissolution vessels containing 1000 mL of SGF.Samples were withdrawn from the dissolution vessels at 5,10,20,30,60,90,and 120 min and passed through 0.45-μm cellulose membrane.Then,the analysis for ITZ content was done by HPLC assay,as described above.
2.9.Stability of dried nanoparticles
The selected formulations of pectin nanoparticles were kept at ambient conditions(25°C)for 12 months before characterization by PXRD,redispersion and dissolution tests.The dissolution profles of the nanoparticles kept at the ambient conditions for 12 months were also compared with those of freshly prepared nanoparticles by the model-independent method,dissolution effciency(DE).The DE was computed bya curve ftting software,KinetDS,which is an open source software and available at http://sourceforge.net/projects/kinetds/, using the following equation.

whereyis the percentage of drug dissolved at timet[16].DE results were subjected to Student’s t-test employing Microsoft Excel 2010(Microsoft,USA).Differences between means were considered statistically signifcant atP<0.05.
3.Results and discussion
3.1.Preparation of nanoemulsion templates
In this study,nanoemulsions were formed by mechanical homogenization using Ultra-Turrax(rotor–stator type)homogenizer.In general,the size of emulsion droplet formed by homogenization is controlled by the interplay between droplet breakup and droplet coalescence[15,17].Droplet breakup is controlled by the type and amount of shear applied to the droplets as well as the droplets’resistance to deformation,which is determined by the surfactant.The rate of droplet coalescence is determined by the ability of the surfactant to adsorb on the surface of newly formed droplets;this is governed by the surfactant concentration and the surface activity[15].
In preliminary studies,the effect of homogenization speed on the droplet size was clearly observed.The emulsion droplet size decreased when the homogenization speed was increased from 8000 to 24,000 rpm(data not shown).Although the report of Maa and Hsu[18]suggested the optimum homogenization time of 5 min,in this study,the homogenization time was fxed at 20 min to ensure the homogeneous dispersion of internal droplet in the high-viscosity pectin solution. In CCT-based formulations,the diameter of emulsion droplets decreased from 15–22 μm to about 8 μm for LMP and ALMP, and 2 μm for HMP when the concentration of pectin was increased from 0.5 to 3%(w/w).For the formulations that used chloroform as an internal phase,3%(w/w)pectin yielded nanosized emulsions with the droplet size of about 500–600 nm. These results indicated that the size of emulsion droplets decreased with the increased concentration of the pectin solution. The relationship between emulsion droplet size and pectin concentration can be explained in terms of steric effect or surface coverage of the polymeric surfactant[8].As a hydrocolloid,it contains hydrophobic groups(e.g.,methyl ester groups and amide groups)that are numerous enough and suffciently accessible on a short timescale to enable the adsorbing molecules to adhere to and spread out at the interface,thereby protecting the newly formed droplets[7].However,the limited emulsifying capacity of pectin may result from the insuffcient amphiphilic character of pectin to produce substantial and rapid lowering of the interfacial tension during droplet breakup.
Kravtchenko et al.[19]reported that pectin can stabilize casein micelles above a concentration called‘the critical pectin level’by interacting with the protein,thus preventing their aggregation as a result of steric repulsion forces which prevent the micelles from approaching each other.Kalsta et al.[20]also observed that HMP can stabilize emulsions containing polysorbate 20/whey protein isolate against creaming and coalescence,and this was attributed to the formation of a pectin layer on the polysorbate 20/whey protein isolate containing oil droplet as a result of interactions with absorbed whey protein molecules through electrostatic forces.Pectin may stabilize the emulsion system against phase separation by increasing the viscosity of the aqueous phase and,therefore,retard droplet or particle movement.This stabilizes the system as long as its value remains high enough to counteract the forces responsible for phase separation[21].In this case,pectin adsorption leading to steric stabilization of oil droplets can take place.
Therefore,from the results mentioned above,the homogenization speed of 24,000 rpm and the pectin concentration of 3%(w/w)were chosen for further investigation.
3.2.Morphology of nanoparticles obtained from freeze-drying of nanoemulsions
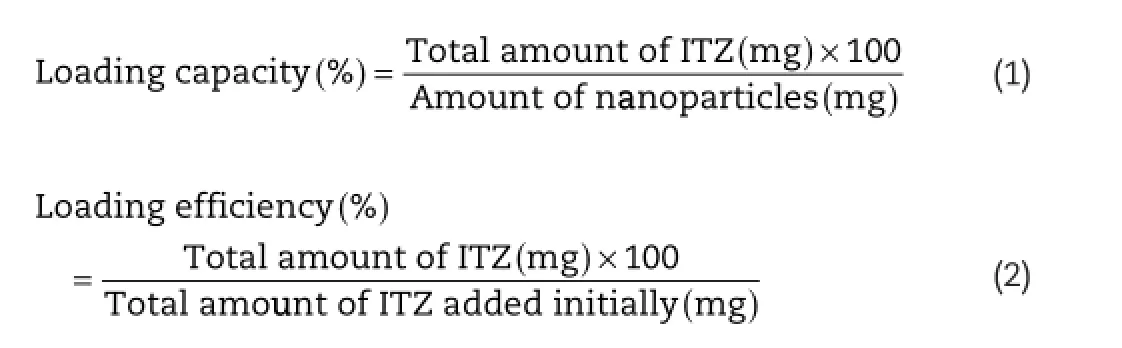
The nanoemulsions were dried by freeze-drying process.The products recovered had a cake-like structure,a typical characteristic of polymeric freeze-dried products[22].The freezedried products were different,depending on the type of internal phase.In the case of chloroform-based nanoemulsions,the freeze-dried products were completely dried and had a fragile characteristic.The products from CCT-based emulsions were oily and dense because high amount of oil remained in the formulations.The characteristics of dried products were investigated to adjust the most suitable formulation for oral administration.
Freeze-dried products from both CCT-based and chloroformbased emulsions contained no observable pore,indicating no large ice crystal formed in the freeze-drying process[23],although about 75%(w/w)of water was removed from the formulations.The cakes were sliced to thin layer and observed by a light microscope(Fig.1).It was clearly seen that CCT-based particles contained large oil droplets in polydispersed cluster for all types of pectin,corresponding to the emulsion droplet size before drying which were about 5–15 μm.The oily particles with low drug loading of CCT-based particles limit their use as a high amount of product is required to maintain therapeutic concentration.Therefore,CCT-based particles were excluded from this study.The nanoparticles prepared from chloroform-based nanoemulsions containing ITZ showed the fne particles embedded in the pectin flm.In the case of nanoemulsions using ALMP,after freeze-drying,the particles seem to be larger than those using HMP and LMP.This might be due to the instability of the emulsions during freezedrying process.The results suggested that using HMP or LMP resulted in more stable emulsions.The polymer layer formed at the nanoparticle surface may help to stabilize the nanoparticles and improve their freezing resistance.
The morphology of dried emulsion powders was investigated by SEM as also shown in Fig.1.The freeze-dried products revealed a porous,sponge-like structure with round-shaped, submicron-sized globules spreading over pectin flm.No ITZ crystal was seen in all formulations.These results confrm the morphology of dried powders and also the stability of the emulsions through freeze-drying.The size of nanoemulsions in allformulations was similar,i.e.,around 500–600 nm before drying. However,after freeze-drying process,particle size was increased due to coalescence that can occur during freezedrying[24].The ALMP-based dried powders showed the largest droplet size,while LMP and HMP formulations gave quite tiny particles with a particle size of about 1 μm.The SEM images of CCT-based particles could not be taken due to the presence of oil in the chamber.
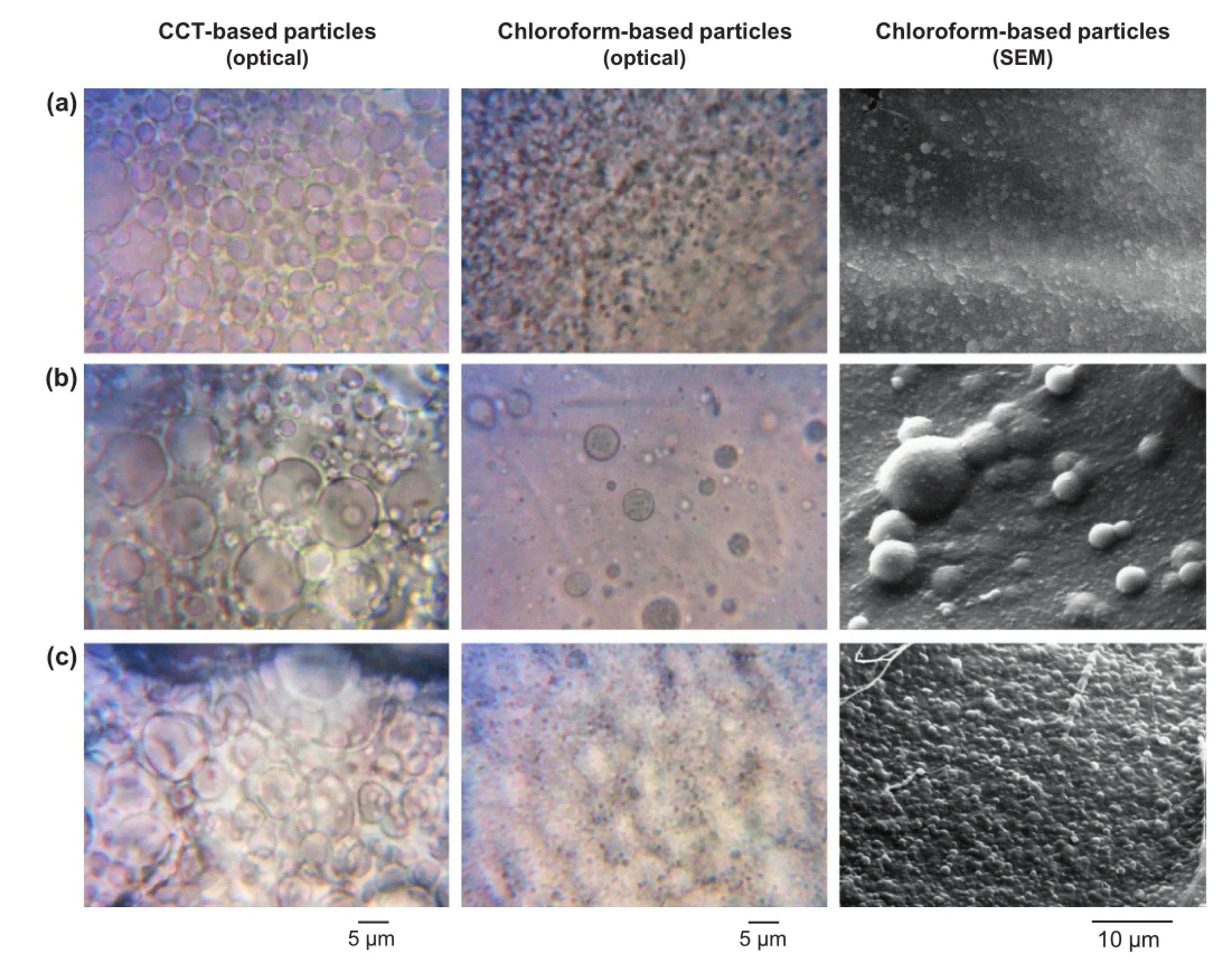
Fig.1–Optical images(left column and middle column)and SEM images(right column)of particles prepared from CCT-based and chloroform-based nanoemulsions using(a)HMP,(b)ALMP and(c)LMP.
3.3.Thermal properties and crystalline state of ITZ in nanoparticles
Fig.2a shows DSC thermograms of physical mixtures and nanoparticles containing ITZ.The melting peak of ITZ crystals can be observed around 166–168°C[14].The thermal properties of physical mixtures of ITZ and various types of pectin at a ratio of 1:6 were compared to those of the nanoparticles.All physical mixtures investigated showed a melting endothermic peak associated with the occurrence of crystalline ITZ.In the case of nanoparticles,no melting peak of ITZ was found,indicating that the drug is molecularly dispersed in the polymer.
Freeze-dried ITZ using chloroform as the crystallizing solvent caused the glassy form of ITZ(data not shown),which showed an endothermic peak at approximately 85–90°C.This peak is due to the transition of the chiral nematic mesophase to the isotropic liquid phase,typical for glassy ITZ[25].In this case, the protective effect of the polymer is clear since no exothermic recrystallization peak was detected,indicating that the glassy fraction remained in its metastable state[26].
The drug crystallinity in all formulations was confrmed by PXRD method,as shown in Fig.2b.Pectin exhibited a halopattern[27],indicating that the drug was molecularly dispersed in polymer,while ITZ crystals showed very high crystallinity. Untreated ITZ presented sharp crystalline peaks,with the major peaks at 17.45 and 17.95(doublet),20.30,and 23.45 2θdegree. The PXRD patterns of physical mixtures showed the peak at the same position as untreated ITZ,indicating no change in drug crystallinity during the mixing process.The PXRD patterns of nanoparticles showed a broad typical hump ofamorphous material with an absence of the characteristic crystalline ITZ peaks,indicating that ITZ was changed to a noncrystalline form by the preparation process.This is also supported by DSC analysis in which no melting endotherm was observed.

Fig.2–(a)DSC thermograms of physical mixture of ITZ and various types of pectin and nanoparticles,and(b)powder X-ray diffraction patterns of ITZ,physical mixture of ITZ and various types of pectin and nanoparticles prepared from nanoemulsion templates.
3.4.FTIR spectrum of nanoparticles
The FTIR spectrum of untreated ITZ is shown in Fig.3a.The spectrum showed the characteristic peaks of ITZ which appeared at 3127.1,3068.5,2936.0,2823.6,1698.4,1510.8 and 1451.3 cm−1.The absorption bands between 2800 and 3200 cm−1corresponded to the alkane,aromatic CH and amine groups [28].The absorption of the NH2groups were found at 3442.0, 3127.1,3068.5 cm−1.The frst band was assigned to be due to stretching vibration of free N—H in the drug molecule.The other two bands were caused by the amino group and the sharp peak occurring at 1698.4 cm−1was due to C—O of the drug.This is in agreement with the previously recorded spectrum of the pure drug[28].The IR region from 1400 to 600 cm−1,which is termed the fngerprint region,usually contains a large number of unassigned vibrations.
The spectrum of all pectins showed a broad strong area of absorption between 3600 and 2500 cm−1represented by the stretching of O—H groups of carboxylic acid.The peak at 2943 cm−1,which was outstanding for HMP,related to C—H stretching vibration.In the case of esterifed pectin,O—CH3stretching band would be expected between 2950 and 2750 cm−1due to methyl esters of galacturonic acid but,due to a large O—H stretching response,the O—CH3activity was not seen. Stronger bands occurring at approximately 1745 cm−1,and between 1640 and 1620 cm−1,indicated the ester carbonyl(C—O)groups and carboxylate ion stretching band(COO−),respectively.The absorption patterns between 1300 and 800 cm−1are referred to as the fngerprint region that is unique to pectin [29].For amidated pectin,three addition bands of amide groups were shown at 1681(N—H bending),1545(N—O asymmetric stretching)and 1413(C—N)cm−1[30].
Fig.3 also shows the FTIR spectra of the physical mixture of ITZ and pectin(HMP,LMP,and ALMP),nanoparticles and pectin alone.In the spectra of nanoparticles,there was no new absorption peak or shifting observed from the FTIR analysis, indicating minor or no interaction between ITZ and pectin molecules.The nanoparticles prepared from both HMP,LMP,and ALMP showed a similar pattern,which was a combination of ITZ and pectin spectra.However,there were some differences in the FTIR spectra of physical mixture and nanoparticles. In the spectra of nanoparticles,the absorption peak corresponding to C—O of pectin increased,while the C—O peak of ITZ decreased signifcantly,compared to the physical mixture of ITZ and pectin at the same ratio.Moreover,the fngerprint regions of the nanoparticles were similar to pectin,althoughthe physical mixture showed the ITZ-like fngerprint.These observations suggested the possibility of weak physical interactions between ITZ and pectin[31]and the amorphous or molecular level dispersion of ITZ in pectin[32].
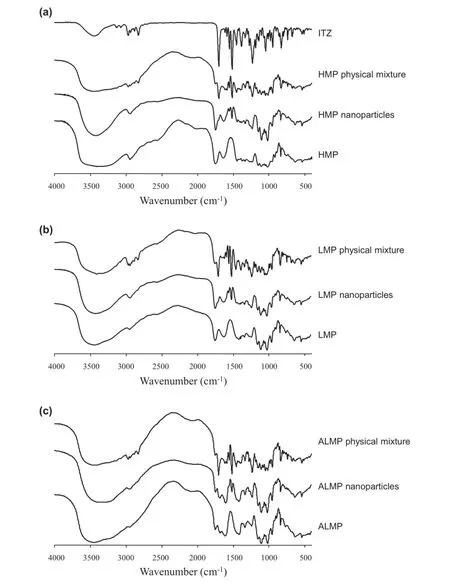
Fig.3–FTIR spectra of ITZ,physical mixture of ITZ and pectin,pectin-based nanoparticles and pure pectin;(a)HMP, (b)ALMP and(c)LMP.
3.5.Redispersibility of nanoparticles
Redispersibility of freeze-dried products is a critical characteristic for drug administration.To rehydrate(reconstitute)the freeze-dried nanoparticles,the same volume of water lost during freeze-drying was added to the nanoparticles.The particle size of nanoemulsions and nanoparticles after redispersion in water and SGF is shown in Fig.4.HMP-based nanoparticles demonstrated the excellent redispersibility within 30 min of stirring in both water and SGF.The particle size of freshly prepared nanoemulsions showed the size of 396±25 nm while that after redispersion in water and SGF was 377±17 and 372±21 nm,respectively.LMP-based nanoparticles could also be redispersed easily in water.The particle size was 256±59 nm and 268±34 nm for LMP-based nanoparticles before freezedrying and after redispersion,respectively.However,LMP-based nanoparticles could not be redispersed in SGF within 1 h and still showed the large particle size and wide size distribution(3.53±2.21 μm).ALMP-based nanoparticles showed the poorest redispersibility in both distilled water and SGF.In SGF, the nanoparticles were not completely dispersed and the cake structure still remained after 1 h of stirring.
These results suggested low redispersibility of ALMP and LMP,which corresponded to their gelation properties.In acidic condition,LMP and ALMP turn into gel clusters,which cannot be redispersed or dissolved.ALMP is LMP in which some of the carboxylic acid groups are amidated.Amidation of LMP increases its gel-forming ability due to the hydrogen bonding between amide groups[2].When the pH is decreased to below 3,the strong gels are formed for ALMP and much weaker gels are formed for LMP[33].Amidation has little infuence on the sensitivity to calcium ion,but strongly favors acid induced gelation[34].The difference in gelation of LMP and ALMP is only signifcant at low pH,where amidation enhances gelation. Therefore,redispersion of ALMP-based nanoparticles in SGF (pH 1.2)is limited.In contrast,HMP-based nanoparticles could redisperse freely in both water and SGF,indicating no gel formations during the redispersion.Basically,HMP requires high concentrations of a low molecular weight cosolute(normally sucrose)and acidifcation(typically to pH 3)to form gels by hydrogen bond and hydrophobic interaction between hydrophobic groups on the junction zones of HMP[1,2].In the case of HMP-based nanoparticles,the most hydrophobic parts of HMP may incorporate in the internal droplet[8]when hydrophilic parts covered the nanoparticle surface.The hydrophilic layer of HMP-based nanoparticles was less than that of LMP/ALMP-based nanoparticles,leading to less hydrogen bonding between the polymers on the nanoparticle surface.The acid medium without the presence of the cosolute could not induce the strong enough gel;therefore,HMP-based nanoparticles could disperse easily in acidifed medium like SGF.
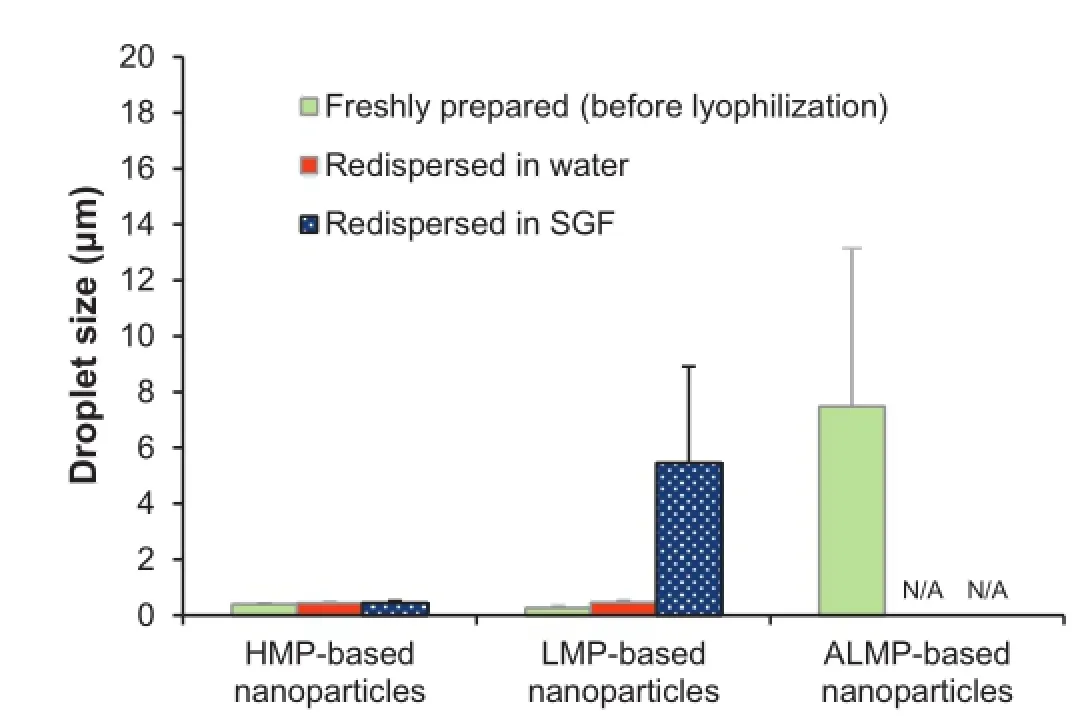
Fig.4–Droplet size of freshly prepared nanoparticles and nanoparticles after redispersion in water and SGF(pH 1.2).
3.6.Loading effciency and dissolution properties of ITZ in nanoparticles
The loading capacity and loading effciency of ITZ in each pectin-based nanoparticles are shown in Table 1.The commercial product of ITZ marketed in Thailand was used for comparison purpose.The coated pellets were taken out of capsule to be investigated for the drug amount.Practically,one capsule of commercial product contains 100 mg of ITZ,or approximately 21.17%of the pellets.In this study,the ITZ commercial product had a loading capacity of 19.67±0.10%. Pectin-based nanoparticles had a loading capacity and loading effciency of 11–13%and 80–88%,respectively.HMP-based nanoparticles gave the highest loading capacity and loading effciency(Table 1).
Fig.5 shows the dissolution profles of ITZ from commercial product and various formulations containing different types of pectin.ITZ powders dissolved only 3–5%within 2 h,while all nanoparticles showed signifcantly improvement.HMP-based nanoparticles showed about 60%of drug dissolution within 2 h while LMP-based nanoparticles and ALMP-based nanoparticles showed 66%and 80%of drug dissolution,respectively.These results indicated that freeze-dried products prepared from nanoemulsions could be used as an effective carrier for improvement of ITZ dissolution.However,the commercial product demonstrated a better drug dissolution with almost 100%drug dissolved within 60 min.
Although HMP-based nanoparticles and LMP-based nanoparticles showed good results in size and redispersibility, the percentage of drug dissolved was only 60%within 120 min, whereas the ALMPs expressed the highest amount of drug dissolved,even if their redispersibility was limited.These behaviors could be corresponding to the wall strength.The rupture ofpectin wall was found in ALMP-based nanoemulsions when investigated with TEM,as also demonstrated in our previous report[14].This may cause the leakage of nanoparticles,leading to immediate release of ITZ,even though the polymer turned to be hydrogel due to the acidity of SGF.Therefore,the fragile wall components of ALMP-based nanoparticles should also be considered as the factor infuencing drug dissolution from the nanoparticles.
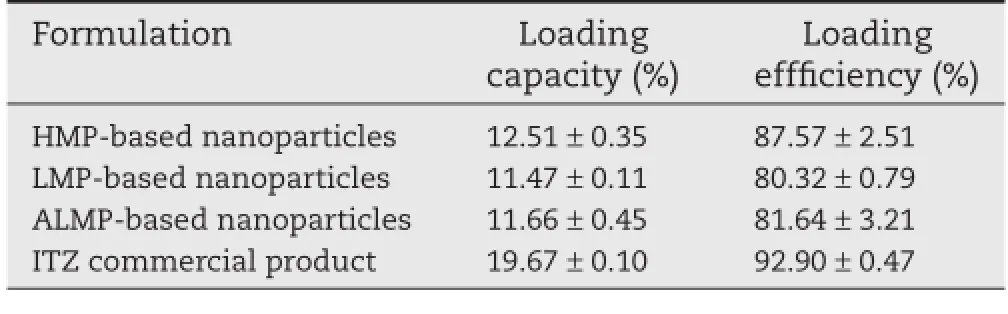
Table 1–Loading capacity and loading effciency of ITZ in nanoparticles and commercial product.

Fig.5–Dissolution profles of ITZ from nanoparticles prepared from nanoemulsion templates.
3.7.Stability of nanoparticles
The stability of pectin-based nanoparticles was performed by investigation of the drug crystalline state,redispersibility and dissolution properties.The PXRD results(Fig.6a)showed the halo-pattern of molecularly dispersed amorphous drug; however,there were some crystallinity peaks presented at approximately 12 and 21 2θdegrees,indicating the start of transformation from amorphous to crystalline solid.
The redispersibility of nanoparticles after 1-year storage at ambient conditions was investigated.Both HMP-based nanoparticles and LMP-based nanoparticles could redisperse easily in water and remained in the original size with narrow distribution(Fig.6b).Redispersion of HMP-based nanoparticles in SGF also demonstrated the preferable characteristics while the gelation of LMP-based nanoparticles and ALMP-based nanoparticles was observed,as discussed above.These results suggested that the nanoparticles were stable when tested for their redispersibility.
Even though PXRD patterns showed a little change in crystallinity of ITZ after storage(Fig.6a),all the nanoparticles after storage showed the same or slightly higher amount of ITZ dissolved than the freshly prepared ones.Table 2 shows the DE of ITZ-loaded nanoparticles after 1-year storage at ambient condition(25°C)compared to that of freshly prepared nanoparticles. No signifcant difference(P>0.05)in DE was observed in the case of HMP and ALMP-based nanoparticles.However,the DE of LMP-based nanoparticles after 1-year storage was signifcantly higher(P<0.01)than the freshly prepared ones.These results indicated the ability of the pectin carrier system to maintain the molecularly dispersed amorphous behavior over 1-year storage at ambient conditions.
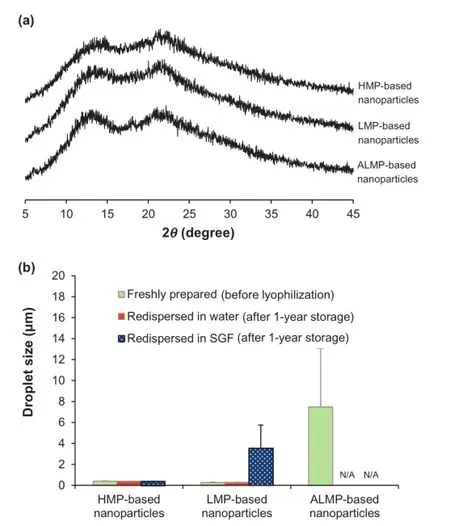
Fig.6–(a)Powder X-ray diffraction patterns of various nanoparticles prepared from nanoemulsion templates, using mechanical homogenizer,after 1-year storage at ambient condition(25°C),and(b)droplet size of redispersed nanoparticles(in water and pH 1.2 SGF)after 1-year storage at ambient condition(25°C).
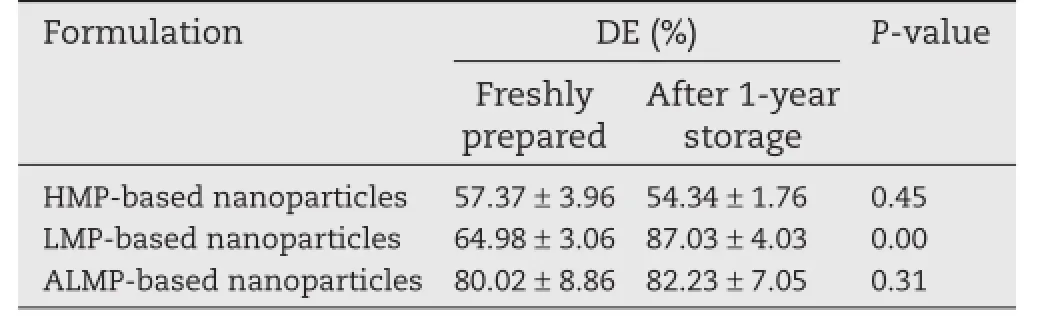
Table 2–Dissolution effciency of ITZ-loaded nanoparticles after 1-year storage at ambient condition (25°C)compared to that of freshly prepared nanoparticles.
4.Conclusions
Pectin nanoparticles were prepared from nanoemulsion templates through mechanical homogenization(i.e.,rotor–stator type homogenizer)in order to enhance drug dissolution. Nanoemulsions were achieved when chloroform was used as an internal phase.The obtained nanoemulsions were then freeze-dried to produce dried nanoparticles.The alteration ofITZ crystalline state was clearly observed from powder X-ray diffractogram while minor or no interaction between ITZ and pectin was found in the nanoparticles.The ITZ-loaded nanoparticles prepared from nanoemulsion templates showed high amounts of drug dissolved in SGF,i.e.,about 80%within 2 h,more than 10 folds over ITZ powder.The nanoparticles could maintain their good dissolution properties over 1-year storage.Therefore,the pectin nanoparticles prepared from nanoemulsion templates are a promising carrier to improve the dissolution of ITZ.
Acknowledgements
The authors wish to acknowledge theThailand Research Fund and Offce of Small and Medium Enterprises Promotion,Thailand,for the fnancial support(grant number IUG5080020).KB isfnanciallysupportedbytheRoyalGoldenJubileePh.D.Program (grant number PHD/0029/2549).Thanks to Pharma Nueva,Co., Ltd.(Thailand)andSasolGmbH(Germany)whichkindlydonated itraconazole and Miglyol®812 samples respectively.
R E F E R E N C E S
[1]Mohnen D.Pectin structure and biosynthesis.Curr Opin Plant Biol 2008;11:266–277.
[2]Sriamornsak P.Application of pectin in oral drug delivery. Expert Opin Drug Deliv 2011;8:1009–1023.
[3]Van Buren JP.Function of pectin in plant tissue structure and frmness.In:Walter RH,editor.The chemistry and technology of pectin.New York:Academic Press;1991.p. 1–17.
[4]Piriyaprasarth S,Sriamornsak P.Flocculating and suspending properties of commercial citrus pectin and pectin extracted from pomelo(Citrus maxima)peel. Carbohydr Polym 2011;83:561–568.
[5]Sriamornsak P,Thirawong N,Weerapol Y,et al.Swelling and erosion of pectin matrix tablets and their impact on drug release behavior.Eur J Pharm Biopharm 2007;67:211–219.
[6]Sungthongjeen S,Sriamornsak P,Pitaksuteepong T,et al. Effect of degree of esterifcation of pectin and calcium amount on drug release from pectin-based matrix tablets. AAPS PharmSciTech 2004;5:E9.
[7]Sriamornsak P,Thirawong N,Puttipipatkhachorn S. Morphology and buoyancy of oil-entrapped calcium pectinate gel beads.AAPS J 2004;6:e24.
[8]Burapapadh K,Kumpugdee-Vollrath M,Chantasart D,et al. Fabrication of pectin-based nanoemulsions loaded with itraconazole for pharmaceutical application.Carbohydr Polym 2010;82(2):384–393.
[9]Willems L,van der Geest R,de Beule K.Itraconazole oral solution and intravenous formulations:a review of pharmacokinetics and pharmacodynamics.J Clin Pharm Ther 2001;26:159–169.
[10]Peeters J,Neeskens P,Tollenaere JP,et al.Characterization of the interaction of 2-hydroxypropyl-b-cyclodextrin with itraconazole at pH 2,4 and 7.J Pharm Sci 2002;91:1414–1422. [11]Timpe C.Drug solubilization strategies applying nanoparticulate formulation and solid dispersion approaches in drug development.Pharm Rev 2010;13:12–21.
[12]Qian C,McClements DJ.Formation of nanoemulsions stabilized by model food-grade emulsifers using high pressure homogenization:factors affecting particle size. Food Hydrocolloid 2011;25:1000–1008.
[13]Dhankhar P.Homogenization fundamentals.IOSR J Eng 2014;4(5):1–8.
[14]Burapapadh K,Takeuchi H,Sriamornsak P.Novel pectinbased nanoparticles prepared from nanoemulsion templates for improving in-vitro dissolution and in-vivo absorption of poorly water-soluble drug.Eur J Pharm Biopharm 2012;82:250–261.
[15]McClements DJ.Lipid-based emulsions and emulsifers.In: Akoh CC,Min DB,editors.Food lipids:chemistry,nutrition, and biotechnology.Boca Raton:CRC;2008.p.77.
[16]Khan CA,Rhodes CT.The concept of dissolution effciency. J Pharm Pharmacol 1975;27:48–49.
[17]Dickinson E.Hydrocolloids at interfaces and the infuence on the properties of dispersed systems.Food Hydrocolloid 2003;17:25–39.
[18]Maa YF,Hsu C.Liquid-liquid emulsifcation by rotor/stator homogenization.J Control Release 1996;38:219–228.
[19]Kravtchenko TP,Parker A,Trespoey A.Colloidal stability and sedimentation of pectin-stabilized acid milk drinks.In: Dickinson E,Lorient D,editors.Food macromolecules and colloids.Cambridge:Royal Society of Chemistry;1995.p. 349–355.
[20]Kalsta O,Paximada P,Mandala I,et al.Physical characteristics of submicron emulsions upon partial displacement of when protein by a small molecular weight surfactant and pectin addition.Food Res Int 2014;66:401–408.
[21]Parker A,Gunning PA,Ng K,et al.How does xanthan stabilize salad dressings?Food Hydrocolloid 1995;9:333–342.
[22]Rey L.Glimpses into the realm of freeze-drying:classical issues and new ventures.In:Louis R,May JC,editors. Freeze drying/lyophilization of pharmaceutical and biological products.London:Informa Healthcare;2010.p. 1–28.
[23]Teagarden DL,Wang W,Baker DS.Practical aspects of freeze-drying of pharmaceutical and biological products using non-aqueous cosolvent system.In:Louis R,May JC, editors.Freeze drying/lyophilization of pharmaceutical and biological products.London:Informa Healthcare;2010.p. 254–287.
[24]Vanapalli S,Palanuwech J,Coupland JN.Stability of emulsions to dispersed phase crystallization:effect of oil type,dispersed phase volume fraction,and cooling rate. Colloid Surf A 2002;204:227–237.
[25]Six K,Verreck G,Peeters J,et al.Investigation of thermal properties of glassy itraconazole:identifcation of a monotropic mesophase.Thermochim Acta 2001;376:175–181.
[26]Wang X,Michoel A,Mooter GV.Study of the phase behavior of polyethylene glycol 6000-itraconazole solid dispersions using DSC.Int J Pharm 2004;272:181–187.
[27]Ghaffani A,Navaee K,Oskoui M,et al.Preparation and characterization of free mixed-flm of pectin/chitosan/ Eudragit®RS intended for sigmoidal drug delivery.Eur J Pharm Biopharm 2007;67(1):175–186.
[28]Shim SY,Ji CW,Sah H,et al.Characterization of itraconazole semisolid dosage forms prepared by hot melt technique. Arch Pharm Res 2006;29(11):1055–1060.
[29]Gnanasambandam R,Proctor A.Determination of pectin degree of esterifcation by diffuse refectance Fouriertransform infrared spectroscopy.Food Chem 2000;68:327–332.
[30]Sinitsya A,Copikova J,Prutyanov V,et al.Amidation of highly methoxylated citrus pectin with primary amines. Carbohydr Polym 2000;42:359–368.
[31]Mandal B,Alexander KS,Riga AT.Sulfacetamide loaded Eudragit RL100 nanosuspension with potential for ocular delivery.J Pharm Pharm Sci 2010;13(4):510–523.
[32]Erdal MS,Gungor S,Ozsoy Y,et al.Preparation andin vitroevaluation of indomethacin loaded solid lipid microparticles.Acta Pharm Sci 2009;51:203–210.
[33]Lootens D,Capel F,Durand D,et al.Infuence of pH,Ca concentration,temperature and amidation on the gelation of low methoxyl pectin.Food Hydrocolloid 2003;17:237–244.
[34]Capel F,Nicolai T,Durand D,et al.Calcium and acid induced gelation of(amidated)low methoxyl pectin.Food Hydrocolloid 2006;20:901–907.
*< class="emphasis_italic">Corresponding author.
.Department of Pharmaceutical Technology,Faculty of Pharmacy,Silpakorn University,Nakhon Pathom 73000, Thailand.Tel.:+66 34 255800;fax:+66 34 255801.
E-mail address:sriamornsak_p@su.ac.th(P.Sriamornsak).
1Present address:Faculty of Pharmacy,Rangsit University,Pathum Thani 12000,Thailand.
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.07.003
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- PEGylation in anti-cancer therapy:An overview
- Near-infrared light-responsive inorganic nanomaterials for photothermal therapy
- Povacoat affecting solid-state polymorphic changes of indomethacin after co-evaporation from different types of solvents via conventional and microwave drying techniques
- Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying:Physicochemical and in silico studies
- Preparation and evaluation of PEGylated phospholipid membrane coated layered double hydroxide nanoparticles
- Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study
