Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study
2017-01-19
College of Pharmacy and Health Sciences,St.John’s University,Queens,NY,USA
Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study
Navneet Sharma,Parshotam Madan,Senshang Lin*
College of Pharmacy and Health Sciences,St.John’s University,Queens,NY,USA
A R T I C L EI N F O
Article history:
Received 8 June 2015
Received in revised form 1
September 2015
Accepted 6 September 2015
Available online 1 October 2015
Paclitaxel
Co-surfactant
Nanoparticles
Biodegradable
Solvent evaporation
The purpose of this study was to evaluate the effect of process(homogenization speed and evaporation time)and formulation(aqueous/organic phase ratio,surfactant concentration,polymer type and concentration,and drug amount)variables on the preparation of paclitaxel-loaded biodegradable polymeric nanoparticles using modifed solvent evaporation technique.Thereafter,a formulation was selected and subjected to evaluation of inclusion of a co-surfactant for further reduction of particle size.Particle size,encapsulation effciency andin-vitrodrug release kinetics were evaluated.It was observed that the inclusion of vitamin E TPGS(0.01%),Poloxamer 188(0.5%)or Tween 80(0.25%)reduced the particle size of nanoparticles to 230,244 or 301 nm from 438 nm,respectively.Encapsulation effciency increased for both vitamin E TPGS and Poloxamer 188 up to concentration at 0.010% and 0.25%,respectively,while this was not the case forTween 80.Comparison of drug release kinetics demonstrated that drug release accelerated from paclitaxel-loaded biodegradable nanoparticles prepared with the inclusion of Tween 80 but was delayed for Poloxamer 188 and vitamin ETPGS.Thus,it was concluded that the particle size of the nanoparticles could be reduced further and the paclitaxel release kinetics could easily be adjusted by taking advantage by the inclusion of a co-surfactant.
©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Recent years have seen signifcant effort devoted to formulate therapeutic agents in biocompatible nanocomposites such as nanoparticles,nanocapsules,micellar systems and conjugates as drug delivery systems.Application of nanotechnology for diagnosis,monitoring,disease therapy,and control of biological systems was referred to as“nanomedicine”,and it has been receiving extensive attention over the past decade[1].Among these drug delivery systems,nanoparticles have received a considerable attention for the delivery of wide variety of drugs as well as biological macromolecules and vaccines[2]. Nanoparticles have been defned as submicron sized drug carriers,where the drug is either adsorbed on the surface or encapsulated within the particle[3].These nanoparticles can be prepared from natural and synthetic polymers that may or may not be biodegradable depending on their route of administration.Biodegradable polymeric nanoparticles administered intravenously have been successfully used to control and target drug to specifc site of action at the therapeutically optimal rate. Owing to the size in nanometric range,these biodegradable nanoparticles have a potential to escape the reticulo-endothelial system and thus an increase in the residence time is seen with these drug delivery systems[4].Nanoparticles can also overcome the multiple drug resistance phenotype mediated by glycoprotein-P(P-gP)[5],resulting in an increased drug content inside the cells and because of this reason biodegradable nanoparticles have been widely studied for P-gP substrates like paclitaxel[6].
Paclitaxel is a taxane originally derived from the bark of the Pacifc yew treeTaxus brevifoliaand has shown to exhibit a signifcant activity against a variety of solid tumors including breast cancer,advanced ovarian carcinoma,lung cancer and neck carcinomas[7].However,the successful clinical application is mainly restricted by its high hydrophobicity and limited availability.Due to its low solubility in water and other pharmaceutical solvents acceptable for parenteral administration, Taxol(Bristol-Myers Squibb,New York,NY)is marketed as a co-solvent system of Cremophor EL and dehydrated alcohol at a 50:50(v/v)ratio.These co-solvents have been reported to have serious side effects such as hypersensitivity,nephrotoxicity and neurotoxicity as well as physical instability and incompatibility with common polyvinyl chloride(PVC)intravenous administration sets[8].In order to eliminate the Cremophor based vehicle and in an attempt to increase the therapeutic effcacy,biodegradable polymeric nanoparticles have been widely studied[9,10].
Poly-lactide(PLA)and poly-lactide-co-glycolide(PLGA)are the most widely used biodegradable polymers because hydrolysis of these leads to monomers(i.e.,lactic and glycolic acid) which are endogenous and are metabolized by the body via Kreb’s cycle resulting in minimal systemic toxicity[11].The degradation of these polymers varies from several days to several months depending on the molecular weight and co-polymer composition,which increases patient compliance[12].Biodegradable polymeric nanoparticles are usually prepared either by anionic polymerization of monomers or by dispersion of the dissolved polymers to give nanoparticles via various methods,such as solvent evaporation/extraction,spontaneous emulsifcation/solvent diffusion,salting out/emulsifcationdiffusion,and supercritical technology[13].In solvent evaporation method,the polymer is dissolved or dispersed in an organic solvent(e.g.,dichloromethane,chloroform or ethyl acetate),which is then emulsifed in an aqueous solution using different surfactants to form stable oil-in-water emulsion.High shear homogenization or sonication is used for emulsifcation.The organic phase is then allowed to evaporate resulting in a suspension of nanoparticles.The nanoparticles are later recovered by fltration or lyophilization.Various process variables,such as homogenization speed and solvent evaporation time,as well as formulation parameters,such as organic and disperse phase ratio,polymer type and concentration,surfactant type and concentration,and drug amount,can infuence the characteristics of nanoparticles prepared by solvent evaporation/extraction method.
A surfactant is used to reduce the surface tension and stabilize the droplet phase during emulsifcation process.Different types of surfactants,such as ionic surfactants and non-ionic polymer surfactants,may be used to stabilize the oil-inwater emulsions[14].Selection of the surfactant is critical to obtain particles in nanometric range.The type and concentration of surfactant are not only important in stabilizing emulsions during microemulsion process,but also helpful in preventing aggregation of the droplets,thus maintaining a low polydispersity index.Biocompatibility and toxicity of the surfactants are also important considerations for pharmaceutical application.Most researchers have fabricated nanoparticles using a single surfactant in varying concentrations.The role of a surfactant in the system is to reduce the interfacial tension between oil and water.Sometimes it is not possible for a surfactant to reduce surface tension to a required level either because of its properties or due to low concentration.If, however,a second amphiphile is added to the system,the effect of both surfactants may be additive provided that the adsorption of one surfactant on the surface of nanodroplets does not affect that of the other and also the mixed micelle formation does not reduce the available concentration of surfactant molecules.The second amphiphile,referred to as co-surfactant, need not necessarily be capable of forming association structures of its own[14].The inclusion of a co-surfactant may also reduce the amount of the surfactant needed,thus avoiding the potential toxicity issue due to the larger amount of the individual surfactant needed for preparing the formulation.The effect of a mixture of surfactant and co-surfactant has not been researched in detail and only limited literature is available in this regard[15].Combination of surfactant and co-surfactant may be benefcial by altering the physicochemical properties of the nanoparticles.Therefore,the effects of various process as well as formulation variables were carried out frst to identify,in terms of small particle size and high encapsulation effciency,a suitable formulation which was then subjected to the study of effect of inclusion of a co-surfactant on particle size,encapsulation effciency andin-vitrodrug release kineticsfrompaclitaxel-loadedbiodegradablepolymeric nanoparticles prepared by the modifed solvent evaporation technique.
2.Materials and methods
2.1.Materials
Paclitaxel was obtained from LC Laboratories(Woburn,MA). Poly-vinyl alcohol(PVA,MW 146,000–186,000),poly(L-lactide) (PLA,MW 75,000),poly(D,L-lactide-co-glycolide)(PLGA 50:50,MW 75,000 and 5000 as well as PLGA 75:25,MW 75,000 and 5000), D-α-tocopherol polyethylene glycol succinate(vitamin ETPGS), and Tween 80 were supplied by Sigma-Aldrich(St.Louis,MO). Poloxamer 188 was purchased from Spectrum Chemicals andLaboratory Products(Gardena,CA).All other chemicals and solvents were of analytical grade and used as received without further purifcation or treatment.
2.2.Analytical methodology
Paclitaxel was determined based on the reported literature with modifcation to avoid any interference from solvents used in this investigation[16].Briefy,the method employed a reverse phase HPLC(Hewlett Packard HP 1100 series)with Waters Spherisorb ODS1 Column(5 μm,4.6 mm×250 mm)and a mixture of 47%v/v acetonitrile,20%v/v methanol and 33%v/v distilled water as the mobile phase.Fifty microliters of standard drug solutions or sample solutions were injected.The fow rate was 2 ml/min and paclitaxel was detected at 254 nm with a retention time of 2.9 min.A standard plot of paclitaxel was generated over the concentration range of 0.5–500 μg/ml and was found to be linear with r2value of 0.9997.
2.3.Preparation of paclitaxel-loaded biodegradable polymeric nanoparticles
The preparation of paclitaxel-loaded nanoparticles was based on oil/water emulsifcation solvent evaporation method.Both polymer and the drug were dissolved in dichloromethane as organic solvent.The organic phase so formed was added drop wise to an aqueous phase,containing PVA as surfactant,using a high-speed microprocessor(Virtis Tempest I.Q.2 Homogenizer,Sentry Microprocessor,Kent City,MI).The emulsion formed was magnetically stirred to evaporate dichloromethane. After evaporation of the solvent,the nanoparticles were recovered by ultracentrifugation(Beckman Coulter Ultracentrifuge/ Beckman Coulter Inc.,Brea,CA)at~40,000gfor 30 min.The pellet obtained was re-suspended in distilled water and centrifuged again.The washing step was repeated twice and the pellet obtained was lyophilized for 8 h using Labconco Shell Freeze Dry Equipment(Labconco Corporation,Kansas City,MO) to obtain free fowing powder.
2.4.Evaluation of process and formulation variables
In a typical emulsifcation-solvent evaporation process,various process and formulation variables govern the physicochemical properties of nanoparticles(e.g.,particle size,encapsulation effciency and drug release kinetics).Effects of process variables(i.e.,homogenization speed and evaporation time)on the preparation of paclitaxel-loaded biodegradable polymeric nanoparticles were studied frst in this investigation.After the selection of homogenization speed and evaporation time for small particles size and high encapsulation effciency of nanoparticles,fve formulation variables(i.e.,aqueous/organic phase ratio,surfactant concentration,polymer type and concentration,and drug amount)were evaluated for further optimization of the formulation(Table 1).Only one variable wasstudied at a time while keeping all other variables and geometry of the fabrication system constant.

Table 1–Effect of formulation variables on the preparation of paclitaxel-loaded biodegradable polymeric nanoparticles.
2.4.1.Effect of homogenization speed
Energy density,which is energy applied per unit total volume has a direct effect on droplet size of the emulsion produced [17].Since the magnitude of shear stress is inversely related to the droplet size of the emulsion,increasing the shear stress may result in reduced droplet size to produce nanodroplets. Therefore,emulsifcation was performed at different homogenization speeds of 1000,5000,10,000 or 15,000 rpm for 15 min with evaporation time of organic phase set at 12 h.
2.4.2.Effect of evaporation time
During solvent evaporation,the nanodroplets are solidifed to produce nanoparticles and the drug is adsorbed on the surface and/or entrapped within the polymer matrix of these nanoparticles.Also,during the transition of nanodroplets to form nanoparticles,the drug may have a tendency to diffuse out into the external aqueous phase.Thus,it is important to control the solidifcation rate of the nanodroplets in order to achieve small particle size and high encapsulation effciency. The evaporation of the organic phase was carried out for 3,6 or 12 h with constant magnetic stirring.
2.4.3.Effect of aqueous/organic phase ratio
Since change in the aqueous/organic phase ratio may result in change in the viscosity of the system,which in turn can change the characteristics of the nanoparticles(e.g.,particle size,encapsulation effciency,surface morphology,etc.),therefore,various aqueous/organic phase ratios(i.e.,1:1,2:1,4:1,8:1 and 12:1)were evaluated.
2.4.4.Effect of surfactant concentration
The effect of surfactant concentration was studied because the presence of surfactant at the aqueous/organic phase interface governs the effectiveness of formation of emulsion and stabilization of the oil nanodroplets during the emulsifcation solvent evaporation process.Based on the preliminary experiments,poly-vinyl alcohol was selected as surfactant and concentration at 0.1,0.25,0.50,0.75,1.0 or 2%w/v was studied.
2.4.5.Effect of polymer type
Lactide and glycolide content of the biodegradable polymers affects the degradation rate of the polymeric nanoparticles and consequently the impact on drug release kinetics.Thus,three different polymers of the same biodegradable class viz PLA(MW 75,000),PLGA(75:25,MW 75,000)and PLGA(50:50,MW 75,000) were used.
2.4.6.Effect of polymer concentration
Increased amount of hydrophobic polymer may result in encapsulation of hydrophobic drug to a greater extent.Hence, PLGA(50:50,MW 75,000)was studied in three different concentrations of 10%,15%and 20%.
2.4.7.Effect of drug amount
To achieve maximum encapsulation,variable amount of drug, ranging from 1 mg to 5 mg,was added to the organic phase to evaluate the effect of drug amount in the nanoparticles.
2.5.Evaluation of the inclusion of a co-surfactant
Upon understanding the effects of various process as well as formulation variables on the preparation of paclitaxel-loaded biodegradable polymeric nanoparticles,a suitable formulation,in terms of small particle size and high encapsulation effciency,was selected for further experiments to study the effect of inclusion of a co-surfactant.By keeping all other formulation and process variables constant,vitamin E TPGS at concentrations of 0.005%,0.010%,0.025%or 0.050%,Poloxamer 188 at concentrations of 0.10%,0.25%,0.50%,0.75%,or 1.0%and Tween 80 at concentrations of 0.05%,0.10%,0.25%,0.50%or 1.0%, respectively,were evaluated.
2.6.Characterization of paclitaxel-loaded biodegradable polymeric nanoparticles
2.6.1.Particle size analysis
The mean particle diameter and polydispersity index was determined by photon correlation spectroscopy using Delsa Nano Series Zeta Potential and Submicron Particle SizeAnalyzer(Brea, CA).The analysis was performed at room temperature at a scattering angle of 90°.
2.6.2.Determination of encapsulation effciency
The encapsulation effciency was determined by dissolving 10 mg of lyophilized nanoparticles in 2 ml of dichloromethane and then mixed with 3 mL of acetonitrile:water(50:50 v/v).The solution was vortexed vigorously and dichloromethane was evaporated under a nitrogen stream until a clear solution was obtained.The fnal solution was diluted to 5 ml with acetonitrile:water(50:50 v/v)and analyzed using the HPLC method described previously.Drug encapsulation effciency was expressed as the percentage of drug in the fabricated nanoparticles with respect to the initial amount of drug used for the preparation of nanoparticles.

2.6.3.In-vitro drug release kinetics
Thein-vitrodrug release kinetics were carried out by suspending 20 mg of the paclitaxel-loaded nanoparticles in 10 ml of phosphate buffered saline solution(PBS,pH 7.4)containing 0.1% v/v ofTween 80.Screw capped glass tubes were used to conduct thein-vitrorelease studies.The glass tubes were shaken at 37°C in Forma Scientifc water shaker bath(Asheville,NC)at 120 cycles/min.Samples(2 ml each)were collected from each bottle at predetermined time intervals for at least 15 d and up to 24 d. The tubes were centrifuged at~14,000gfor 15 min before sampling and the same volume of fresh PBS was replaced into the release bottles immediately after each sampling of thesupernatant.The supernatant was analyzed for drug content by the HPLC method described previously.
3.Results and discussion
3.1.Characterization of paclitaxel-loaded biodegradable polymeric nanoparticles
The comparisons of particle size,encapsulation effciency,and cumulative amount of drug released from paclitaxel-loaded biodegradable polymeric nanoparticles prepared using various process and formulation variables are summarized in Table 2.
3.1.1.Effect of homogenization speed
第二乐章的音乐风格发生了戏剧性的变化。维瓦尔第用规律的、舒缓的广板描写淅淅沥沥的冬雨。旁边的独奏小提琴奏出祥和的曲调,这说明屋外的人们回到了屋里。窗外的万物还沉浸在风雨中,室内的人们则悠闲地围坐在炉火旁,享受着火堆带来的温暖。
Nanoparticles prepared at lower homogenization speeds(1000, 5000,and 10,000 rpm,formulations X1,X2,and X3)resulted in larger particle size(913 nm,856 nm,and 899 nm,respectively), as compared to 15,000 rpm(formulation X4)for which the particle size was 438 nm(Table 2).Emulsifcation at high speed would result in the reduction of emulsion globules and consequently,reduction of the emulsion globule size allowed the formation of smaller size of nanoparticles.With the higher speed of homogenization,more energy is released in the process that leads to a rapid dispersion of polymeric organic phase and because of which nanoparticles of small size and monomodal distribution are obtained.A bimodal distribution was seen in case of lower homogenization speeds at 1000 and 5000 rpm, which may be due to insuffcient dispersion of the organic phase.On the other hand,above 15,000 rpm,it was diffcult to control the process in terms of formation of froth and air bubbles.
The encapsulation effciency increased from 65.3%to 77.5% when the speed of emulsifcation was increased from 1000 rpm (formulation X1)to 15,000 rpm(formulation X4).Although not linear,the increase in encapsulation effciency was greater when homogenization speed was increased from 10,000 rpm to 15,000 rpm(72.1%to 77.5%)than when the homogenizationspeed was increased from 5000 rpm to 10,000 rpm(67.1%to 72.1%).The increase in encapsulation effciency may have been due to the fact that a unidirectional and less turbulent fow in the case of lower speed may have resulted in the loss of drug from the organic phase.
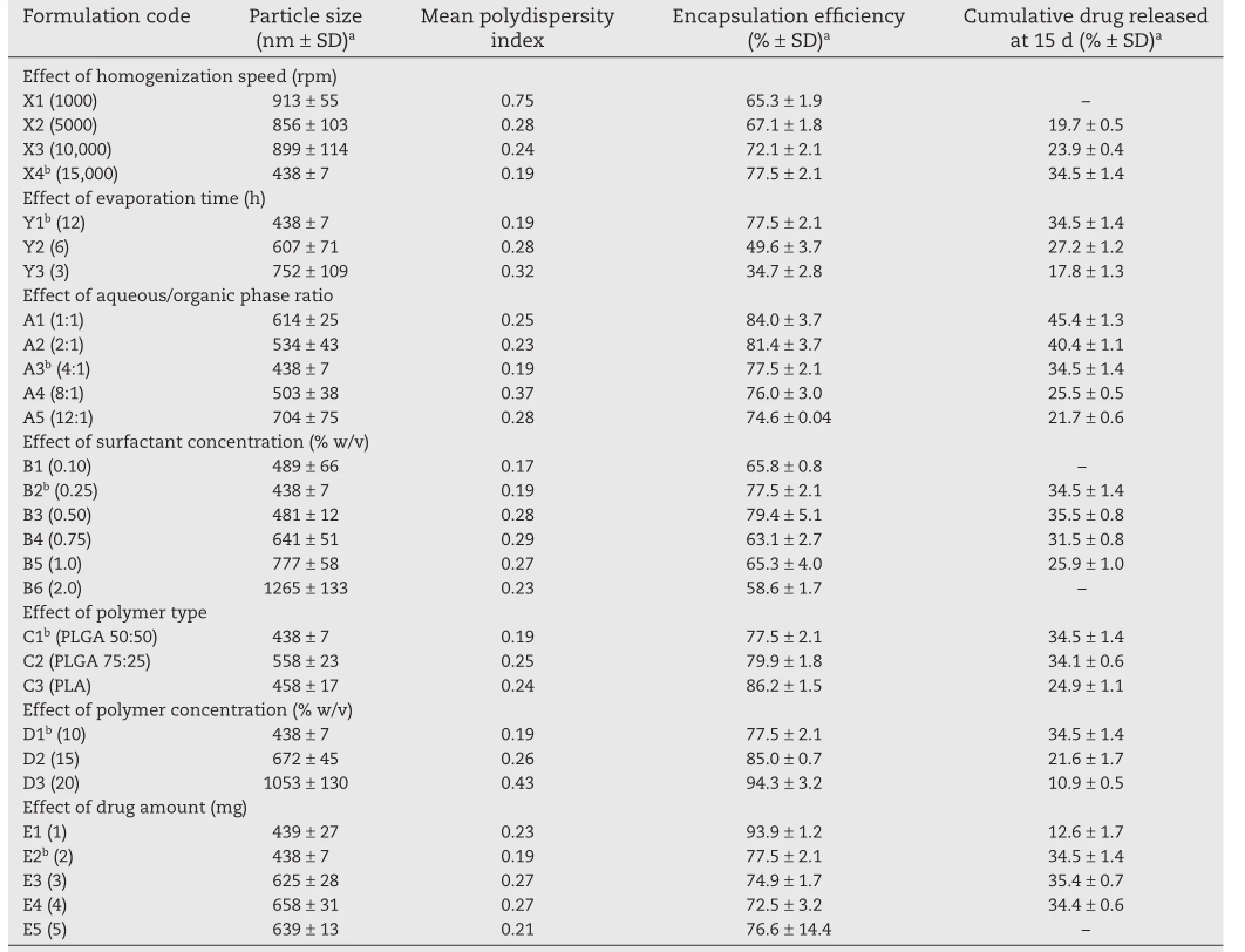
Table 2–Comparison of particle size,encapsulation effciency,and cumulative amount of drug released from paclitaxelloaded biodegradable polymeric nanoparticles prepared using various process and formulation variables.
The cumulative amount of drug released at 15 d increased with increasing homogenization speed.Owing to a large particle size and low yield resulting from low homogenization speed,formulation X1 was not evaluated for drug release.The difference in the release profles can be attributed to the difference in the particle size of the nanoparticles(Fig.1a).The difference in the mean particle size of formulations X2 and X3 was very small(856 nm versus 899 nm)and so was the cumulative amount of drug released at 15 d(67.1%versus 72.1%). The mean particle size of formulation X4 was less than 50% (i.e.,438 nm)the particle size of formulations X2 and X3,and the difference in cumulative amount of drug released from formulation X4 was high(34.5%versus 19.7%and 23.9%).Larger particles exhibited slower rate of release of the drug,due to the longer diffusion pathways that the drug had to travel to reach dissolution medium.
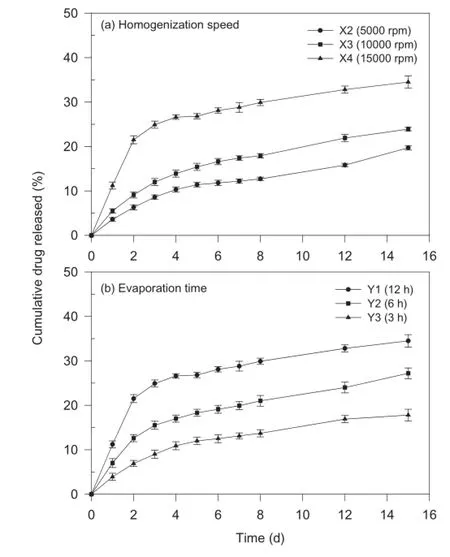
Fig.1–Effect of(a)homogenization speed and(b) evaporation time on in-vitro drug release kinetics of paclitaxel-loaded biodegradable polymeric nanoparticles (data represent mean±standard deviation,n=3).
3.1.2.Effect of evaporation time
As shown in Table 2,an increase in the evaporation time resulted in a decrease in particle size,increase in encapsulation effciency,and decrease in cumulative amount of drug released at the end of the experiment.A critical parameter determining the particle size is diffusion of organic solvent through the interface of emulsion droplets[17].When evaporation is carried out for a shorter period of time(i.e.,faster evaporation rate),the diffusion of the organic solvent out of oil droplets may not have been complete before the droplets start to harden,thus resulting in larger particles.On the other hand,when the organic solvent is allowed to evaporate for a longer time,the extent of diffusion of organic solvent out of the droplets is greater and results in smaller particle size.During the organic solvent evaporation process,there is a gradual decrease of the dispersion volume and consequently an increase of the viscosity of the dispersed droplets.This may have affected the droplet size equilibrium,involving the processes of droplet coalescence and agglomeration during the early step of the organic solvent removal.
Reduced encapsulation effciency in the case of shorter evaporation time may be due to the rapid replacement of the organic solvent with the aqueous medium before the droplet hardening occurs.As concluded previously,smaller particle size of nanoparticles is a factor for the faster drug release from the nanoparticles.It was observed that smaller particle size of formulation X4 provides an increased surface area for dissolution contributing to a greater release.Furthermore,it has been reported that rapid solvent evaporation leads to a smoother surface of the particles as compared to the particles obtained by delayed solvent evaporation[18].Thus,the smoother surface of the polymer matrix could have resulted in much slower release(Fig.1b).
Based on the results obtained,process variables of formulation X4 was selected and used for the evaluation of various formulation variables described below.
3.1.3.Effect of aqueous/organic phase ratio
The particle size of nanoparticles exhibited an initial decrease from 704 nm to 438 nm when the concentration of the organic phase was increased from 7.7%(formulation A5)to 20% (formulation A3).On further increasing the concentration from 20%to 50%(formulation A1),the particle size increased from 438 nm to 614 nm(Table 2).These fndings may be explained on the basis of change in the viscosity of the emulsion formed during processing.Increased viscosity of the emulsion by alternation of aqueous/organic phase ratio resulted in high viscous resistance against the shear force during the forming of nanodroplets[19].The external energy in the form of homogenization and the amount of emulsifer causing droplet breakdown were kept constant;thus on increasing the aqueous phase volume,the same amount of energy must now be distributed in the large volume,leading to less droplet breakdown, and hence bigger nanoparticles were obtained.
The encapsulation effciency was within a narrow range of 74.6%(formulation A5)and 84.0%(formulation A1).Higher encapsulation effciency of formulations A1 and A2 may be because of increased viscosity of the emulsion formed which would have resulted in high viscous resistance against the shear force during the emulsifcation.Upon increasing the aqueous phase volume,the amount of drug dissolved in the aqueous phase increases and,therefore,increasing the drug loss formorganic phase results in the reduction of the encapsulation effciency[17].
The cumulative amount of drug released at 15 d increased with increasing concentration of organic phase used in the preparation of nanoparticles(Table 2).The difference in the release profles(Fig.2a)can be attributed to the difference in the surface of nanoparticles regardless of the difference of particle size.It has been reported that large amount of aqueous phase results in faster precipitation of the polymer with a smoother and less porous surface of the nanoparticles[20]. The porosity decreases with increasing aqueous/organic phase ratio and thus lower aqueous/organic phase ratio could have resulted in more porous nanoparticles leading to faster release rates[21].Furthermore,the burst release at lower aqueous/organic phase ratios can probably be explained by the difference in the distribution of drug in nanoparticles due to different evaporation rates of the organic solvent.The solubility of dichloromethane in water is considered low.At lower aqueous/organic phase ratio(formulation A1),dichloromethane will distribute into the aqueous phase slowly,which is controlled by dichloromethane evaporation rate;therefore,the drug will tend to migrate onto the nanoparticle surface together with dichloromethane because of more solubility in the organic solvent,resulting in drug-rich nanoparticle surface.When the aqueous/organic phase ratio is high(formulation A5), dichloromethane distributes in water quickly and the drug solidifes in the polymer matrix rapidly.The drug near the surface will diffuse out of the nanoparticle frst,causing burst release.These fndings are in agreement with the published research where the effect of process and formulation variables on the internal morphology of PLGA microparticles was evaluated[22].
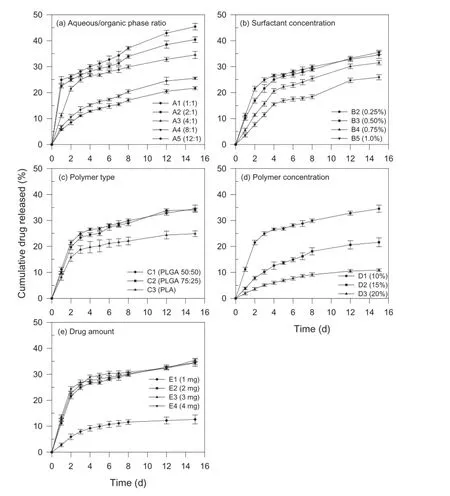
Fig.2–Effect of(a)aqueous/organic phase ratio,(b)surfactant concentration,(c)polymer type,(d)polymer concentration, and(e)drug amount on in-vitro drug release kinetics of paclitaxel-loaded biodegradable polymeric nanoparticles(data represent mean±standard deviation,n=3).
3.1.4.Effect of surfactant concentration
In this investigation,PVA was selected and used as the surfactant.It was observed that at 0.10%PVA concentration (formulation B1),the emulsion formed was not stable and phase separation occurred after a few hours of emulsifcation resulting in the formation of polymer aggregates.Increasing PVA concentration from 0.10%to 0.25%(formulation B2)and 0.50% (formulation B3)resulted in particle sizes of 438 nm and 481 nm, respectively.Increase in particle size from 481 nm to 1265 nm was seen with further increase in concentration from 0.50% to 2.0%(formulation B6).An increase in PLGA nanoparticle size with increase in PVA concentration has also been reported in the literature[23].In the emulsion solvent evaporation method, the emulsifcation and stabilization of the globules are two crucial factors.The amount of surfactant plays an important role,because it can avoid the coalescence of the oil globules. The surfactant molecules tend to align themselves at the droplet surface lowering the free energy at the interface between two phases and resisting coalescence of the droplets.Smaller nanodroplets have large surface area and thus need more surfactant to stabilize the emulsion nanodroplets.Less amount of surfactant may result in formation of unstable emulsion implying that 0.1%PVA may not be suffcient to stabilize the emulsion nanodroplets,leading to phase separation after a few hours.
The encapsulation effciency increased from 65.8%to 79.4% and then reduced to 58.6%when the surfactant concentration increased from 0.10%(formulation B1)to 0.50%(formulation B3)and further to 2.0%(formulation B6),respectively.Decrease in encapsulation effciency of the drug from 79.4%to 58.6%was seen with increasing surfactant concentration. Paclitaxel being a hydrophobic molecule will tend to stay in the oil nanodroplets.But with an increase in PVA concentration in the external aqueous phase,paclitaxel may diffuse out from the oil nanodroplets and solubilize as micelles in the aqueous phase.More solubilization of the drug in the external aqueous phase will result in decreased amount of surfactant available at the aqueous/organic phase interface and thus agglomeration of nanodroplets may take place.A secondary explanation could be on the basis of gelatinization of PVA molecules.Due to strong hydrogen bonds via hydroxyl group between inter-or intra-molecules of PVA,gelatinization of PVA at the oil/water interface may occur during the nanoparticle formation process[24].As a result,increasing particle size and decreasing encapsulation effciency were observed with increasing surfactant concentration.
The cumulative amount of drug released at surfactant concentrations of 0.25%,0.50%,0.75%and 1.0%(formulations B2-B5)were 34.5%,35.5%,31.5%and 25.9%,respectively(Table 2). Although the drug release profles demonstrated slower release with increasing surfactant concentration,the release of drug from formulations B2 and B3 prepared containing 0.25%and 0.50%PVA were essentially similar(Fig.2b).The amount of drug released decreased with an increase in the surfactant concentration.This phenomenon could be attributed to the difference in particle size at different concentrations.As the surfactant concentration increased from 0.25%to 1.0%,the particle size increased from 438 nm to 777 nm.The increase in surface area due to smaller size could have been contributing to a greater release.Burst release amount decreased with increasing surfactant concentration.Similar fndings have been reported[25]. The surfactant concentration at 0.10%level resulted in an unstable emulsion,a fake like mass was obtained after freezedrying and hence formulation B1 was not characterized for drug release.In addition,due to particle size in micron range,formulation B6 was also not characterized for drug release.
3.1.5.Effect of polymer type
The mean particle size of the nanoparticles prepared using PLGA 50:50,PLGA 75:25,and PLA(formulations C1-C3)were 438 nm, 558 nm,and 458 nm,respectively(Table 2).The particle size of nanoparticles formulated with these three different polymers was almost similar despite the difference in their lactide content.These results indicate that the type of polymer did not infuence the particle size of the nanoparticles.This fnding is in agreement with a previous study using PLA and PLGA polymers to prepare oral microparticles for budesonide delivery to ileum and colon[26].The similarity in particle size can be attributed to the fact that both polymers used belong to the same class of biodegradable polymers exhibiting the same inherent viscosity(0.9 dl/g).
The encapsulation effciency was found to be dependent on the solid-state solubility of drug in the polymer.The mean encapsulation effciencies of the nanoparticles prepared using PLGA 50:50,PLGA 75:25 and PLA(formulations C1–C3)were 77.5%,79.9%and 86.2%,respectively.The encapsulation effciency for nanoparticles formulated with PLGA 75:25 and PLGA 50:50 were almost similar,despite the difference in their lactide content.One possibility may be that the change in lactide content of the polymer from 75%to 50%does not signifcantly affect the properties of nanoparticles.In the case of PLA, because the lactide:glycolide ratio is increased from 50%to 100%,the hydrophobic interaction of the lipophilic drug with PLGA is increased,thus resulting in higher encapsulation effciency[27].
The cumulative amount of drug released at 15 d was essentially similar between formulation C1 and formulation C2 (34.5%versus 34.1%)while a much lower value(24.9%)was observed for formulation C3(Table 2).From the drug release profles shown in Fig.2c,formulations C1 and C2 incorporated with PLGA 50:50 and PLGA 75:25,respectively,resulted in higher release than that of formulation C3(PLA).In-vitrorelease of the drug from nanoparticles has been reported to have an inverse relationship with the solid-state drug-polymer solubility[28].Higher percentage of drug was released from the nanoparticles formulated with PLGA 50:50 and PLGA 75:25,since the polymers exhibiting lower solid-state drug solubility as compared to PLA with high solid-state drug solubility.Moreover, PLA is crystalline polymer while PLGA is amorphous.Due to the rigid structure of the nanoparticles prepared using crystalline PLA,the diffusion of the drug is reduced[26].The glycolide content determines the hydrophilicity and degradation of the polymers.PLGA is more hydrophilic with a faster degradation rates as compared to PLA which lacks any glycolic acid and hence more hydrophobic in nature,resulting in a much slower degradation rate[29].Additionally,the drugrelease from nanoparticles formulated using PLGA 50:50 and PLGA 75:25 had similar release properties despite the difference in their lactide content.There is a possibility that the change in the lactide content does not signifcantly affect the properties of nanoparticles[10].
3.1.6.Effect of polymer concentration
The mean particle size of formulations prepared using 10%, 15%,or 20%(formulations D1-D3)of PLGA 50:50 resulted in 438 nm,672 nm and 1053 nm,respectively(Table 2).A bimodal size distribution was seen in the case of polymer concentrations of 15%and 20%.As the polymer concentration increased from 10%to 15%and 20%,the mean encapsulation effciency increased from 77.5%to 79.9%and 86.2%,respectively.The cumulative amount of drug released at 15 d from formulations D1–D3 with polymer concentrations of 10%,15%and 20%were 35.5%,21.6%and 10.9%,respectively.The drug release decreased with increase in the polymer concentration as seen in Fig.2d and suggested that polymer concentration play a signifcant role in determining the drug release from the paclitaxelloaded PLGA 50:50 nanoparticles.
Increasing the polymer concentration,while keeping the volume of organic phase constant at 10 ml,the viscosity of the organic phase is increased,which results in increase in the viscous forces resisting droplet breakdown and thus bigger oil droplets are formed,resulting in increased particle size[23]. Similarly the increase in polymer concentration increases the organic phase viscosity,which increases the diffusional resistance to drug molecules from organic phase to the aqueous phase,thereby entrapping more drug in the polymer nanoparticles.Increasing polymer concentration also increases particle size and drug content is known to increase with particle size in other systems[30].An increase in particle size increases the length of diffusional pathways into the aqueous phase,thereby reducing the drug loss through diffusion and increasing the drug content.Also,the time required for polymer precipitation decreases at higher polymer concentration,so there is less time for drug molecules to diffuse out of nanoparticles,which increases the drug content[27].Another reason could be the availability of a greater amount of polymer to encapsulate the drug,thus not causing saturation of encapsulation[26].The decreased percentage of cumulative drug released could be due to the increased particle size and thus smaller surface area at higher polymer concentration.Another explanation for lower cumulative amount of drug release at higher polymer concentration could be the increased concentration of the polymer present which hinders the drug release by diffusion[26].
3.1.7.Effect of drug amount
The mean particle size of formulations E1 and E2 prepared using 1 mg or 2 mg of drug was about 440 nm(Table 2),but the particle size increased to above 600 nm when the amount of drug was increased to 3 mg,4 mg,or 5 mg(formulations E3–E5).The increase in particle size can be attributed to the increased content of drug present in the emulsion nanodroplets.These results are in agreement with the previously published study where a 50%increase in particle size was observed when the drug concentration was increased from 0.2%to 1.0%[26]. Because only a fxed amount of drug can be incorporated in a given amount of the polymer,it would appear that further increase in amount of drug may have resulted in a more viscous dispersed phase resulting in larger particles[22].
The encapsulation effciency exhibited a slight downward trend(from about 94%to about 77%)with increasing amount of drug in the formulation.The drug content in the nanoparticles is affected by the drug–polymer interactions and the drug miscibility in the polymer[28].Higher drug miscibility leads to higher drug incorporation.Almost constant encapsulation effciency was seen in this study,which might be due to a fxed amount of polymer available in the formulation to encapsulate the drug.Thus it can be implied that amount of drug does not play a very pivotal role in infuencing the encapsulation effciency of paclitaxel-loaded PLGA nanoparticles as long as the amount of polymer is kept constant.Studies show that the internal micro-morphology of the microparticles is not affected by increase in drug loading[22].
It was observed that the cumulative amount of drug released at 15 d from the formulations containing 1 mg,2 mg, 3 mg or 4 mg of drug were 12.6%,34.5%,35.4%and 34.4%,respectively(Table 2).The rate of drug release was slower when the drug content was 1 mg(formulation E1),and faster when the amount of drug was increased(Fig.2e).The slower rate of release as well as cumulative amount released at 15 d may be attributed to relatively higher proportion of polymer in the formulation that hindered diffusion of drug from the nanoparticles. These results indicate that at higher drug content,drug release is independent of amount of drug present in the formulation.Hence,it was assumed that formulation E5 with 5 mg drug will also show a similar pattern and,therefore,formulation E5 was not characterized for drug release.
3.2.Evaluation of the inclusion of a co-surfactant
Although the release of paclitaxel from the biodegradable nanoparticles is mainly attributed to the degradation of polymer, particle size of nanoparticle is another important factor on the drug release.Therefore,the inclusion of a co-surfactant might help in the formation of smaller droplets of microemulsion by reducing the surface tension and subsequently might affect the drug release from nanoparticles formed.Therefore,upon understanding the effects of various process as well as formulation variables on the preparation of paclitaxel-loaded biodegradable nanoparticles,formulation A3 was selected for further experiments to study the effect of inclusion of a co-surfactant.
Ten different non-ionic surfactants,namely,Poloxamer 188, vitamin E TPGS,Tween 80,polyethylene glycol 2000,dipalmitoylphosphatidylcholine,Poloxamer 407,Pluronic 127, lecithin,dextran and cholesterol were investigated as the cosurfactant coupled with 0.25%PVA(formulation A3)as the surfactant at three different concentrations(data not shown). Only three of these,namely,Poloxamer 188,vitamin E TPGS, and Tween 80,were able to reduce the particle size less than that obtained with PVA alone(i.e.,438 nm)and hence were considered for further experiments to study the effect of inclusion of these co-surfactants at various concentration levels.The comparisons of particle size,encapsulation effciency,andcumulative amount of drug released form these experiments are summarized in Table 3.

Table 3–Comparison of particle size,encapsulation effciency,and cumulative amount of drug released from formulation A3 with the inclusion of a co-surfactant.
3.2.1.Particle size
It was observed that the particle sizes of the formulations I2–I5 having vitamin E TPGS at concentration of 0.005%,0.010%, 0.025%and 0.050%were 1296 nm,230 nm,248 nm and 290 nm, respectively(Table 3).There was a reduction of almost 200 nm form 438 nm(formulation I1)when vitamin E TPGS was used as the co-surfactant with PVA.At low concentration of 0.005%, a bimodal distribution of the particles was seen with wider standard deviation.For formulations I3 and I4,the monomers of vitamin E TPGS may have aligned on the oil/water interface along with PVA to cover the oil nanodroplets more effciently and thus reducing the interfacial tension between the oil and water phase and resulting in lower particles size.Similar results have been reported when vitamin E TPGS was used as emulsifer to prepare paclitaxel:PLGA nanoparticles[10].
When Poloxamer 188 was used as the co-surfactant,the particle size decreased from 438 nm(no co-surfactant)to 362 nm, 300 nm,and 244 nm at concentrations of 0.10%,0.25%,and 0.50%(formulations J2–J4),respectively,but further increase in concentration to 0.75%and 1.0%(formulations J5 and J6)resulted in increase in particle size.It has been known that increasing the viscosity of water exerts the stabilizing effect of Poloxamer 188.The suffciently high viscosity prevents emulsifed multiple nanodroplets from interfowing.Poloxamer 188, as a non-ionic emulsifer,has been reported to act as a coemulsifer during the emulsifcation process,resulting in smaller particle size and narrower size distribution[31].An interaction between Poloxamer 188 and polyester linkage of PLGA in methylene chloride solution has been emphasized by an associative thickening effect[32].The hydrophobic propylene chain of Poloxamer 188,like vitamin ETPGS,may have aligned on the oil/water interface along with PVA to cover the oil nanodroplets more effciently and thus reducing the interfacial tension between the oil and water phase and resulting in lower particles size.But,as the concentration of poloxamer 188 was increased,the hydrophilic chains of one particle may have interacted with hydrophilic chains of the other particle and this inter-particle interaction of chains may have resulted in agglomeration at higher concentrations.
The effect of Tween 80 on the particle size was essentially similar to that of Poloxamer 188.Increase in the concentration of Tween 80 exhibited an initial decrease in particle size followed by an increase in particle size with increasing concentration of Tween 80.This could be due to the alignment of the hydrophobic monooleate part on the oil/water interface along with PVA to cover the oil nanodroplets more effciently and thus reducing the interfacial tension between the oil and water phase and resulting in lower particle size.
3.2.2.Encapsulation effciency
The encapsulation effciency ranged between 71.7%(formulation I5)and 81.6%(formulation I3)when vitamin ETPGS was used as the co-surfactant.The encapsulation effciency increased at low vitamin E TPGS concentrations(0.005%and 0.010%),then exhibited a decline with increasing concentration to 0.050%.This can be explained on the basis of critical micellar concentration(CMC)value ofTPGS.The reported CMC value of vitamin E TPGS is 0.025%[10].Initially there is an increase in the encapsulation that may be due to enhanced stabilization of the oil nanodroplets due to co-surfactant that may have prevented the leaching out of the drug from the oilnanodroplets.As the concentration is slowly increased,more and more monomers of vitamin E TPGS leaving the oil/water interface tend to form micelles in the aqueous phase.As the number of monomers decrease at the interface leaving the oil nanodroplet surface more exposed to aqueous phase,drug may have leached out of the oil nanodroplets,thus reducing encapsulation effciency.
Poloxamer 188 had essentially no effect on the encapsulation effciency.The hydrophobic propylene chain of Poloxamer 188 may have aligned on the oil/water interface along with PVA to cover the oil nanodroplets more effciently,thus sterically stabilizing the oil nanodroplets.As all the nanodroplets were stabilized by the presence of surfactant and co-surfactant on the interface,drug may not partition out to the aqueous phase and hence no change in the encapsulation effciency was seen.
The encapsulation effciency in the presence of Tween 80 decreased with increasing concentration ofTween 80.The encapsulation effciency of nanoparticles without co-surfactant was 77.5%(formulation K1)which was reduced to 41.1%and 34.1%(formulations K3 and K4)with a co-surfactant concentration of 0.10%and 0.25%,respectively.Similar to vitamin E TPGS,this behavior could be explained on the basis of the CMC value of Tween 80.The reported CMC value of Tween 80 is 0.0014%[33]and is known to form core shell cylinder micelles in aqueous solutions[34].As the concentration ofTween 80 is slowly increased,more and more monomers similar to vitamin E TPGS,many monomers of Tween 80 leave the oil/ water interface and form micelles.As the number of monomers decrease at the interface leaving the oil droplet surface more exposed to aqueous phase,drug may have diffused out of the oil nanodroplets,thus reducing encapsulation effciency.
3.2.3.In-vitro drug release kinetics
As the inclusion of a co-surfactant was to further reduce the particle size of nanoparticles,in-vitrodrug release kinetics from formulations with smallest particle size were performed and is represented in Fig.3.It can be seen that drug release is accelerated in case of Tween 80(formulations K3 and K4),but is delayed in case of vitamin ETPGS(formulations I3 and I4)and Poloxamer 188(formulations J3 and J4).Vitamin ETPGS has recently been established as an emulsifer that possesses a dual nature similar to an amphiphile[10].Although the exact portion of the hydrophilic polar head and the lipophilic alkyl tail is not elucidated,it is assumed that the polyethylene glycol portion behaves as the polar head and the tocopherol succinate portion behaves as the lipophilic tail.The cumulative amount of drug released at 24 d from the formulations I3 and I4 with 0.01% and 0.025%vitamin E TPGS were 37.6%and 33.3%,respectively,which were less as compared to the formulation I1 (48.3%).This retarded drug release may be due to complete distribution of the vitamin E TPGS on the surface of the nanoparticle.It has been reported via X-ray photoelectron microscopy that the surface of the nanoparticles fabricated using vitamin E TPGS as emulsifer is independent of the type of polymer used and the emulsifer surrounds the nanoparticle surface completely[10].
Poloxamer 188,a triblock polymer of polypropylene oxide and polyethylene oxide,when used as the co-surfactant retards the release of the drug from formulations J3 and J4 as compared to formulation I1.The cumulative amount of drug released at 24 d from the formulations J3 and J4 with 0.25%and 0.50%of Poloxamer 188 at 24 d were found to be 35.7%and 35.0%,respectively.The drug release was independent of the concentration of Poloxamer 188 used.Various hydrolytic processes are involved in the degradation of PLGA,which leads to the formation of acidic oligomers and monomers because of which an acidic microenvironment is generated to facilitate the degradation process and thus more drug is released. Poloxamers and poloxamines could possibly retard the degradation by neutralizing the acidity generated during the polymer degradation and could also prevent unwanted interactions between the drug and the PLGA resulting in controlled release of drug form polymer matrix[35].It has also been reported that the stability by poloxamer is due to steric mechanism produced by the free hydrophilic polyethylene chains.These free chains may interact with the free PVA chains to further stabilize the nanoparticles and retard the release of the drug[35].
The cumulative amount of drug released at 24 d from the formulations K3 and K4 with 0.10%and 0.25%Tween 80 were 64.6%and 60.9%,respectively.The drug release profles were also found to be sensitive to co-surfactant concentration.A burst effect was seen in both formulations K3 and K4 that could be due to the presence of the drug onto or near the nanoparticle surface.The HLB value of Tween 80 is 15 because of which it has a strong tendency to form oil-in-water emulsion and thus Tween 80 may migrate at oil/water interface along with the drug, thereby increasing the drug concentration at the surface of the nanoparticles[36].The repulsion exerted by monooleate group ofTween 80 may have been counterbalanced by the hydrocarbon chains of PVA.Another possible reason for a faster release of drug may be the smaller particle size of 355 nm and 301 nm for formulations K3 and K4,respectively.Increased surface area for dissolution may be contributing to a greater release.
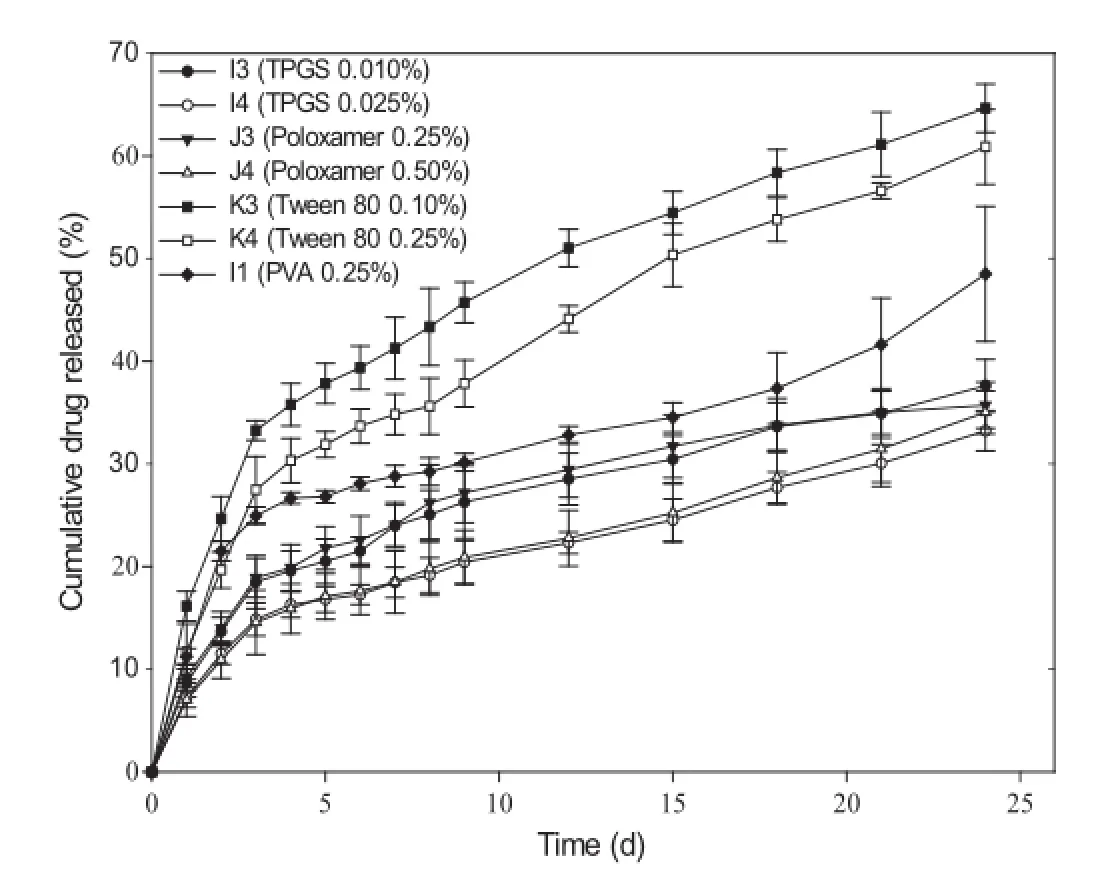
Fig.3–Effect of inclusion of a co-surfactant on in-vitro drug release kinetics of paclitaxel-loaded biodegradable polymeric nanoparticles(formulation A3)(data represent mean±standard deviation,n=3).
4.Conclusion
The results of this investigation elucidates that the process and formulation variables could be effectively altered to achieve the desired characteristics,such as particle size,entrapment effciency,andin vitrodrug release kinetics,of paclitaxelloaded biodegradable polymeric nanoparticles.Additionally, particle size of the nanoparticles could be reduced further and the paclitaxel release kinetics could easily be adjusted by taking advantage of synergistic action of surfactants by the inclusion of a co-surfactant.
Acknowledgments
The authors acknowledge St.John’s University for providing fnancial assistance and research facilities to carry out this research.
R E F E R E N C E S
[1]Moghimi SM,Hunter AC,Murray JC.Nanomedicine:current status and future prospects.FASEB J 2005;19:311–330.
[2]Mahapatro A,Singh DK.Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines.J Nanobiotechnology 2011;9:55.
[3]Pinto RC,Neufeld RJ,Ribeiro AJ,et al.Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles.Nanomedicine 2006;2:8–21.
[4]Acharya S,Sahoo SK.PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect.Adv Drug Deliv Rev 2011;63:170–183.
[5]Brigger I,Dubernet C,Couvreur P.Nanoparticles in cancer therapy and diagnosis.Adv Drug Deliv Rev 2002;54:631–651.
[6]Chavanpatil MD,Patil Y,Panyam J.Susceptibility of nanoparticle-encapsulated paclitaxel to P-glycoproteinmediated drug effux.Int J Pharm 2006;320:150–156.
[7]Desai A,Vyas T,Amiji M.Cytotoxicity and apoptosis enhancement in brain tumor cells upon coadministration of paclitaxel and ceramide in nanoemulsion formulations. J Pharm Sci 2008;97:2745–2756.
[8]Mitra A,Lin S.Effect of surfactant on fabrication and characterization of paclitaxel-loaded polybutylcyanoacrylate nanoparticulate delivery systems.J Pharm Pharmacol 2003;55:895–902.
[9]Ibrahim NK,Desai N,Legha S,et al.Phase I and pharmacokinetic study of ABI-007,a Cremophor-free, protein-stabilized,nanoparticle formulation of paclitaxel. Clin Cancer Res 2002;8:1038–1044.
[10]Mu L,Feng SS.A novel controlled release formulation for the anticancer drug paclitaxel(Taxol):PLGA nanoparticles containing vitamin E TPGS.J Control Release 2003;86:33–48.
[11]Lu JM,Wang X,Marin-Muller C,et al.Current advances in research and clinical applications of PLGA-based nanotechnology.Expert Rev Mol Diagn 2009;9:325–341.
[12]Fu Y,Kao WJ.Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems.Expert Opin Drug Deliv 2010;7:429–444.
[13]Soppimath KS,Aminabhavi TM,Kulkarni AR,et al. Biodegradable polymeric nanoparticles as drug delivery devices.J Control Release 2001;70:1–20.
[14]Lawrence MJ,Rees GD.Microemulsion-based media as novel drug delivery systems.Adv Drug Deliv Rev 2000;45:89–121.
[15]Mu L,Feng SS.Fabrication,characterization andin vitrorelease of paclitaxel(Taxol)loaded poly(lactic-co-glycolic acid)microspheres prepared by spray drying technique with lipid/cholesterol emulsifers.J Control Release 2001;76:239–254.
[16]Bala S,Uniyal GC,Chattopadhyay SK,et al.Analysis of taxol and major taxoids in Himalayan yew,Taxus wallichiana. J Chromatogr A 1999;858:239–244.
[17]Budhian A,Siegel SJ,Winey KI.Haloperidol-loaded PLGA nanoparticles:systematic study of particle size and drug content.Int J Pharm 2007;336:367–375.
[18]Izumikawa S,Yoshioka S,Aso Y,et al.Preparation of poly(llactide)microspheres of different crystalline morphology and effect of crystalline morphology on drug release rate. J Control Release 1991;15:133–140.
[19]Quintanar-Guerrero D,Fessi H,Allémann E,et al.Infuence of stabilizing agents and preparative variables on the formation of poly(D,L-lactic acid)nanoparticles by an emulsifcation-diffusion technique.Int J Pharm 1996;143:133–141.
[20]Yang Q,Owusu-Ababio G.Biodegradable progesterone microsphere delivery system for osteoporosis therapy.Drug Dev Ind Pharm 2000;26:61–70.
[21]Choi HS,Seo SA,Khang G,et al.Preparation and characterization of fentanyl-loaded PLGA microspheres:in vitro release profles.Int J Pharm 2002;234:195–203.
[22]Mao S,Shi Y,Li L,et al.Effects of process and formulation parameters on characteristics and internal morphology of poly(D,L-lactide-co-glycolide)microspheres formed by the solvent evaporation method.Eur J Pharm Biopharm 2008;68:214–223.
[23]Zweers ML,Grijpma DW,Engbers GH,et al.The preparation of monodisperse biodegradable polyester nanoparticles with a controlled size.J Biomed Mater Res B Appl Biomater 2003;66:559–566.
[24]Murakami H,Kawashima Y,Niwa T,et al.Infuence of the degrees of hydrolyzation and polymerization of poly(vinylalcohol)on the preparation and properties of poly(dl-lactide-co-glycolide)nanoparticle.Int J Pharm 1997;149:43–49.
[25]Yang YY,Chung TS,Ng NP.Morphology,drug distribution, and in vitro release profles of biodegradable polymeric microspheres containing protein fabricated by doubleemulsion solvent extraction/evaporation method. Biomaterials 2001;22:231–241.
[26]Krishnamachari Y,Madan P,Lin S.Development of pH-and time-dependent oral microparticles to optimize budesonide delivery to ileum and colon.Int J Pharm 2007;338:238–247.
[27]Budhian A,Siegel SJ,Winey KI.Production of haloperidolloaded PLGA nanoparticles for extended controlled drug release of haloperidol.J Microencapsul 2005;22:773–785.
[28]Panyam J,Williams D,Dash A,et al.Solid-state solubility infuences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles.J Pharm Sci 2004;93:1804–1814.
[29]Kranz H,Ubrich N,Maincent P,et al.Physicomechanical properties of biodegradable poly(D,L-lactide)and poly(D,L-lactide-co-glycolide)flms in the dry and wet states.J Pharm Sci 2000;89:1558–1566.
[30]Gorner T,Gref R,Michenot D,et al.Lidocaine-loaded biodegradable nanospheres.I.Optimization of the drug incorporation into the polymer matrix.J Control Release 1999;57:259–268.
[31]Yan F,Zhang C,Zheng Y,et al.The effect of poloxamer 188 on nanoparticle morphology,size,cancer cell uptake,and cytotoxicity.Nanomedicine 2010;6:170–178.
[32]Nihant N,Schugens C,Grandfls C,et al.Polylactide microparticles prepared by double emulsion/evaporation technique.I.Effect of primary emulsion stability.Pharm Res 1994;11:1479–1484.
[33]Wan LS,Lee PF.CMC of polysorbates.J Pharm Sci 1974;63:136–137.
[34]Aizawa H.Morphology of polysorbate 80(Tween 80)micelles in aqueous dimethyl sulfoxide solutions.J Appl Crystallogr 2010;43:630–631.
[35]Santander-Ortega MJ,Jodar-Reyes AB,Csaba N,et al. Colloidal stability of pluronic F68-coated PLGA nanoparticles:a variety of stabilisation mechanisms. J Colloid Interface Sci 2006;302:522–529.
[36]Rosa GD,Iommelli R,La Rotonda MI,et al.Infuence of the co-encapsulation of different non-ionic surfactants on the properties of PLGA insulin-loaded microspheres.J Control Release 2000;69:283–295.
*< class="emphasis_italic">Corresponding author.
.College of Pharmacy and Health Sciences,St.John’s University,Queens,NY,USA.Tel.:+1(718)990 5344;fax:+1 (718)990 1877.
E-mail address:linse@stjohns.edu(S.Lin).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.09.004
1818-0876/©2016 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
猜你喜欢
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- PEGylation in anti-cancer therapy:An overview
- Near-infrared light-responsive inorganic nanomaterials for photothermal therapy
- Development of pectin nanoparticles through mechanical homogenization for dissolution enhancement of itraconazole
- Povacoat affecting solid-state polymorphic changes of indomethacin after co-evaporation from different types of solvents via conventional and microwave drying techniques
- Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying:Physicochemical and in silico studies
- Preparation and evaluation of PEGylated phospholipid membrane coated layered double hydroxide nanoparticles
