间充质干细胞对移植物的保护作用*
2017-01-12黄江鹏陈忠华宫念樵
黄江鹏, 陈忠华, 宫念樵△, 时 军△
1南昌大学第一附属医院普外一科,南昌 3300062华中科技大学同济医学院附属同济医院器官移植研究所,武汉 430030
间充质干细胞对移植物的保护作用*
黄江鹏1, 陈忠华2, 宫念樵2△, 时 军1△
1南昌大学第一附属医院普外一科,南昌 3300062华中科技大学同济医学院附属同济医院器官移植研究所,武汉 430030
间充质干细胞; 移植物; 免疫调节; 内质网应激
器官移植是治疗终末期脏器功能衰竭的有效方法,但移植过程中可能出现缺血再灌注损伤、免疫排斥反应、炎症反应、氧化应激以及内质网应激等多种损伤,严重影响移植物的功能和存活。例如,胰岛移植后3 d内可能发生瞬时血液介导的炎症反应(instant blood-mediated inflammatory reaction,IBMIR),并可导致超过50%胰岛细胞凋亡或者失活[1]。
间充质干细胞(mesenchymal stem cells,MSCs)又称为基质细胞或间充质祖细胞,在人类和啮齿类动物中,可以从外周血、胎盘组织、脐带血、脂肪组织、骨髓腔组织、关节液、腱、牙髓、肺、胎儿肝脏等多种组织和器官中获得。其中,MSCs从骨髓中获取相对容易,也是许多研究人员分离、培养MSCs时的首选。在设定的分化环境中,MSCs可以成脂、成骨、成软骨化[2-3]。MSCs能抑制免疫排斥,旁分泌多种营养因子,减弱内质网应激,并且向移植区域迁徙,改善移植区微环境,有效提高移植物的存活,改善移植物功能。自Bartholomew等发现MSCs能显著延长非人类灵长类动物皮肤移植物存活后,在一系列啮齿类动物的胰岛、肾脏、肝脏、心脏移植的实验中,这种保护作用也得到验证[4-9]。其中涉及到3个主要的机制。
1 调节免疫应答作用
炎症和排斥反应可严重损害移植物各种功能。MSCs能有效减弱相关损害,提高移植物的存活率和改善移植物功能。MSCs与多种免疫细胞通过细胞-细胞直接接触或旁分泌细胞因子及趋化因子的方式,直接或间接调节免疫细胞的增殖和功能状态,抑制排斥反应。MSCs的保护机制尚未在体内实验中得到完全阐释,然而在体外实验中已有进展。MSCs通常被认为具有低免疫原性,这和它限制性表达MHC-Ⅱ、协同刺激分子并且不刺激T细胞的增殖有关。尽管低浓度干扰素可刺激MSCs上调MHC-Ⅱ的表达并可能发挥非专职抗原提呈作用,然而,稍高浓度干扰素即可扭转MSCs这一功能,并发挥强大免疫抑制作用[10]。
1.1 MSCs与T淋巴细胞的相互作用
T淋巴细胞是在移植后参与急性免疫排斥反应的主要细胞群。MSCs在和T淋巴细胞的相互作用中,一方面可以通过细胞-细胞直接接触抑制T细胞的增殖,如抑制分子程序性死亡1(programmed cell death 1,PD-1)及其配体PD-L1。在体外实验,MSCs经干扰素的刺激可上调表面分子PD-L1的表达并抑制T细胞的增殖[11-12]。在体内,前期糖尿病NOD鼠的胰腺中MSCs能高表达PD-L1并且抑制T细胞增殖从而延缓1型糖尿病的发展[13]。另外,粘附分子ICAM1和VCAM1,共刺激分子B7-H4均可在MSCs表达上调,通过细胞-细胞直接接触作用,调节T细胞的增殖[14-15]。这些表明,MSCs可以通过细胞-细胞直接接触作用抑制T细胞的增殖而参与免疫调节。
另一方面,MSCs还能够旁分泌多种可溶性分子如转化生长因子β(transforming growth factor β,TGF-β)、IL-10、前列腺素E2(prostaglandin E2,PGE2)、基质金属蛋白酶(matrix metalloproteinases,MMPs)、半乳糖凝集素家族、吲哚胺2,3双加氧酶(indoleamine 2,3,dioxygenase,IDO)等,抑制T细胞的激活或者增殖。体外被γ-干扰素(interferon-γ,IFN-γ)刺激后,MSCs能分泌TGF-β,抑制T细胞增殖;静脉内注射MSCs后,血清中的TGF-β和IL-10也可升高,并抑制T细胞增殖[16-18]。MSCs介导的PGE2的免疫抑制效果虽具有争议性,然而越来越多研究表明,PGE2在MSCs抑制T细胞的增殖方面具有重要作用[19-20]。例如,移植肺间充质干细胞(LR-MSCs)经过IL-1β刺激可以大幅提高PGE2的分泌,并抑制T细胞的激活和增殖[21]。MMP-2、MMP-9裂解T细胞表面分子CD25,使得T细胞对IL-2的刺激无反应,抑制T细胞的激活[22];半乳糖凝集素家族通过多种方式引起T细胞的凋亡,并影响T细胞的激活、功能、细胞因子的分泌,进而调节免疫作用[23-26]。由此可见,MSCs可以通过旁分泌的方式分泌多种可以调节T细胞增殖和分化的分子,调节免疫耐受。
1.2 MSCs和树突状细胞的相互作用
MSCs可以抑制树突状细胞(DCs)成熟,减弱其抗原提呈功能,诱生调节性T细胞。其机制包括:①抑制单核细胞分化为未成熟DCs[27-28];②通过IL-6的分泌抑制炎性DCs的产生[29];③MSCs分泌IL-6,下调DCs表面共刺激分子CD80、CD86及MHC-Ⅱ类分子表达,进而抑制DCs成熟、阻断DCs抗原提呈功能[30]。最近发现,MSCs分泌IL-6并上调细胞因子信号抑制物1(suppressor of cytokine signaling 1,SOCS1)的表达,阻断DCs中的TLR4信号通路,从而抑制DCs成熟分化[31]。可见,IL-6在MSCs调节DCs的作用中至关重要。此外,MSCs调节DCs的作用也可能和IL-10密切相关。MSCs能够激活JAK/STAT通路,从而活化SOCS3,增加产生依赖IL-10的调节性DCs,继而发挥抗炎效应和免疫调节作用[32-33]。另有研究表明,在和MSCs共培养的DCs体系中,IL-10的分泌上调,TNF-α、INF-γ、IL-12等炎性因子的分泌则会下调[28,34-35]。而这些炎性因子的改变可能在炎症介导的移植物免疫微环境中起重要调节作用。
DCs迁移至淋巴结区域,将抗原呈递给T细胞,这一过程与趋化因子受体CCR7密切关联。MSCs通过下调DCs的CCR7的表达,减弱DCs的迁移作用[36]。因此,MSCs可能通过减弱DCs的迁移作用减弱免疫排斥,保护移植物的功能和存活。
1.3 MSCs和巨噬细胞的相互作用
在移植肾中可以活检出活化的M2型巨噬细胞,但激活方式与经典的M1型巨噬细胞不同。在移植肾微环境中,M2型巨噬细胞可通过Th2细胞分泌的细胞因子如IL-13、IL-14激活[37],而不是通过经典的Th1细胞分泌的干扰素等细胞因子激活。MSCs被IFN-γ和TNF-α刺激后,可上调IDO的分泌,激活M2型巨噬细胞,从而抑制T细胞的增殖,调节免疫应答[38-39]。
1.4 MSCs和自然杀伤细胞的相互作用
MSCs还可以调节自然杀伤细胞(NK细胞)的功能。NK细胞可选择性杀伤未成熟DCs,忽视成熟DCs并增强免疫杀伤力;另外还可以分泌多种毒性细胞因子和趋化因子。MSCs抑制NK细胞功能的机制复杂,可能通过分泌PGE2和TGF-β来发挥作用[40]。另外MSCs还可下调NK细胞表面激动性受体如NKp44、NKG2D和NKp30的表达,抑制NK细胞的增殖、细胞毒性及因子的分泌[41]。
1.5 MSCs和B淋巴细胞的相互作用
MSCs可以抑制B细胞表面相关趋化因子的受体CXCR4、CXCR5、CXCR7及其配体CXCL12、CXCL13的表达,进而影响B细胞的趋化能力。MSCs还可使B细胞停滞于G0/G1期,抑制B细胞的增殖[42]。
1.6 MSCs和调节性淋巴细胞群的相互作用
调节性淋巴细胞群在诱导免疫耐受和减弱免疫排斥方面具有重要作用。MSCs能够诱导和扩增调节性淋巴细胞群。MSCs治疗实体器官移植(mesenchymal stem cells in solid organ transplantation,MISOT)的研究发现,MSCs调节移植后排斥反应的主要机制包括扩增调节性T细胞[43-44]和抑制效应T细胞及记忆性T细胞的增殖[45]。MSCs高效诱生Treg细胞可能和部分细胞因子密切相关[46]。然而,通过细胞接触方式上调IL-10、TGF-β和PGE2的分泌,是人MSCs诱导产生CD4+CD25hiFOXP3+Treg细胞的关键[47]。在体内,FOXP3+Treg细胞的产生与IDO表达有关,这个因子由IFN-γ刺激MSCs产生[48]。此外,MSCs还可促进生成其它调节性细胞如调节性巨噬细胞(Mreg)、调节性B细胞(Breg)、Tr1 IL-10+和Th3 TGF-β+等,参与移植免疫调节[49-55]。
2 修复受损组织
MSCs具有多向分化、修复及再生受损组织的潜能。它可迁移至受损区域,分泌多种细胞因子,甚至可分化为具有功能的细胞,保护或者替代受损的细胞,以维持甚至改善组织的功能。研究表明,MSCs经静脉输注治疗,虽然大量滞留于肺毛细血管床,但在各种疾病模型如心肌梗死、脑外伤、肝纤维化、肺化学损伤、各类肿瘤和移植中,仍有部分细胞趋向于受损组织区域并发挥修复作用[56-57]。然而,MSCs主要通过分化替代还是旁分泌效应发挥修复作用一直存在争议。
一方面,MSCs可以分化成有功能的细胞或者实质细胞替代受损细胞。将雄性小鼠MSCs经外周血注入雌性小鼠体内,能够得到具有胰岛素分泌功能的胰腺β细胞[58]。链脲菌素诱导的糖尿病小鼠接受BMC移植后,在其体内检测到供者来源的胰岛素阳性细胞[59]。将MSCs经肝细胞生长培养液处理后可出现肝细胞的形态和功能,再将预处理的MSCs给予肝移植术后免疫抑制或者缺陷的受体,这种肝细胞样细胞仍有肝细胞的相关功能,如糖原储存、尿素合成、表达磷酸烯醇式丙酮酸羧激酶(phosphoenolpyruvate carboxykinase,PCK1)、合成白蛋白等[60]。在肝移植中,MSCs可分化为有功能的肝细胞样细胞,这在体内实验和体外均可证明[61-62]。因此,MSCs可能通过直接分化来替代受损细胞并改善受体移植物的功能。
另一方面,有学者认为MSCs的旁分泌效应或许才是修复的主要原因。MSCs可分泌许多种可溶性营养细胞因子和生长因子、含mRNA和miRNA的微泡和招募受损区域的祖细胞而发挥修复作用[63-66]。MSCs在很多疾病模型中几乎仅在急性期才有明显疗效,直接分化的证据也十分有限,这表明MSCs的治疗效果在很大程度上可能依赖于本身改善受损组织微环境的能力[10]。在减体积大鼠肝移植实验中,MSCs保护肝脏功能可能是通过分泌VGEF等许多营养因子实现,而非直接分化[67]。胰岛移植中,MSCs共移植后的移植物功能改善与周围血管再生率升高有关,而与MSCs可能分化为极少量的胰岛β细胞无关[68]。
MSCs的旁分泌效应可保护移植物,除了上述免疫调节作用外,还主要表现在促血管生成、抗凋亡、抗炎、内生修复、抗纤维化等方面[69]。①MSCs促进内皮细胞的增殖,改善组织血运,提高组织修复能力。MSCs能分泌VEGF,是最重要的营养性因子之一,能促进血管再生、内皮细胞的增殖和存活以及血运重建,提高胰岛移植物的生存[70-73]。除了VEGF,MSCs还可分泌TGF-β、MMP-2、MMP-14作用于内皮细胞,增加周围组织血管密度,改善血供[74-75]。②MSCs分泌营养因子,调节炎症分子,增加抗凋亡蛋白,促进细胞增殖保护移植物。在小体积肝移植中,MSCs上调HGF、IL-10、Bcl-2,并增加c-Jun N端激酶(c-Jun N-terminal Kinase,JNK/c-Jun)、细胞周期蛋白D1(Cyclin D1,CyD1)和NF-κB的表达,促进肝细胞增殖[76]。③MSCs在各种器官移植中具有抗纤维化的作用,如心、肺、肝、肾移植[77-80],而这可能与多种旁分泌细胞因子有关,如:BMP-7,肝细胞生长因子(hepatocyte growth factor,HGF)和TGF-β[81-83]。MSCs通过旁分泌效应改变微环境,修复损伤的作用已被广泛接受。
3 调节内质网应激作用
内质网应激(endoplasmic reticulum stress,ERS)可以严重影响各种移植物的存活和功能。胰岛移植物早期大量凋亡、功能失活仅仅用免疫排斥的原因来解释是不够的[84-85]。移植物遭受受体高血糖毒性和移植后的“非正常环境”也是非常重要的因素[86]。在移植后早期,移植物处于缺血、缺氧、缺血再灌等“非正常环境”中,这会引起严重的内质网应激[85]。而持久或严重的内质网应激是引起早期移植物凋亡、功能失活的重要原因[87]。此外,内质网应激能影响移植肝术后移植物的质量,内质网应激阻滞剂腹腔注射后,大鼠肝移植术成功率明显提高,术后肝损害有所减轻[88-89]。内质网应激在胰岛移植、心脏移植、肾脏移植和其他实体器官移植中同样影响着术后移植物的存活率和存活时间[90-93]。而MSCs可减弱内质网应激,改善移植物的存活和功能[94-95]。
MSCs减轻内质网应激的机制尚未完全阐明。在严重内质网应激中,PI3K/Akt可调节内质网应激PERK-eIF2α/CHOP通路[96],并调节细胞的凋亡;而MSCs可调节PI3K/Akt通路[97-98]。因此PI3K/Akt可能是MSCs调节ERS保护移植物的桥梁。
MSCs减弱内质网应激的效应可能与MSCs直接接触作用有关[94]。经毒胡萝卜素(thapsigargin)处理的肾小管细胞处于内质网应激状态,内质网应激相关分子CHOP和Caspase-3明显高表达;而与MSCs共培养的实验组肾小管细胞的CHOP和Caspase-3表达相对低,肾小管细胞的凋亡也显著减少;使用插入式细胞培养皿(transwell)隔绝MSCs和肾小管细胞的接触,尽管培养液共用,内质网应激的相关指标无明显减少。也有研究表明MSCs减轻内质网应激的作用可能和MSCs的旁分泌有关[95]。对经高浓度衣霉素(Tunicamycin)诱导后处于强内质网应激状态下的大鼠视网膜神经节细胞系(RGC-5),再使用脐带间充质干细胞(hUC-MSCs)条件培养液可对其有一定的保护作用。相比使用普通培养液的RGC-5,细胞内CHOP和PERK的表达明显下降,而生长速度、细胞的粘附能力以及存活情况等都有提高。
4 问题与展望
本文将目前关于MSCs对移植物的保护作用机制进行了综述,并归纳于图1。虽然针对此机制已有许多研究,但仍有许多亟需解决的问题。目前关于免疫调节的研究集中在体外细胞实验上,体内的调节效果仍不稳定。MSCs在基础研究和临床应用中的主要问题包括:①MSCs的免疫调节效应和机制在各种动物模型中并不一致。②MSCs与微环境的关系不详。MSCs可调节微环境,微环境也可以影响MSCs的功能发挥,因此需要进一步确定和掌控MSCs在适当范围内调节宿主排斥反应[44]。③MSCs输液输注可能引起的急性移植物相关功能障碍(植入综合征)。④临床器官移植术后,如何确定MSCs的输注时机、剂量以及途径[99]。⑤MSCs与免疫抑制剂如何联用,以减少免疫抑制剂的副作用。MSCs在器官移植中的应用,今后仍是重要的研究方向。
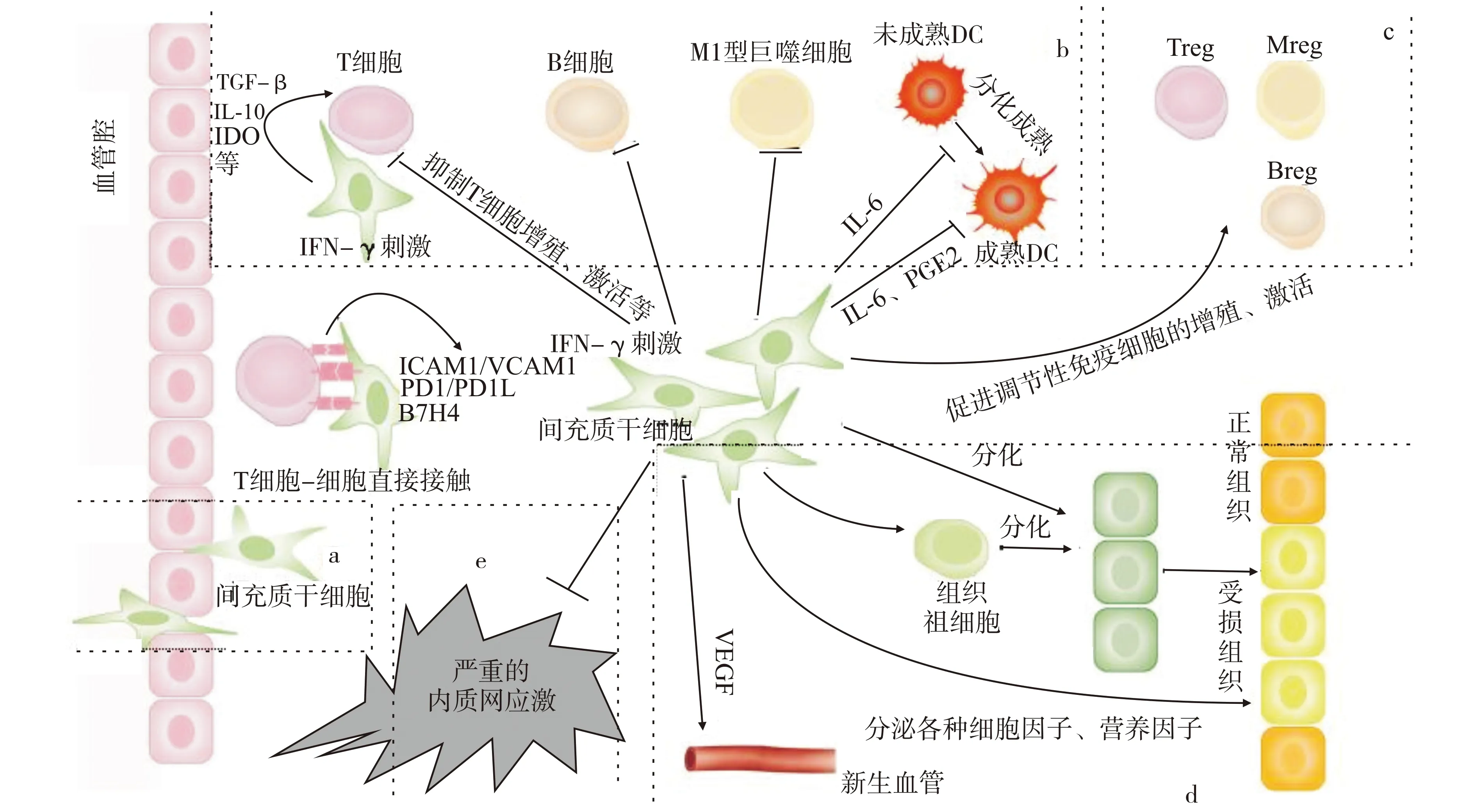
a:输注在血管内的MSCs迁徙至移植区域;b:MSCs通过细胞-细胞直接接触或者旁分泌抑制各种免疫细胞的增殖和激活等;c:MSCs扩增或激活调节性细胞群的活动;d:MSCs通过旁分泌各种细胞因子、营养因子修复受损组织,促进血管生成,促进区域组织祖细胞分化替代受损组织,直接分化替代受损组织;e:抑制严重的内质网应激。(注:各细胞、组织大小比例不代表真实情况)图1 间充质干细胞(MSCs)在移植区域发挥保护作用Fig.1 Mesenchymal stem cells(MSCs)play a protective role in the transplant area
[1] Xu Y,Wang L,He J,et al.Prevalence and control of diabetes in Chinese adults[J].JAMA,2013,310(9):948-959.
[2] Prockop D J.Marrow stromal cells as stem cells for nonhematopoietic tissues[J].Science,1997,276(5309):71-74.
[3] Da Silva Meirelles L,Chagastelles P C,Nardi N B.Mesenchymal stem cells reside in virtually all post-natal organs and tissues[J].J Cell Sci,2006,119(11):2204-2213.
[4] Bartholomew A,Sturgeon C,Siatskas M,et al.Mesenchymal stem cells suppress lymphocyte proliferationinvitroand prolong skin graft survivalinvivo[J].Exp Hematol,2002,30(1):42-48.
[5] Ding Y,Xu D,Feng G,et al.Mesenchymal stem cells prevent the rejection of fully allogeneic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9[J].Diabetes,2009,58(8):1797-1806.
[6] Xu D M,Yu X F,Zhang D,et al.Mesenchymal stem cells differentially mediate regulatory T cells and conventional effector T cells to protect fully allogeneic islet grafts in mice[J].Diabetologia,2012,55(4):1091-1102.
[7] Ge W,Jiang J,Arp J,et al.Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression[J].Transplantation,2010,90(12):1312-1320.
[8] Wang Y,Zhang A,Ye Z,et al.Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion[J].Transplant Proc,2009,41(10):4352-4356.
[9] Chabannes D,Hill M,Merieau E,et al.A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells[J].Blood,2007,110(10):3691-3694.
[10] Uccelli A,Moretta L,Pistoia V.Mesenchymal stem cells in health and disease[J].Nat Rev Immunol,2008,8(9):726-736.
[11] Jurewicz M,Yang S,Augello A,et al.Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes[J].Diabetes,2010,59(12):3139-3147.
[12] Augello A,Tasso R,Negrini S M,et al.Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway[J].Eur J Immunol,2005,35(5):1482-1490.
[13] Fiorina P,Jurewicz M,Augello A,et al.Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes[J].J Immunol,2009,183(2):993-1004.
[14] Ren G,Zhao X,Zhang L,et al.Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression[J].J Immunol,2010,184(5):2321-2328.
[15] Xue Q,Luan X Y,Gu Y Z,et al.The negative co-signaling molecule b7-h4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity[J].Stem Cells Dev,2010,19(1):27-38.
[16] Ryan J M,Barry F,Murphy J M,et al.Interferon-γ does not break,but promotes the immunosuppressive capacity of adult human mesenchymal stem cells[J].Clin Exp Immunol,2007,149(2):353-363.
[17] Zhao W,Wang Y,Wang D,et al.TGF-beta expression by allogeneic bone marrow stromal cells ameliorates diabetesm in NOD mice through modulating the distribution of CD4+T cell subsets[J].Cell Immunol,2008,253(1/2):23-30.
[18] Cuerquis J,Romieu-Mourez R,François M,et al.Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli[J].Blood,2002,99(10):3838-3843.
[19] Yanez R,Oviedo A,Aldea M,et al.Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells[J].Exp Cell Res,2010,316(19):3109-3123.
[20] English K,Ryan J M,Tobin L,et al.Cell contact,prostaglandin E(2)and transforming growth factor beta 1 play nonredundant roles in human mesenchymal stem cell induction of CD4+CD25(High)forkhead box P3+ regulatory T cells[J].Clin Exp Immunol,2009,156(1):149-160.
[21] Jarvinen L,Badri L,Wettlaufer S,et al.Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator[J].J Immunol,2008,181(6):4389-4396.
[22] Ding Y C,Xu D,Feng G,et al.Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and-9[J].Diabetes,2009,58(8):1797-1806.
[23] Perillo N L,Pace K E,Seilhamer J J,et al.Apoptosis of T cells mediated by galectin-1[J].Nature,1995,378(6558):736-739.
[24] Curciarello R,Steele A,Cooper D,et al.The role of Galectin-1 and Galectin-3 in the mucosal immune response to citrobacter rodentium infection[J].PLoS One,2014,9(9):e107933.
[25] Norambuena A,Metz C,Vicua L,et al.Galectin-8 induces apoptosis in Jurkat T cells by phosphatidic acid-mediated ERK1/2 activation supported by protein kinase A down-regulation[J].J Biol Chem,2009,284(19):12670-12679.
[26] Sioud M,Mobergslien A,Boudabous A,et al.Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins[J].Int J Oncol,2011,38(2):385-390.
[27] Jiang X X,Zhang Y,Liu B,et al.Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells[J].Blood,2005,105(10):4120-4126.
[28] Nauta A J,Kruisselbrink A B,Lurvink E,et al.Mesenchymal stem cells inhibit generation and function of both CD34(+)-derived and monocyte-derived dendritic cells[J].J Immunol,2006,177(4):2080-2087.
[29] Jurewicz M,Yang S,Augello A,et al.Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes[J].Diabetes,2010,59(12):3139-3147.
[30] Djouad F,Charbonnier L M,Bouffi C,et al.Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism[J].Stem Cells,2007,25(8):2025-2032.
[31] Deng Y,Yi S,Wang G,et al.Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOSCS1[J].Stem Cells Dev,2014,23(17):2080-2092.
[32] Liu X,Qu X,Chen Y,et al.Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOSC3 activtion[J].J Immunol,2012,189(3):1182-1192.
[33] Liu W H,Liu J J,Wu J.et al.Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathyway[J].PLoS One,2013,8(1):55487.
[34] Chen L,Zhang W,Yue H,et al.Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+cells[J].Stem Cells Dev,2007,16(5):719-731.
[35] Beyth S,Borovsky Z,Mevorach D,et al.Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness[J].Blood,2005,105(5):2214-2219.
[36] English K,Barry F P,Mahon B P.Murine mesenchymal stem cells suppress dendritic cell migration,maturation and antigen presentation[J].Immunol Lett,2008,115(1):50-58.
[37] Mannon R B.Macrophages:contributors to allograft dysfunction,repair,or innocent bystanders?[J].Curr Opin Organ Transplant,2012,17(1):20-25.
[38] Francois M,Romieu-Mourez R,Li M,et al.Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation[J].Mol Ther,2012,20(1):187-195.
[39] Nakajima H,Uchida K,Rodriguez Guerrero A,et al.Transplantation of mesenchymal stem cells promotes the alternative pathway of macrophage activation and functional recovery after spinal cord injury[J].J Neurotrauma,2012,29(8):1614-1625.
[40] Sotiropoulou P A,Perez S A,Gritzapis A D,et al.Interactions between human mesenchymal stem cells and natural killer cells[J].Stem Cells(Dayton,Ohio),2006,24(1):74-85.
[41] Spaggiari G M,Capobianco A,Abdelrazik H,et al.Mesenchymal stem cells inhibit natural killer-cell proliferation,cytotoxicity,and cytokine production:role of indoleamine 2,3-dioxygenase and prostaglandin E2[J].Blood,2008,111(3):1327-1333.
[42] Corcione A,Benvenuto F,Ferretti E,et al.Human mesenchymal stem cells modulate B-cell functions[J].Blood,2006,107(1):367-372.
[43] Hoogduijn M J,Popp F C,Grohnert A,et al.Advancement of mesenchymal stem cell therapy in solid organ transplantation(MISOT)[J].Transplantation,2010,90(2):124-126.
[44] Casiraghi F,Perico N,and Remuzzi G.Mesenchymal stromal cells to promote solid organ transplantation tolerance[J].Curr Opin Orgin Transplant,2013,18(1):51-58.
[45] Cortinovis M,Casiraghi F,Remuzzi G,et al.Mesenchymal stromal cells to control donor-specific memory T cells in solid organ transplantation[J].Curr Opin Organ Transplant,2015,20(1):79-85.
[46] Franquesa M,Hoogduijn M J,Baan C C.The impact of mesenchymal stem cell therapy in transplant rejection and tolerance[J].Curr Opin Organ Transplant,2012,17(4):355-361.
[47] English K,Ryan J M,Tobin L,et al.Cell contact,prostaglandin E(2)and transforming growth factor beta 1 play nonredundant roles in human mesenchymal stem cell induction of CD4+CD25hiforkhead box P3+regulatory T cells[J].Clin Exp Immunol,2009,156(1):149-160.
[48] Croitoru-Lamoury J,Lamoury F M,Caristo M,et al.Interferon-gamma regulates the proliferation and differentiation of mesenchymal stem cells via activation of indoleamine 2,3 dioxygenase(IDO)[J].PLoS One,2011,6(2):e14698.
[49] Hutchinson J A,Riquelme P,Sawitzki B,et al.Cutting edge:immunological consequences and trafcking of human regulatory macrophages administered to renal transplant recipients[J].J Immunol,2011,187(5):2072-2078.
[50] Nemeth K,Leelahavanichkul A,Yuen P S,et al.Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production[J].Nat Med,2009,15(1):42-49.
[51] Maggini J,Mirkin G,Bognanni I,et al.Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like pro-le[J].PLoS One,2010,5(2):e9252.
[52] Asari S,Itakura S,Ferreri K,et al.Mesenchymal stem cells suppress B-cell terminal differentiation[J].Exp Hematol,2009,37(5):604-615.
[53] Krampera M,Pasini A,Pizzolo G,et al.Regenerative and immunomodulatory potential of mesenchymal stem cells[J].Curr Opin Pharmacol,2006,6(4):435-441.
[54] Rasmusson I,Le Blanc K,Sundberg B,et al.Mesenchymal stem cells stimulate antibody secretion in human B cells[J].Scand J Immunol,2007,65(4):336-343.
[55] Mougiakakos D,Jitschin R,Johansson C C,et al.The impact of inammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells[J].Blood,2011,117(18):4826-4835.
[56] Ren G,Chen X,Dong F,et al.Concise review:mesenchymal stem cells and translational medicine:emerging issues[J].Stem Cells Transl Med,2012,1(1):51-58.
[57] Devine S M,Cobbs C,Jennings M,et al.Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into non-human primates[J].Blood,2003,101(8):2999-3001.
[58] Ianus A,Holz G G,Theise N D,et al.Invivoderivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion[J].J Clin Invest,2003,111(6):843-850.
[59] Hess D,Li L,Martin M,et al.Bone marrow-derived stem cells initiate pancreatic regeneration[J].Nat Biotechnol,2003,21(7):763-770.
[60] Aurich I,Mueller L P,Aurich H,et al.Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers[J].Gut,2007,56(3):405-415.
[61] Stock P,Brückner S,Ebensing S,et al.The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver[J].Nat Protoc,2010,5(4):617-627.
[62] Puglisi M A,Tesori V,Lattanzi W,et al.Therapeutic implications of mesenchymal stem cells in liver injury[J].J Biomed Biotechnol,2011,2011:860578.
[63] Wang Y,Chen X,Cao W,et al.Plasticity of mesenchymal stem cells in immunomodulation:pathological and therapeutic implications[J].Nat Immunol,2014,15(11):1009-1016.
[64] Quesenberry P J,Aliotta J M.Cellular phenotype switching and microvesicles[J].Adv Drug Deliv Rev,2010,62(12):1141-1148.
[65] Li Y,Chen X,Chen X G,et al.Human marrow stromal cell therapy for stroke in rat:neurotrophins and functional recovery[J].Neurology,2002,59(4):514-523.
[66] Munoz J R,Stoutenger B R,Robinson A P,et al.Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice[J].Proc Natl Acad Sci USA,2005,102(50):18171-18176.
[67] Du Z,Wei C,Cheng K,et al.Mesenchymal stem celleconditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation[J].J Surg Res,2013,183(2):907-915.
[68] Borg D J,Weigelt M,Wilhelm C,et al.Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model[J].Diabetologia,2014,57(3):522-531.
[69] Nesselmann C,Ma N,Bieback K,et al.Mesenchymal stem cells and cardiac repair[J].J Cell Mol Med,2008,12(5B):1795-1810.
[70] Ito T,Itakura S,Todorov I,et al.Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function[J].Transplantation,2010,89(12):1438-1445.
[71] Figliuzzi M,Bonandrini B,Silvani S,et al.Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes[J].World J Stem Cells,2014,6(2):163-172.
[72] Kinnaird T,Stabile E,Burnett M S,et al.Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms[J].Circulation,2004,109(12):1543-1549.
[73] Haynesworth S E,Baber M A,Caplan A I.Cytokine expression by human marrow-derived mesenchymal progenitor cellsinvitro:effects of dexamethasone and IL-1 alpha[J].J Cell Physiol,1996,166(3):585-592.
[74] Ahlborg H G,Johnell O,Turner C H,et al.Bone loss and bone size after menopause[J].N Engl J Med,2003,349(4):327-334.
[75] Beyth S,Borovsky Z,Mevorach D,et al.Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unrespon-siveness[J].Blood,2005,105(5):2214-2219.
[76] Wang W,Du Z,Yan J,et al.Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts:involvement of C-Jun N-terminal kinase,cyclin D1,and NF-κB[J].PLoS One,2014,9(12):e112532.
[77] Ohnishi S,Sumiyoshi H,Kitamura S,et al.Mesenchymal stem cells attenuate Diabetes,2005,54:100-106.cardiacbroblast proliferation and collagen synthesis through paracrine actions[J].FEBS Lett,2007,581(21):3961-3966.
[78] Ortiz L A,Gambelli F,McBride C,et al.Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates itsbrotic effects[J].Proc Natl Acad Sci USA,2003,100(14):8407-8411.
[79] Abdel Aziz M T,Atta H M,Mahfouz S,et al.Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liverbrosis[J].Clin Biochem,2007,40(12):893-899.
[80] Ninichuk V,Gross O,Segerer S,et al.Multipotent mesenchymal stem cells reduce interstitialbrosis but do not delay progression of chronic kidney disease in collagen 4A3-decient mice[J].Kidney Int,2006,70(1):121-129.
[81] Villanueva S,Ewertz E,Carrion F,et al.Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model[J].Clin Sci(Lond),2011,121(11):489-499.
[82] Li L,Zhang Y,Li Y,et al.Mesenchymal stem cell transplantation attenuates cardiacbrosis associated with isoproterenol-induced global heart failure[J].Transpl Int,2008,21(12):1181-1189.
[83] Ueno T,Nakashima A,Doi S,et al.Mesenchymal stem cells ameliorate experimental peritonealbrosis by suppressing inammation and inhibiting TGF-beta1 signaling[J].Kidney Int,2013,84(2):297-307.
[84] Zhu M,Guo M,Fei L,et al.4-Phenylbutyric acid attenuates endoplasmic reticulum stress-mediated pancreatic β-cell apoptosis in rats with streptozotocin-induced diabetes[J].Endocrine,2014,47(1):129-137.
[85] Potter K J,Westwell-Roper C Y,Klimek-Abercrombie A M,et al.Death and dysfunction of transplanted beta-cells:lessons learned from type 2 diabetes?[J].Diabetes,2014,63(1):12-19.
[86] Rickels M R,Schutta M H,Markmann J F,et al.{beta}-Cell function following human islet transplantation for type 1 diabetes[J].Diabetes,2005,54(1):100-106.
[87] Negi S,Park S H,Jetha A,et al.Evidence of endoplasmic reticulum stress mediating cell death in transplanted human islets[J].Cell Transplant,2012,21(5):889-900.
[88] Pallet N,Fougeray S,Beaune P,et al.Endoplasmic reticulum stress:an unrecognized actor in solid organ transplantation[J].Transplantation,2009,88(5):605-613.
[89] Lu H,Lu L,Xu Z C,et al.Tauroursodeoxycholic acid and 4-phenyl butyric acid alleviate endoplasmic reticulum stress and improve prognosis of donation after cardiac death liver transplantation in rats[J].Hepatobiliary Pancreat Dis Int,2014,13(6):586-593.
[90] Mizukami H,Takahashi K,Inaba W,et al.Involvement of oxidative stress-induced DNA damage,endoplasmic reticulum stress,and autophagy deficits in the decline of β-cell mass in Japanese type 2 diabetic patients[J].Diabetes Care,2014,37(7):1966-1974.
[91] Xiang J,Gu X,Qian S,et al.Endoplasmic reticulum stress-mediated apoptosis involved in indirect recognition pathway blockade induces long-term heart allograft survival[J].J Biomed Biotechnol,2010,2010:705431.
[92] Fougeray S,Loriot M A,Nicaud V,et al.Increased body mass index after kidney transplantation in activating transcription factor 6 single polymorphism gene carriers[J].Transplant Proc,2011,43(9):3418-3422.
[93] Palle N,Bouvier N,Beaune P,et al.Involvement of endoplasmic reticulum stress in solid organ transplantation[J].Med Sci(Paris),2010,26(4):397-403.
[94] Zhu X Y,Urbieta-Caceres V,Krier J D,et al.Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms[J].Stem Cells,2013,31(1):117-125.
[95] 胡婕,江冰.干细胞条件培养液对RGCs内质网应激的保护作用及机制[D].长沙:中南大学,2013.
[96] Mounir Z,Krishnamoorthy J L,Wang S,et al.Akt determines cell fate through the negative regulation of the PERK-eIF2α phosphorylation pathway[J].Sci Signal,2011,4(192):ra62.
[97] Gnecchi M,He H,Liang O D,et al.Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells[J].Nat Med,2005,11(4):367-368.
[98] Yulyana Y,Ho I A,Sia K C,et al.Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1 R/PI3K/Akt signaling[J].Mol Ther,2015,23(4):746-756.
[99] Casiraghi F,Azzollini N,Todeschini M,et al.Localization of mesenchymal stromal cells dictates their immune or proinammatory effects in kidney transplantation[J].Am J Transplant,2012,12(9):2373-2383.
(2015-12-03 收稿)
*国家自然科学基金资助项目(No.81570678);国家卫生和计划生育委员会公益性行业科研专项基金资助项目(No.201302009)
R392.4
10.3870/j.issn.1672-0741.2016.06.022
黄江鹏,男,1989年生,医学硕士,E-mail:529664106@qq.com
△通讯作者,Corresponding author,E-mail:shijundoc@yahoo.com(时军),nqgong@tjh.tjmu.edu.cn(宫念樵)
