大碳笼内嵌钆氧化物团簇金属富勒烯Gd2O@C88:实验和理论研究
2017-01-12徐时清吕延超李燕丽刘子阳
徐时清,张 沛,吕延超,李燕丽,杨 华,刘子阳
(中国计量大学 材料科学与工程学院,浙江 杭州 310018)
大碳笼内嵌钆氧化物团簇金属富勒烯Gd2O@C88:实验和理论研究
徐时清,张 沛,吕延超,李燕丽,杨 华,刘子阳
(中国计量大学 材料科学与工程学院,浙江 杭州 310018)
利用改进的电弧放电技术合成及多步高效液相色谱(HPLC)分离方法,得到了大碳笼含钆氧化物团簇的金属富勒烯Gd2O@C88.激光解吸电离碰撞诱导解离质谱/质谱的研究结果表明,氧化钆团簇位于碳笼内部.密度泛函理论计算结果表明,金属富勒烯结构为Gd2O@D2(35)-C88.内嵌团簇向碳笼转移4个电子,价态+4,碳笼-4价,金属富勒烯的电子结构表示为[Gd2O]4+@[D2(35)-C88]4-.
金属富勒烯;氧化物团簇;大碳笼;密度泛函理论

1 实验部分
1.1 金属富勒烯的电弧放电制备
金属富勒烯的制备采用自制的电弧放电装置进行.放电装置主要由真空水冷腔体、20 mm直径金属钨阴极、5根φ8 mm石墨棒阳极以及可调节进料速度步进电机等部分组成.为了得到氧化钆团簇内嵌金属富勒烯,向钻有6 mm孔的石墨棒中充填Gd2O3、Co3O4和石墨粉混合物,其中Gd/C/Co摩尔比为1∶40∶0.1.在真空条件下,首先对以上石墨阳极进行200 A电流活化5 min.然后,在0.027 MPa的氦气和氧气(He和O2体积比9∶1)压力下,80 A电流条件下放电,制得电弧放电烟炱.
1.2 金属富勒烯的提取与分离
原始烟炱用邻二氯苯在超声波震荡条件下,于40 ℃重复提取3次.提取物以氯苯为流动相,经过三轮高效液相色谱(HPLC)分离,得到Gd2O@C88.第一轮分离使用Buckyprep-M柱,取8~12 min流出组分,该组分用Buckyprep柱分离,取19.5~22.6 min组分,第三轮使用5PBB柱,取40.1~44.6 min色谱柱,得到纯Gd2O@C88.分离使用的色谱柱均为φ10 mm×250 mm规格,氯苯流动相流速4.5 mL/min,色谱检测波长450 nm.
1.3 光谱与质谱表征
紫外-可见-近红外光谱由日本岛津公司UV-3600,测定波长范围400~1 600 nm.
质谱表征在AB Sciex公司3700 TOF/TOF质谱系统上进行.采用激光解吸电离(LDI)方式.在串联质谱(MS/MS)实验中,选择碰撞能量3 keV,碰撞气体为Ar气.
1.4 密度泛函理论计算
所有密度泛函理论计算均使用Gaussian09软件.符合独立五边形规则的C88全部初始构型由螺旋算法生成[14].该构型经过半经验理论优化后,作为密度泛函水平优化的输入构型.使用B3LYP混合密度泛函对空心富勒烯和金属富勒烯进行计算,碳采用B3LYP/6-31G(d)基组,Gd采用CEP-31赝势模型式和赝势基组.
2 结果与讨论
金属富勒烯提取物以氯苯为流动相,使用三种不同保留行为的色谱柱,经过三轮HPLC分离,得到纯度较高的Gd2O@C88异构体.图1显示了该异构体的色谱和质谱分析图.色谱分析中,采用Buckyprep-M色谱柱,甲苯流动相.由图1可见,色谱流出曲线在21.3~23.6 min呈高斯峰形状,表明所分离的Gd2O@C88异构体纯度可能较高.质谱分析检验中,激光解吸电离飞行时间质谱图上仅出现强度较大的Gd2O@C88分子离子峰,其它杂质峰非常小.由质谱峰的强度计算可知,分离到的Gd2O@C88纯度大于99%.
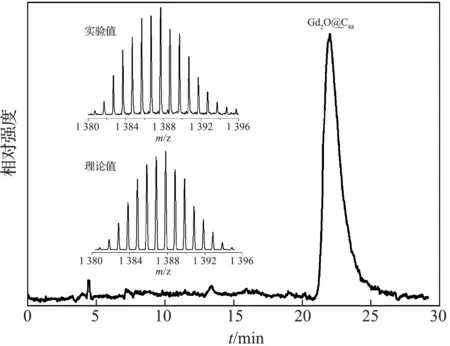
HPLC条件:色谱柱Buckyprep-M 10×250 mm,甲苯流动相流速4.5 mL/min,检测波长450 nm.质谱条件:激光解吸电离,插图上图为展宽的分子离子峰,下图为Gd2O@C88峰同位素的理论分布图1 Gd2O@C88异构体的HPLC谱图和激光解吸 电离飞行时间质谱图Figure 1 HPLC profile and LDI-TOF-MS of the purified Gd2O@C88
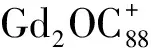

图2 Gd2O@C88的激光解吸电离串联质谱图Figure 2 Spectrum LDI-MS/MS of Gd2O@C88
Gd2O@C88的紫外-可见-近红外光谱图示于图3.由图可见,在400~1 600 nm光谱范围内,光谱图显示了了一系列特征的谱带或谱峰.在1 095 nm出现一个非常强的吸收峰,在555 nm处有一弱峰.而在623 nm和671 nm处有两个中等强度的肩带,此外,在800和920 nm附近有两个强度较弱的宽带.值得指出的是,Gd2O@C88的光谱特征与已报道的基于C88碳笼形成的金属富勒烯M3N@C88(M=Gd, Tb, Nd等)[17-19],M2@C88(M=Dy, Er)[20-21]and M@C88(M=Ca, Yb)[22-23]没有任何相似之处,然而与M2C90(M=Er, Dy, Gd)[20-21]有一定的相似性.从图3的光谱上可以看出,吸收转折点在1 382 nm处,对应于光谱带隙0.89 eV,与区分富勒烯带隙大小的1.0 eV的标准相比,Gd2O@C88属于小带隙富勒烯,这正是金属富勒烯的一个特点.然而,其带隙比具有特殊电化学可逆性,且带隙非常小的Gd3N@C88稍大.
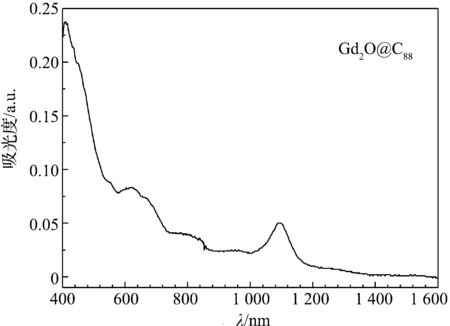
图3 Gd2O@C88在CS2溶液中的紫外-可见-近红外光谱图Figure 3 UV-vis-NIR absorption spectrum of Gd2O@C88 in CS2

表1 最稳定的10个C88和的相对能量 (ΔE, 4.186 8×103J/mol)和HOMO-LUMO带隙(eV)Table 1 Relative energies (ΔE, 4.186 8×103J/mol) and HOMO-LUMO gaps (gap, eV) of the ten most stable C88, computed at the DFT Level
注:1kcal=4.186 8×103J

表2 密度泛函理论计算得到的最稳定的5个Gd2O@C88异构体的相对能量(ΔE, 4.186 8×103J/mol) 和HOMO-LUMO带隙(eV)Table 2 Relative energies (ΔE, 4.186 8×103J/mol) andHOMO-LUMO gaps (eV) of Gd2O cluster encapsulatedinside the five most stable C88 tetra-anionscomputed at the DFT Level
注:1 kcal=4.1868×103J
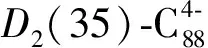
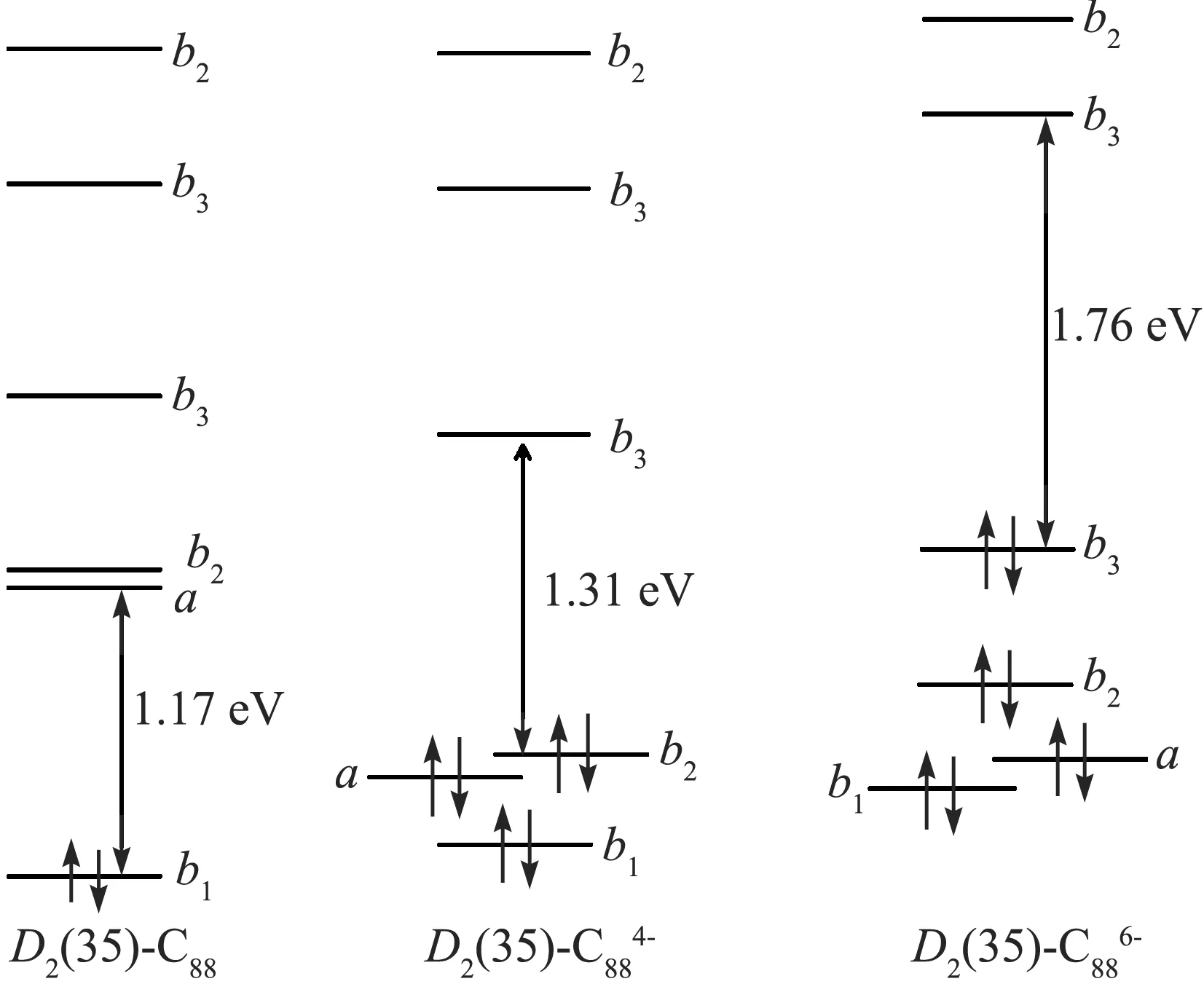
图4 D2(35)-C88碳笼的中性、负4价和6价离子的能级 和HOMO-LUMO带隙图,计算在B3LYP/6-31G(d)水平Figur 4 Orbital levels and HOMO-LUMO gap scheme of neutral, tetra-, and hexa-anionicD2(35)-C88calculated at DFT B3LYP/6-31G(d) level
密度泛函理论计算结果显示,金属富勒烯Gd2O@D2(35)-C88的整体的对称性属于D2点群,与碳笼的D2对称性保持一致,这种一致性在金属富勒烯领域尚属首次.金属富勒烯的D2对称性使得Gd2O为线形结构,即Gd-O-Gd键角为180°,2个Gd原子位于三个C2轴中的一个轴上,如图5所见.这种线形结构与已鉴定结构的Sc2O团簇金属富勒烯完全不同,所有Sc2O单元都形成弯曲结构[7-10].此外,文献报道的硫化钪团簇金属富勒烯Sc2S@Cs(10528)-C72中,Sc2S也是弯曲结构.在Gd2O@D2(35)-C88中,Gd位于六元环下方,到该环上碳原子的距离分别为2.62、2.67和2.68 Å,比Gd3N@C80中相应的距离稍大39.Gd-O键长2.07 Å,稍小于常见Gd的有机化合物中Gd-O键的长度,这可能由于受到碳笼空间大小的限制所引起的.通过电荷布居分析可知,Gd为正3价,O为负2价,这样,金属富勒烯Gd2O@D2(35)-C88的电子结构可以确定表示为[Gd2O]4+@[D2(35)-C88]4-.

图5 优化的Gd2O@D2(35)-C88两个垂直方向的结构图Figure 5 Two orthogonal views of the optimized Gd2O @D2(35)-C88
3 结 语
通过向石墨阳极引入强氧化剂Co3O4以及在气相中引入氧气成分,成功合成并分离得到了大碳笼含钆氧化物团簇的金属富勒烯.对该样品的激光解吸电离碰撞诱导解离质谱/质谱的研究表明,氧化钆团簇位于碳笼内部,紫外可见近红外光谱表明Gd2O@C88具有典型的金属富勒烯特征,带隙小于空心富勒烯.密度泛函理论计算结果显示,金属富勒烯采用D2点群对称性的D2(35)-C88碳笼,金属富勒烯Gd2O@D2(35)-C88中,Gd2O单元呈线性结构,位于碳笼的一个C2轴上,整个分子也呈D2点群对称性.内嵌团簇向碳笼转移4个电子,价态+4,金属富勒烯的电子结构表示为[Gd2O]4+@[D2(35)-C88]4-.
[1] AKASAKA T, NAGASE S. Endofullerenes: A New Family of Carbon Clusters[M]. London: Kluwer Academic Publishers,2005:50-70.
[2] POPOV A A, YANG S, DUNSCH L. Endohedral fullerenes[J]. Chemical Reviews,2013,113(8):5989-6113.
[3] AKASAKA T, WAKAHARA T, NAGASE S, et al. La@C82anion. An unusually stable metallofullerene[J]. Journal of the American Chemical Society,2000,122:9316.
[4] PAVANELLO M, JALBOUT A F, TRZASKOWSKI B. et al. Fullerene as an electron buffer: charge transfer in Li@C60[J]. Chemical Physics Letters,2007,442(4-6):339-343.
[5] CAO B P, SUENAGA K, OKAZAKI T, et al. Production, isolation, and EELS characterization of Ti2@C84dititanium metallofullerenes[J]. Journal of Physical Chemistry,2002,106(36):9295-9298.
[6] STEVENSON S, MACKEY M A, STUART M A, et al. A Distorted tetrahedral metal oxide cluster inside an icosahedral carbon cage. synthesis, isolation, and structural characterization of Sc4((3-O)2@Ih-C80[J]. Journal of the American Chemical Society,2008,130(36):11844-11845.
[7] TANG Q Q, ABELLA L, HAO Y J, et al. Sc2O@C2v(5)-C80: dimetallic oxide cluster inside a C80fullerene cage[J]. Inorganic Chemistry,2015,54(20):9845-9852.
[8] MERCADO B Q, STUART M A, MACKEY M A, et al. Balch, Sc2(μ2-O) trapped in a fullerene cage: the isolation and structural characterization of Sc2(μ2-O)@Cs(6)-C82and the relevance of the thermal and entropic effects in fullerene isomer selection[J]. Journal of the American Chemical Society,2010,132(34):12098-12105.
[9] TANG Q Q, ABELLA L, HAO Y J, et al. Sc2O@C3v(8)-C82: a missing isomer of Sc2O@C82[J]. Inorganic Chemistry,2016,55(4):1926-1933.
[10] YANG T, Y. HAO J, ABELLA L, et al. Sc2O@Td(19151)-C76: hindered cluster motion inside a tetrahedral carbon cage probed by crystallographic and computational studies[J]. Chemistry-A European Journal,2015,21(31):11110-11117.
[11] CHEN N, C. BEAVERS M, MULET-GAS M. Echegoyen, et al. Sc2S@Cs(10528)-C72: a dimetallic sulfide endohedral fullerene with a non isolated pentagon rule cage[J]. Journal of the American Chemical Society,2012,134(18):7851-7860.
[12] ZHAO P, LI M Y, GUO Y J, et al. Single step stone-wales transformation linking two thermodynamically stable Sc2O@C78isomers[J]. Inorganic Chemistry,2016,55(5):2220-2226.
[13] GUO Y J, ZHAO X, ZHAO P, et al. Theoretical insight into Sc2O@C84: interplay between small cluster and large carbon cage[J]. Journal of Physical Chemistry,2015,119(41):10428-10439.
[14] FOWLER P W, MANOLOPOULOS D E. An Atlas of Fullerenes[M]. Oxford: Clarendon Press,1995:30-50.
[15] HETTICH R, LAHAMER A, ZHOU L, et al. Investigation of the fragmentation and oxygen reactivity of endohedral metallofullerenes M@C60[J]. International Journal of Mass Spectrometry,1999,183:335.
[16] WEISS F D, ELKIND J L, O’BRIEN S C, et al. Photophysics of metal complexes of spheroidal carbon shells[J]. Journal of the American Chemical Society,1988,110:4464.
[17] CHAUR M N, MELIN F, ELLIOTT B, et al. Gd3N@C2n(n=40, 42, and 44): remarkably low HOMO-LUMO gap and unusual electrochemical reversibility of Gd3N@C88[J]. Journal of the American Chemical Society,2007,129:14826.
[18] ZUO T M, BEAVERS C M, DUCHAMP J C, et al. Isolation and structural characterization of a family of endohedral fullerenes including the large, chiral cage fullerenes Tb3N@C88and Tb3N@C86as well as the Ihand D5hisomers of Tb3N@C80[J]. Journal of the American Chemical Society,2007,129:2035.
[19] MELIN F, CHAUR M N, ENGMANN S, et al. The large Nd3N@C2n(40 ( n ( 49) cluster fullerene family: preferential templating of a C88cage by a trimetallic nitride cluster[J]. Angewandte Chemie-International Edition,2007,46:9032.
[20] TAGMATARCHIS N, SHINOHARA H. Production, separation, isolation, and spectroscopic study of dysprosium endohedral metallofullerenes[J]. Chemical Materials,2000,12:3222.
[21] TAGMATARCHIS N, ASLANIS E, PRASSIDES K, et al. Mono-, di- and trierbium endohedral metallofullerenes: Production, separation, isolation, and spectroscopic study[J]. Chemical Materials,2001,13:2374.
[22] ZHANG Y, XU J X, HAO C, et al. Synthesis, isolation, spectroscopic and electrochemical characterization of some calcium-containing metallofullerenes[J]. Carbon,2006,44:475.
[23] XU J X, WANG Z Y, SHI Z J, et al. Synthesis, isolation and spectroscopic characterization of Yb-containing high metallofullerenes[J]. Chemical Physics Letters,2005,409:192.
[24] LEE C, YANG W, PARR R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density[J]. Physical Reviews B,1988.37:785.
[25] SUN G Y. Assigning the major isomers of fullerene C88by theoretical13C NMR spectra[J]. Chemical Physics Letters,2003,367(1-2):26-33.
[26] POPOV A A, DUNSCH L. Structure, stability, and cluster-cage interactions in nitride clusterfullerenes M3N@C2n(M = Sc, Y; 2n=68-98): a density functional theory study[J]. Journal of the American Chemical Society,2007,129(38):11835-11849.
[27] VALENCIA R, RODRIGUEZ-FORTEA A, POBLET J M. Large fullerenes stabilized by encapsulation of metallic clusters[J]. Chemical Communications,2007(40):4161-4163.
[28] DUNSCH L, YANG S. Metal nitride cluster fullerenes: Their current state and future prospects[J]. Small,2007,3(8):1298-1320.
High-carbon cage encasing gadolinium oxide cluster metallofulerene Gd2O@C88: an experimental and theoretical study
XU Shiqing, ZHANG Pei, LU Yanchao, LI Yanli, YANG Hua, LIU Ziyang
(College of Material Science and Technology, China Jiliang University, Hangzhou 310018, China)
The metallofullerene Gd2O@C88with Gd2O cluster encased in the high carbon cage C88was synthesized using arc discharging and isolated to isomer pure by the multi-step HPLC method. Laser desorption ionization collisional induced dissociation MS/MS indicated the Gd2O cluster was located inside the carbon cage. Density functional theory computation suggests that the whole structure can be depicted as Gd2O@D2(35)-C88and a further electronic population representation[Gd2O]4+@[D2(35)-C88]4-, with 4 electrons transferred from the encased cluster to the carbon cage. So the inside cluster shows +4 valent,while the carbon cage -4.
metallofullerene; oxide cluster; high-carbon cage; density functional theory
2096-2835(2016)04-0465-06
10.3969/j.issn.2096-2835.2016.04.019
2016-08-18 《中国计量大学学报》网址:zgjl.cbpt.cnki.net
国家自然科学基金资助项目(No.11274283,21271162),浙江省科技厅国际合作项目资助项目(No.2013C24017),浙江省自然科学基金资助项目(No.LR1ZB01001).

O661.1
A
